3. Experimental Section
3.1. General Methods
Commercial reagents were used without further purification. All solvents were distilled before use. Hexanes refer to the fraction boiling at 60–65 °C. Flash column liquid chromatography (FLC) was performed on silica gel Kieselgel 60 (40–63 μm, 230–400 mesh, Merck, Darmstadt, Germany) and analytical thin-layer chromatography (TLC) was performed on aluminium plates precoated with either 0.2 mm (DC-Alufolien, Merck) or 0.25 mm silica gel 60 F254 (ALUGRAM® SIL G/UV254, Macherey-Nagel, Fisher Scientific, Loughborough, UK). The compounds were visualized by UV fluorescence and by dipping the plates in an aqueous H2SO4 solution of cerium sulphate/ammonium molybdate followed by charring with a heat gun. Melting points were obtained using a Boecius apparatus (Büchi® melting point apparatus Model B-545, BÜCHI Labortechnik AG, Flawil, Switzerland) and are uncorrected. Optical rotations were measured with a JASCO P-2000 polarimeter (JASCO, Easton, MD, USA) and are given in units of 10−1 deg.cm2.g−1. FTIR spectra were obtained on a Nicolet 5700 spectrometer (Thermo Electron, Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Smart Orbit (diamond crystal ATR) accessory, using the reflectance technique (4000–400 cm−1).1H and 13C NMR spectra were recorded on either 300 (75) MHz or 600 (150) MHz Varian spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shifts (δ) are quoted in ppm and are referenced to tetramethylsilane (TMS, δ = 0 ppm) as internal standard for 1H NMR and to CDCl3 peak (δ = 77.16 ppm in case of 13C NMR). High-resolution mass spectra (HRMS) were recorded on an OrbitrapVelos mass spectrometer (Thermo Scientific, Waltham, MA, USA; Bremen, Germany) with a heated electrospray ionisation (HESI) source. The mass spectrometer was operated with full scan (50–2000 amu) in positive or negative FT mode (at a resolution of 100,000). The analyte was dissolved in methanol and infused via syringe pump at a rate of 5 mL/min. The heated capillary was maintained at 275 °C with a source heater temperature of 50 °C and the sheath, auxiliary, and sweep gases were at 10, 5, and 0 units, respectively. Source voltage was set to 3.5 kV.
Data collection and cell refinement of
4 and
9 were made on a Stoe StadiVari diffractometer (Stoe & Cie GmbH, Darmstadt, Germany) using a Pilatus3R 300K HPAD detector and the microfocus source Xenocs Genix3D Cu HF (λ = 1.54186 Å). The structures were solved using SHELXT [
17] and refined by the full-matrix least-squares procedure with SHELXL (ver. 2018/3) (for
4) [
18] or CRYSTALS (ver. 14.61) (
9) [
19]. The structures were drawn using the OLEX2 package [
20]. The absolute configurations of both compounds were determined. The Flack parameter x = −0.08(5) for
9 was calculated by Parsons method [
21]. The absolute structure of very small crystal of
4 is impossible to determine based on the Flack parameter, however, using Hooft parameter [
22] with (Gaussian) statistics led to the conclusive value of y = 0.07(11). The deposition numbers CCDC 1892452 (
4) and CCDC 1892453 (
9) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from
http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK.
