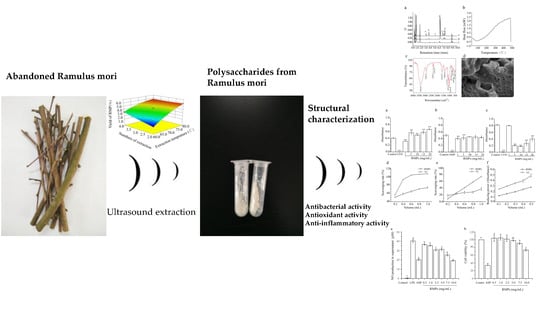
Preparation of Polysaccharides from Ramulus mori, and Their Antioxidant, Anti-Inflammatory and Antibacterial Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
2.2. Model Fitting and Statistical Analysis
2.3. Optimization of RMP Extraction
2.4. Identification of Monosaccharides
2.5. Differential Scanning Calorimetry (DSC) Analysis
2.6. FT-IR Spectroscopy Analysis
2.7. Morphological Analysis
2.8. Antibacterial Activity of RMPs
2.9. Antioxidant Activity of RMPs
2.10. Effect of RMPs on NO Production Inhibition
2.11. Effect of RMPs on the Cell Viability of RAW 264.7 Cells
3. Materials and Methods
3.1. Materials
3.2. Extraction of RMPs
3.3. Experimental Design and Statistical Analysis
3.4. UPLC Analysis of The Monosaccharide Composition of RMPs
3.5. FT-IR Analysis of RMPs
3.6. Antibacterial Experiments In Vitro
3.7. Antioxidant Activity of RMPs
3.8. Determination of Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jian, Q.; He, N.; Wang, Y.; Xiang, Z. Ecological issues of mulberry and sustainable development. J. Resour. Ecol. 2012, 3, 330–339. [Google Scholar] [CrossRef]
- Liu, Y. Application prospect of mulberry plants to vegetation restoration in Three Gorges Reservoir area. Sci. Seric. 2011, 37, 93–97. [Google Scholar]
- Wu, Y.; Liang, Z.; Xing, D. Comparison of the physiological characteristics of paper mulberry (Broussonetia papyrifera) and mulberry (Morus alba) undersimulated drought stress. Guihaia 2011, 31, 92–96. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, C.; Wang, X.; Zhang, Y.; Liu, L.; Wang, R.; Ye, J.; Hu, L.; Kong, L. Uricosuric and nephroprotective properties of Ramulus mori ethanol extract in hyperuricemic mice. J. Ethnopharmacol. 2012, 143, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, R.; Zheng, N.; Xu, L.; Liang, T.; He, Q. Anti-diabetic effect of Ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int. Immunopharmaco. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, B.; Xia, X.; Li, X.; Wang, R.; Sheng, L.; Li, D.; Liu, Y.; Li, Y. Simultaneous quantification of three active alkaloids from a traditional Chinese medicine Ramulus mori (Sangzhi) in rat plasma using liquid chromatography-tandem mass spectrometry. J. Pharmaceut. Biomed. 2015, 109, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liang, T.; He, Q.; Wei, P.; Zheng, N.; Xu, L. Renoprotective effect of Ramulus mori polysaccharides on renal injury in STZ-diabetic mice. Int. J. Biol. Macromol. 2013, 62, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, F.; Wang, J.; Huang, H.; Huang, Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015, 117, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Cook, C.M.; Borror, C.M.; Montgomery, D.C. Response surface design evaluation and comparison. J. Stat. Plan. Infer. 2009, 139, 629–641. [Google Scholar] [CrossRef]
- Granato, D.; Ribeiro, J.C.B.; Castro, I.A.; Masson, M.L. Sensory evaluation and physicochemical optimisation of soy-based desserts using response surface methodology. Food Chem. 2010, 121, 899–906. [Google Scholar] [CrossRef]
