Luminescent κ-Carrageenan-Based Electrolytes Containing Neodymium Triflate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology
2.2. Thermal Behavior
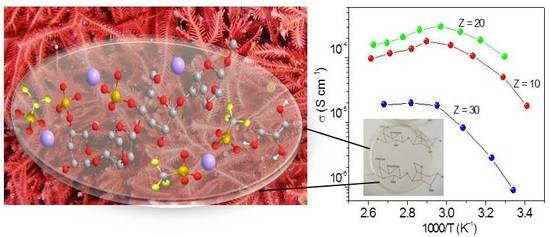
2.3. Ionic Conductivity Study
2.4. Ionic Association Study
2.5. UV/Visible and NIR Analysis
3. Experimental Section
3.1. Materials
3.2. Preparation of the κ-Cg -Based Electrolytes
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, K. Biopolymer Electrolytes-Fundamentals and Applications in Energy Storage, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Samsudin, A.S.; Khairul, W.M.; Isa, M.I.N. Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J. Non-Cryst. Solids 2012, 358, 1104–1112. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, Highly graphitized carbon aerogels based on bacterial cellulose/lignin: Catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Leones, R.; Fernandes, M.; Ferreira, R.A.S.; Cesarino, I.; Lima, J.F.; Carlos, L.D.; Bermudez, V.D.; Magon, C.J.; Donoso, J.P.; Silva, M.M.; et al. Luminescent DNA- and Agar-based membranes. J. Nanosci. Nanotechnol. 2014, 14, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Firmino, A.; Grote, J.G.; Kajzar, F.; M’Peko, J.-C.; Pawlicka, A. DNA-based ionic conducting membranes. J. Appl. Phys. 2011, 110, 033704–033709. [Google Scholar] [CrossRef]
- Avellaneda, C.O.; Vieira, D.F.; Al-Kahlout, A.; Heusing, S.; Leite, E.R.; Pawlicka, A.; Aegerter, M.A. All solid-state electrochromic devices with gelatin-based electrolyte. Sol. Energy Mater. Sol. Cells 2008, 92, 228–233. [Google Scholar] [CrossRef]
- Ramadan, R.; Kamal, H.; Hashem, H.M.; Abdel-Hady, K. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Sol. Energy Mater. Sol. Cells 2014, 127, 147–156. [Google Scholar] [CrossRef]
- Vieira, D.F.; Avellaneda, C.O.; Pawlicka, A. Conductivity study of a gelatin-based polymer electrolyte. Electrochim. Acta 2007, 53, 1404–1408. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.; Fernandes, M.; Bermudez, V.; Pawlicka, A.; Silva, M. Samarium (III) triflate-doped chitosan electrolyte for solid state electrochromic devices. Electrochim. Acta 2018, 267, 51–62. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Pawlicka, A.; Silva, M.M. Innovative electrolytes based on chitosan and thulium for solid state applications: Synthesis, structural, and thermal characterization. J. Electroanal. Chem. 2017, 788, 156–164. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellulose 2013, 20, 3227–3237. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 2015, 21, 111–124. [Google Scholar] [CrossRef]
- Raphael, E.; Avellaneda, C.O.; Aegerter, M.A.; Silva, M.M.; Pawlicka, A. Agar-based gel electrolyte for electrochromic device application. Mol. Cryst. Liq. Cryst. 2012, 554, 264–272. [Google Scholar] [CrossRef]
- Lima, E.; Raphael, E.; Sentanin, F.; Rodrigues, L.C.; Ferreira, R.A.S.; Carlos, L.D.; Silva, M.M.; Pawlicka, A. Photoluminescent polymer electrolyte based on agar and containing europium picrate for electrochemical devices. Mater. Sci. Eng. B 2012, 177, 488–493. [Google Scholar] [CrossRef]
- Tavares, F.C.; Dörr, D.S.; Pawlicka, A.; Oropesa Avellaneda, C. Microbial origin xanthan gum-based solid polymer electrolytes. J. Appl. Polym. Sci. 2018, 135, 46229–46234. [Google Scholar] [CrossRef]
- Pereira, R.F.P.; Sentanin, F.; Pawlicka, A.; Goncalves, M.C.; Silva, M.M.; Bermudez, V.D. Smart windows prepared from Bombyx mori Silk. Chemelectrochem 2016, 3, 1084–1097. [Google Scholar] [CrossRef]
- Nunes, S.C.; Pereira, R.F.P.; Sousa, N.; Silva, M.M.; Almeida, P.; Figueiredo, F.M.L.; de Zea Bermudez, V. Eco-friendly red seaweed-derived electrolytes for electrochemical devices. Adv. Sustain. Syst. 2017, 1, 1700070. [Google Scholar] [CrossRef]
- Silva, M.M.; Bermudez, V.d.Z.; Pawlicka, A. Application of Polymer Electrolytes for Electrochemical Devices. In Polymer Electrolytes: Characterization and Applications; Winie, T., Arof, A.K., Thomas, S., Eds.; Wiley-VCH: Weinheim, Germany, 2019; Volume 2. [Google Scholar]