3.2. (2R,3S)-1,2-O-Isopropylidene-hexane-1,2,3-triol (l-erythro-12) and (2R,3R)-1,2-O-isopropylidene-hexane-1,2,3-triol (d-threo-12)
A solution of propylmagnesium chloride in diethyl ether (2.0 M solution, 5.8 mL, 11.6 mmol) was added dropwise to a stirred solution of freshly distilled 1,2-O-isopropylidene-d-glyceraldehyde (1 g, 7.68 mmol) in dry THF (62 mL) at room temperature. Following the addition, the reaction mixture was stirred for 1 h (TLC control). The reaction was quenched by pouring into a sat. aqueous NH4Cl (62 mL), the aqueous layer was extracted with diethyl ether (3 × 35 mL), and the combined organic layers were dried and concentrated. The residue was purified by MPLC (gradient AcOEt/hexanes 0/100 to 30/70) to give 12 (954 mg, 71%, l-erythro-12/d-threo-12 67:33) as colorless liquid. 1H NMR (600 MHz, CDCl3) δ (l-erythro-12) 0.95 (t, H-6, J = 7.1 Hz, 3H), 1.33–1.44 (m, H-4, H-5a, 2 × CH3, 9H), 1.51–1.59 (m, H-5b, 1H), 1.96 (d, OH, J = 2.9 Hz, 1H), 3.78–3.82 (m, H-3, 1H), 3.91 (dd, H-1a, J = 7.3, 8.0 Hz, 1H), 3.97 (dd, H-1b, J = 6.5, 8.0 Hz, 1H), 4.04 (ddd, H-2, J = 4.0, 6.5, 7.2 Hz, 1H); δ (D-threo-12) 0.94 (t, H-6, J = 7.2 Hz, 3H), 1.30–1.36 (m, H-4a, 1H), 1.37 (s, CH3, 3H), 1.37–1.44 (m, H-5a, 1H), 1.44 (s, CH3, 3H), 1.44–1.48 (m, H-4b, 1H), 1.53–1.59 (m, H-5b, 1H), 2.15 (d, OH, J = 5.2 Hz, 1H), 3.48-3.53 (m, H-3, 1H), 3.73 (dd, H-1a, J = 6.3, 7.7 Hz, 1H), 3.96–4.00 (m, H-2, 1H), 4.02 (dd, H-1b, J = 6.6, 7.8 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-12) 14.2 (C-6), 19.1 (C-5), 25.5 (CH3), 26.6 (CH3), 34.8 (C-4), 64.6 (C-1), 70.5 (C-3), 78.8 (C-2), 109.0 (Cq); δ (D-threo-12) 14.2 (C-6), 18.9 (C-5), 25.5 (CH3), 26.8 (CH3), 36.0 (C-4), 66.3 (C-1), 72.2 (C-3), 79.3 (C-2), 109.5 (Cq); HRMS (ESI) calcd for C9H18O3Na+ [M + Na]+: 197.1148, found: 197.1148.
3.3. (2R,3S)-1,2-O-Isopropylidene-3-O-tert-butyldimethylsilyl-hexane-1,2,3-triol (l-erythro-13) and (2R,3R)-1,2-O-isopropylidene-3-O-tert-butyldimethylsilyl-hexane-1,2,3-triol (d-threo-13)
Imidazole (1.09 g, 16.0 mmol) was added to a solution of diastereomeric mixture 12 (928 mg, 5.33 mmol) in dry CH2Cl2 (11 mL) at room temperature. The mixture was subsequently cooled to 0 °C and tert-butyldimethylsilyl chloride (1.61 g, 10.7 mmol) was added. The reaction mixture was then stirred for 23 h at room temperature. After the dilution with CH2Cl2 (140 mL), the reaction mixture was washed with water (2 × 140 mL), the water phase was extracted with CH2Cl2 (3 × 90 mL), and combined organic layers were dried and concentrated. The residue was purified by MPLC (isocratic AcOEt/hexanes 2/98) to afford 13 (1.42 g, 92%, l-erythro-13/d-threo-13 67:33) as colorless liquid. 1H NMR (600 MHz, CDCl3) δ (l-erythro-13) 0.06 (s, CH3, 3H), 0.07 (s, CH3, 3H), 0.88 (s, tBu, 9H), 0.91 (t, H-6, J = 7.3 Hz, 3H), 1.34 (s, CH3, 3H), 1.35–1.47 (m, H-4a, H-5, CH3, 6H), 1.49–1.52 (m, H-4b, 1H), 3.73–3.76 (m, H-3, 1H), 3.80–3.84 (m, H-1a, 1H), 3.96–4.00 (m, H-1b, H-2c, 2H); δ (D-threo-13) 0.07 (s, CH3, 3H), 0.08 (s, CH3, 3H), 0.89 (s, tBu, 9H), 0.91 (t, H-6, J = 7.2 Hz, 3H), 1.30–1.39 (H-4, H-5a, CH3, 6H), 1.41 (s, CH3, 3H), 1.45-1.50 (m, H-5b, 1H), 3.69 (ddd, H-3, J = 4.2, 6.0, 7.7 Hz, 1H), 3.71 (dd, H-1a, J = 7.4, 8.2 Hz, 1H), 3.94 (dd, H-1b, J = 6.6, 8.2 Hz, 1H), 4.04 (ddd, H-2, J = 6.0, 6.6, 7.3 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-13) −4.2 (CH3), −4.1 (CH3), 14.5 (C-6), 17.8 (C-5), 18.2 (tBu), 25.6 (CH3), 26.0 (tBu), 26.8 (CH3), 37.0 (C-4), 66.6 (C-1), 72.4 (C-3), 78.4 (C-2), 109.0 (Cq); δ (D-threo-13) −4.5 (CH3), −4.1 (CH3), 14.4 (C-6), 18.4 (tBu), 19.0 (C-5), 25.4 (CH3), 26.1 (tBu), 26.6 (CH3), 34.7 (C-4), 65.7 (C-1), 73.2 (C-3), 78.9 (C-2), 109.2 (Cq); HRMS (ESI) calcd for C15H32O3SiNa+ [M + Na]+: 311.2013, found: 311.2013.