- Li, F.; Gao, J.; Xue, F.; Yu, X.; Shao, T. Extraction optimization, purification and physicochemical properties of polysaccharides from Gynura medica. Molecules 2016, 21, 397. [Google Scholar] [CrossRef]
- Yang, R.; Geng, L.; Lu, H.; Fan, X. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina baroni. Ultrason. Sonochem. 2017, 34, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, X.; Lv, G.; Hu, D.; Zhao, J.; Li, S. Optimization of microwave-assisted extraction of bioactive alkaloids from lotus plumule using response surface methodology. J. Pharmaceut. Biomed. 2016, 6, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Guo, Y.; Zhou, Q.; Zhong, X.; Zhu, L.; Piao, J.; Chen, J.; Jiang, J. Optimization of ultrasonic-assisted extraction of total saponins from Eclipta prostrasta L. using response surface methodology. J. Food. Sci. 2012, 77, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.; Wang, J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. Pulp. Carbohydr. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Souza, B.W.S.; Simões, J.; Teixeira, J.A.; Domingues, M.R.M.; Coimbra, M.A.; Vicente, A.A. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr. Polym. 2011, 83, 179–185. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, G.; Liu, B.; Wang, B. Physicochemical and functional properties of fern rhizome (Pteridium aquilinum) starch. Starch-Starke 2011, 63, 468–474. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietrantonio, E.; Pietroletti, M. The roles of carbohydrates, proteins and lipids in the process of aggregation of natural marine organic matter investigated by means of 2D correlation spectroscopy applied to infrared spectra. Spectrochim. Acta Part A 2009, 71, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Kačuráková, M.; Wilson, R.H. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Ma, H.; Wang, Z.; Pei, J. Structural characteristics and antioxidant activity in vivo of a polysaccharide isolated from Phellinus linteus mycelia. J. Taiwan Inst. Chem. E. 2016, 65, 110–117. [Google Scholar]
- Mayer, S.G.; Boyd, J.E.; Heser, J.D. A high-pressure attenuated total reflectance cell for collecting infrared spectra of carbon dioxide mixtures. Vib. Spectrosc. 2010, 53, 311–313. [Google Scholar] [CrossRef]
- Zhu, Z.; Dong, F.; Liu, X.; Lv, Q.; Yang, Y.; Liu, F.; Chen, L.; Wang, T.; Wang, Z.; Zhang, Y. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wu, Z.; Huang, B.; Sun, L.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Zhou, L.; Liu, J.; Huang, Y.; Liao, J.; Yuan, M. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mazarei, F.; Jooyandeh, H.; Noshad, M.; Hojjati, M. Polysaccharide of caper (Capparis spinosa L.) Leaf: Extraction optimization, antioxidant potential and antimicrobial activity. Int. J. Biol. Macromol. 