- Singh, R.; Jadhav, N.A.; Majumder, S.; Bhattacharya, B.; Singh, P.K. Novel biopolymer gel electrolyte for dye-sensitized solar cell application. Carbohydr. Polym. 2013, 91, 682–685. [Google Scholar] [CrossRef]
- Bella, F.; Mobarak, N.N.; Jumaah, F.N.; Ahmad, A. From seaweeds to biopolymeric electrolytes for third generation solar cells: An intriguing approach. Electrochim. Acta 2015, 151, 306–311. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Boopathi, G.; Pugalendhi, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S.; Aristatil, G. Development of proton conducting biopolymer membrane based on agar–agar for fuel cell. Ionics 2017, 23, 2781–2790. [Google Scholar] [CrossRef]
- Christopher Selvin, P.; Perumal, P.; Selvasekarapandian, S.; Monisha, S.; Boopathi, G.; Leena Chandra, M.V. Study of proton-conducting polymer electrolyte based on k-carrageenan and NH4SCN for electrochemical devices. Ionics 2018, 24, 3535–3542. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Rahman, M.Y.A. Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid State Ionics 2012, 224, 51–57. [Google Scholar] [CrossRef]
- Rudhziah, S.; Ahmad, A.; Ahmad, I.; Mohamed, N.S. Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim. Acta 2015, 175, 162–168. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Jumaah, F.N.; Ghani, M.A.; Abdullah, M.P.; Ahmad, A. Carboxymethyl carrageenan based biopolymer electrolytes. Electrochim. Acta 2015, 175, 224–231. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Hemalatha, R.; Boopathi, G. Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J. Solid State Electr. 2018, 22, 3209–3223. [Google Scholar] [CrossRef]
- Jumaah, F.N.; Mobaraka, N.N.; Ahmad, A.; Ramli, N. Characterization of τ -carrageenan and its derivative based green polymer electrolytes. AIP Conf. Proc. 2013, 1571, 768–774. [Google Scholar]
- Nunes, S.C.; Saraiva, S.M.; Pereira, R.F.P.; Pereira, S.; Silva, M.M.; Carlos, L.D.; Fortunato, E.; Ferreira, R.A.S.; Rego, R.; de Zea Bermudez, V. Sustainable dual-mode smart windows for energy-efficient buildings. ACS Appl. Energy Mater. 2019. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Effect of modified natural filler o-methylene phosphonic κ-carrageenan on chitosan-based polymer electrolytes. Energies 2018, 11, 1910. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1—Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–31. [Google Scholar]
- Tanaka, F. Thermoreversible gelation strongly coupled to polymer conformational transition. Macromolecules 2000, 33, 4249–4263. [Google Scholar] [CrossRef]
- Tanaka, F. Thermoreversible gelation driven by coil-to-helix transition of polymers. Macromolecules 2003, 36, 5392–5405. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Doublier, J.-L.; Piculell, L. Viscoelastic properties for kappa- and iota-carrageenan in aqueous NaI from the liquid-like to the solid-like behaviour. Int. J. Biol. Macromol. 2000, 28, 1–14. [Google Scholar] [CrossRef]
- Carrageenans. Thermoreversible Networks: Viscoelastic Properties and Structure of Gels; Springer: Berlin/Heidelberg, Germany, 1997; pp. 203–218. [Google Scholar]
- Gonçalves, A.R.; da Silva, C.J.R.; Silva, M.M.; Smith, M.J. Ionic conduction and thermal properties of poly(ethylene oxide) - Er (CF3SO3)3 films. Ionics 1995, 1, 342–347. [Google Scholar] [CrossRef]
- de Zea Bermudez, V.; Carlos, L.D.; Duarte, M.C.; Silva, M.M.; Silva, C.J.R.; Smith, M.J.; Assunção, M.; Alcácer, L. A novel class of luminescent polymers obtained by the sol–gel approach. J. Alloys Compd. 1998, 275–277, 21–26. [Google Scholar] [CrossRef]
- Silva, M.M.; de Zea Bermudez, V.; Carlos, L.D.; Smith, M.J. Neodymium doped, sol-gel processed polymer electrolytes. Ionics 1998, 4, 170–174. [Google Scholar] [CrossRef]