2017, 95, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shang, X.; Zhou, X.; Zhao, B.; Zhang, J. Ultrasound-assisted extraction of polysaccharides from Rhododendro aganniphum: Antioxidant activity and rheological properties. Ultrason. Sonochem. 2017, 38, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Liu, Y.; Liu, G.; Ding, L.; Lu, X. Construction of a highly sensitive non-enzymatic sensor for superoxide anion radical detection from living cells. Biosens. Bioelectron. 2017, 90, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 35–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Zhu, J.; Xie, W.; Hu, X.; Song, L.; Zi, J.; Yu, R. Purification and characterization of an antibacterial and anti-inflammatory polypeptide from Arca subcrenata. Int. J. Biol. Macromol. 2017, 96, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xiang, X.; Jin, H.; Guo, X.; Liu, L.; Huang, Y.; OuYang, X.; Qu, Y. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hu, J.; Liu, J.; Zhou, Z.; Zhao, A. In vitro antioxidant and antimicrobial activities of flavonoids from Panax notoginseng flowers. Nat. Prod. Res. 2014, 28, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, M.; Yadav, A.; Yadav, J.P. Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. South Afr. J. Bot. 2017, 111, 50–59. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Independent Variables | Symbol | Range and Level | ||||||
|---|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||||
| Extraction temperature (°C) | X1 | 60 | 70 | 80 | ||||
| Extraction time (min) | X2 | 30 | 40 | 50 | ||||
| Solid-liquid ratio (g/mL) | X3 | 1:30 | 1:40 | 1:50 | ||||
| Numbers of extraction | X4 | 2 | 3 | 4 | ||||
| Run | Coded Variable Levels | Extraction Yield (%) | ||||||
| X1 | X2 | X3 | X4 | Experimental | Predicted | |||
| 1 | 60 | 30 | 1:40 | 3 | 1.91 | 1.93 | ||
| 2 | 80 | 30 | 1:40 | 3 | 4.74 | 4.75 | ||
| 3 | 60 | 50 | 1:40 | 3 | 1.63 | 1.65 | ||
| 4 | 80 | 50 | 1:40 | 3 | 5.97 | 5.99 | ||
| 5 | 70 | 40 | 1:30 | 2 | 3.63 | 3.63 | ||
| 6 | 70 | 40 | 1:50 | 2 | 3.22 | 3.24 | ||
| 7 | 70 | 40 | 1:30 | 4 | 4.20 | 4.21 | ||
| 8 | 70 | 40 | 1:50 | 4 | 4.69 | 4.72 | ||
| 9 | 60 | 40 | 1:40 | 2 | 1.12 | 1.12 | ||
| 10 | 80 | 40 | 1:40 | 2 | 5.81 | 5.82 | ||
| 11 | 60 | 40 | 1:40 | 4 | 3.27 | 3.26 | ||
| 12 | 80 | 40 | 1:40 | 4 | 5.73 | 5.73 | ||
| 13 | 70 | 30 | 1:30 | 3 | 3.41 | 3.42 | ||
| 14 | 70 | 50 | 1:30 | 3 | 3.59 | 3.61 | ||
| 15 | 70 | 30 | 1:50 | 3 | 3.22 | 3.20 | ||
| 16 | 70 | 50 | 1:50 | 3 | 3.97 | 3.96 | ||
| 17 | 60 | 40 | 1:30 | 3 | 1.95 | 1.93 | ||
| 18 | 80 | 40 | 1:30 | 3 | 4.85 | 4.82 | ||
| 19 | 60 | 40 | 1:50 | 3 | 1.31 | 1.30 | ||
| 20 | 80 | 40 | 1:50 | 3 | 5.59 | 5.58 | ||
| 21 | 70 | 30 | 1:40 | 2 | 3.15 | 3.14 | ||
| 22 | 70 | 50 | 1:40 | 2 | 4.10 | 4.08 | ||
| 23 | 70 | 30 | 1:40 | 4 | 4.64 | 4.63 | ||
| 24 | 70 | 50 | 1:40 | 4 | 4.67 | 4.65 | ||
| 25 | 70 | 40 | 1:40 | 3 | 4.42 | 4.38 | ||
| 26 | 70 | 40 | 1:40 | 3 | 4.39 | 4.38 | ||
| 27 | 70 | 40 | 1:40 | 3 | 4.37 | 4.38 | ||
| 28 | 70 | 40 | 1:40 | 3 | 4.41 | 4.38 | ||
| 29 | 70 | 40 | 1:40 | 3 | 4.31 | 4.38 | ||
| Source | Sum of Squares | df | Mean Square | F | P |
|---|---|---|---|---|---|
| Model | 48.41 | 14 | 3.46 | 3356.14 | <0.0001 ** |
| X1 | 38.52 | 1 | 38.52 | 37385.9 | <0.0001 ** |
| X2 | 0.68 | 1 | 0.68 | 661.55 | <0.0001 ** |
| X3 | 0.011 | 1 | 0.011 | 11.07 | 0.0050 ** |
| X4 | 3.17 | 1 | 3.17 | 3078.94 | <0.0001 ** |
| X1X2 | 0.57 | 1 | 0.57 | 553.23 | <0.0001 ** |
| X1X3 | 0.48 | 1 | 0.48 | 462.07 | <0.0001 ** |
| X1X4 | 1.24 | 1 | 1.24 | 1206.6 | <0.0001 ** |
| X2X3 | 0.081 | 1 | 0.081 | 78.83 | <0.0001 ** |
| X2X4 | 0.21 | 1 | 0.21 | 205.37 | <0.0001 ** |
| X3X4 | 0.2 | 1 | 0.2 | 196.53 | <0.0001 ** |
| X12 | 1.44 | 1 | 1.44 | 1393.12 | <0.0001 ** |
| X22 | 0.71 | 1 | 0.71 | 687.3 | <0.0001 ** |
| X32 | 1.63 | 1 | 1.63 | 1584.35 | <0.0001 ** |
| X42 | 0.035 | 1 | 0.035 | 33.86 | <0.0001 ** |
| Residual | 0.014 | 14 | 0.001 | ||
| Lack of fit | 0.0068 | 10 | 0.0068 | 0.36 | 0.9138 |
| Pure error | 0.0076 | 4 | 0.0019 | ||
| Cor. total | 48.43 | 28 | |||
| R2 = 0.9997; R2adj = 0.9994; R2pred = 0.9989; RSN = 210.828; CV = 0.83% | |||||
| Monosaccharide | Regression Equations | R2 | Sample Hydrolyzed (μg/mL) | Sample Unhydrolyzed (μg/mL) | RMPs (μg/mL) |
|---|---|---|---|---|---|
| a-Mannose | Y = 1.53e + 0.04X − 7.38 | 0.9998 | 2.79 | 0.34 | 2.45 |
| b-Rhamnose | Y = 1.29e + 0.04X + 1.62 | 0.9999 | 12.07 | 7.67 | 4.40 |
| c-Glucuronic Acid | Y = 1.13e + 0.04X + 1.25 | 0.9996 | 1.15 | 0.25 | 0.90 |
| d- Glucose | Y = 1.31e + 0.04X + 1.44 | 0.9998 | 593.59 | 2.88 | 590.71 |
| e-Xylose | Y = 3.08e + 0.04X − 6.61 | 0.9992 | 2.69 | 0.40 | 2.29 |
| f-Galactose | Y = 1.09e + 0.04X − 7.97 | 0.9979 | 40.33 | 1.05 | 39.28 |
| g-Arabinose | Y = 3.30e + 0.04X − 2.12 | 0.9986 | 9.52 | ND | 9.52 |
| Group | NO Production (μM) | Cell Viability (%) |
|---|---|---|
| Control | 0.40 ± 0.17 f | 100.00 ± 0.00 a |
| LPS | 40.36 ± 1.92 a | — |
| ASP 1.0 mg/mL | 20.14 ± 1.12 e | 33.80 ± 0.32 d |
| RMP 0.5 mg/mL | 36.56 ± 0.77 b | 104.02 ± 8.10 a |
| RMP 1.0 mg/mL | 35.38 ± 0.65 b | 104.42 ± 5.48 a |
| RMP 2.5 mg/mL | 30.79 ± 1.33 c | 101.68 ± 6.54 a |
| RMP 5.0 mg/mL | 31.36 ± 1.37 c | 98.03 ± 1.88 a,b |
| RMP 7.5 mg/mL | 25.23 ± 1.09 d | 90.52 ± 2.28 b |
| RMP 10.0 mg/mL | 19.03 ± 0.22 e | 73.29 ± 2.91 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Chen, H.; Xiang, Z.; He, N. Preparation of Polysaccharides from Ramulus mori, and Their Antioxidant, Anti-Inflammatory and Antibacterial Activities. Molecules 2019, 24, 856. https://doi.org/10.3390/molecules24050856
Yu W, Chen H, Xiang Z, He N. Preparation of Polysaccharides from Ramulus mori, and Their Antioxidant, Anti-Inflammatory and Antibacterial Activities. Molecules. 2019; 24(5):856. https://doi.org/10.3390/molecules24050856
Chicago/Turabian StyleYu, Wansha, Hu Chen, Zhonghuai Xiang, and Ningjia He. 2019. "Preparation of Polysaccharides from Ramulus mori, and Their Antioxidant, Anti-Inflammatory and Antibacterial Activities" Molecules 24, no. 5: 856. https://doi.org/10.3390/molecules24050856