- Pekcan, Ö.; Tari, Ö. A Fluorescence Study on the Gel-to-Sol Transition of κ-Carrageenan; Elselvier: Amsterdam, The Netherlands, 2004; Volume 34, pp. 223–231. [Google Scholar]
- Nishinari, K.; Koide, S.; Ogino, K. On the temperature dependence of elasticity of thermo-reversible gels. J. Phys. Fr. 1985, 46, 793–797. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Kaneko, Y.; Kadokawa, J.-i. Novel gelling systems of κ-, ι- and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol. Biosci. 2009, 9, 376–382. [Google Scholar] [CrossRef]
- Anderson, N.S.; Campbell, J.W.; Harding, M.M.; Rees, D.A.; Samuel, J.W.B. X-ray diffraction studies of polysaccharide sulphates: Double helix models for κ- and ι-carrageenans. J. Mol. Biol. 1969, 45, 85–97. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int. J. Electrochem. Sci. 2008, 3, 597–608. [Google Scholar]
- Tsutsumi, H.; Matsuo, A.; Onimura, K.; Oishi, T. Conductivity enhancement of a polyacrylonitrile-based polymer electrolyte containing cascade nitrile as a plasticizer. Electrochem. Solid State Lett. 1998, 1, 244–245. [Google Scholar] [CrossRef]
- Fujishima, M.; Matsuo, Y.; Takatori, H.; Uchida, K. Proton-conductive acid–base complex consisting of κ-carrageenan and 2-mercaptoimidazole. Electrochem. Commun. 2008, 10, 1482–1485. [Google Scholar] [CrossRef]
- de Zea Bermudez, V.; Ostrovskii, D.; Lavoryk, S.; Cristina Gonçalves, M.; Carlos, L.D. Urethane cross-linked poly(oxyethylene)/siliceous nanohybrids doped with Eu3+ ions Part 2. Ionic association. Phys. Chem. Chem. Phys. 2004, 6, 649–658. [Google Scholar] [CrossRef]
- Oza, M.D.; Meena, R.; Siddhanta, A.K. Facile synthesis of fluorescent polysaccharides: Cytosine grafted agarose and κ-carrageenan. Carbohydr. Polym. 2012, 87, 1971–1979. [Google Scholar] [CrossRef]
- Molina, C.; Ferreira, R.A.S.; Poirier, G.; Fu, L.; Ribeiro, S.J.L.; Messsaddeq, Y.; Carlos, L.D. Er3+-Based Diureasil Organic–Inorganic Hybrids. J. Phys. Chem. C 2008, 112, 19346–19352. [Google Scholar] [CrossRef]
- GENU®. Carrageenan Book; CPKelco ApS: Copenhagen, Denmark, 2001. [Google Scholar]
- Djaeni, M.; Prasetyaningrum, A.; Sasongko, S.B.; Widayat, W.; Hii, C.L. Application of foam-mat drying with egg white for carrageenan: drying rate and product quality aspects. J. Food Sci. Technol. 2015, 52, 1170–1175. [Google Scholar] [CrossRef]
- Peakfit, (Version 4.0); SYSTAT Software Inc.: San Jose, CA, USA, 2007.
Sample Availability: Samples of the compounds are not available from the authors. |







| Sample | z (%) | Tg-s (°C) | Td (°C) |
|---|---|---|---|
| κ-Cg | - | 139 [18] | 188 [18] |
| CG0Nd0 | 0 | 115 [18] | 197 [18] |
| CG50Nd0 | 0 | 124 | 190 |
| CG50Nd10 | 10 | 131 | 195 |
| CG50Nd20 | 20 | 124 | 182 |
| CG50Nd30 | 30 | 129 | 169 |
| CG50Nd40 | 40 | 124 | 170 |
| CGxNdz | |||||
|---|---|---|---|---|---|
| x | z | m (κ-Cg) (g) | V (H2O) (mL) | % Nd/κ-Cg | m (NdTrif) (g) |
| 50 | 0 | 0.3017 | 15 | - | - |
| 10 | 0.3013 | 10 | 0.0316 | ||
| 20 | 0.3020 | 20 | 0.0615 | ||
| 30 | 0.3006 | 30 | 0.0919 | ||
| 40 | 0.3020 | 40 | 0.1211 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, S.C.; Saraiva, S.M.; Pereira, R.F.P.; Silva, M.M.; Carlos, L.D.; Almeida, P.; Gonçalves, M.C.; Ferreira, R.A.S.; Bermudez, V.d.Z. Luminescent κ-Carrageenan-Based Electrolytes Containing Neodymium Triflate. Molecules 2019, 24, 1020. https://doi.org/10.3390/molecules24061020
Nunes SC, Saraiva SM, Pereira RFP, Silva MM, Carlos LD, Almeida P, Gonçalves MC, Ferreira RAS, Bermudez VdZ. Luminescent κ-Carrageenan-Based Electrolytes Containing Neodymium Triflate. Molecules. 2019; 24(6):1020. https://doi.org/10.3390/molecules24061020
Chicago/Turabian StyleNunes, S. C., S. M. Saraiva, R. F. P. Pereira, M. M. Silva, L. D. Carlos, P. Almeida, M. C. Gonçalves, R. A. S. Ferreira, and V. de Zea Bermudez. 2019. "Luminescent κ-Carrageenan-Based Electrolytes Containing Neodymium Triflate" Molecules 24, no. 6: 1020. https://doi.org/10.3390/molecules24061020