3.4. (2R,3S)-3-O-tert-Butyldimethylsilyl-hexane-1,2,3-triol (l-erythro-14) and (2R,3R)-3-O-tert-butyldimethylsilyl-hexane-1,2,3-triol (d-threo-14)
Trifluoroacetic acid (50%, 3.3 mL) was added dropwise to a vigorously stirred solution of compound 13 (1.39 g, 4.81 mmol) in CH2Cl2 (120 mL) at room temperature. The reaction mixture was stirred for 55 min, then diluted with CH2Cl2 (220 mL), and washed with sat. aqueous NaHCO3 (60 mL) and water (2 × 130 mL). The organic phase was dried and concentrated to give crude 14 (1.11 g, 93%, l-erythro-14/d-threo-14 67:33) as colorless oil, which was used in the next step without further purification. 1H NMR (600 MHz, CDCl3) δ (l-erythro-14) 0.09 (s, CH3, 3H), 0.11 (s, CH3, 3H), 0.89 (s, tBu, 9H), 0.91 (t, H-6, J = 7.3 Hz, 3H), 1.26–1.44 (m, H-4a, H-5, 3H), 1.52–1.58 (m, H-4b, 1H), 2.20 (bs, 2x OH, 2H), 3.60 (ddd, H-2, J = 3.5, 3.7, 5.5 Hz, 1H), 3.66 (dd, H-1a, J = 3.5, 11.5 Hz, 1H), 3.79 (dd, H-1b, J = 5.5, 11.5 Hz, 1H), 3.83 (ddd, H-3, J = 3.7, 5.6, 6.6 Hz, 1H); δ (D-threo-14) 0.08 (s, CH3, 3H), 0.09 (s, CH3, 3H), 0.90 (s, tBu, 9H), 0.92 (t, H-6, J = 7.3 Hz, 3H), 1.28–1.44 (m, H-4a, H-5, 3H), 1.61–1.68 (m, H-4b, 1H), 2.20 (bs, 2 × OH, 2H), 3.56-3.60 (m, H-1, H-2, 3H), 3.68 (ddd, H-3, J = 2.8, 4.6, 6.9 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-14) −4.5 (CH3), −4.4 (CH3), 14.4 (C-6), 18.2 (tBu), 18.7 (C-5), 26.0 (tBu), 35.8 (C-4), 63.4 (C-1), 73.2 (C-2), 75.3 (C-3); δ (D-threo-14) −4.6 (CH3), −4.0 (CH3), 14.4 (C-6), 18.2 (tBu), 18.4 (C-5), 26.0 (tBu), 36.2 (C-4), 64.7 (C-1), 72.4 (C-3), 73.1 (C-2); HRMS (ESI) calcd for C12H28O3SiNa+ [M + Na]+: 271.1700, found: 271.1700.
3.5. (2R,3S)-3-O-tert-Butyldimethylsilyl-1-O-trityl-hexane-1,2,3-triol (l-erythro-15) and (2R,3R)-3-O-tert-butyldimethylsilyl-1-O-trityl-hexane-1,2,3-triol (d-threo-15)
A solution of trityl chloride (1.01 g, 3.64 mmol) in dry CH2Cl2 (2.4 mL) was cooled to 0 °C, and triethylamine (0.95 mL, 6.84 mmol) and DMAP (46 mg, 0.37 mmol) were added. Subsequently, a solution of compound 14 (772 mg, 3.11 mmol) in CH2Cl2 (2.4 mL) was added dropwise. After warming to room temperature, the reaction mixture was stirred for 15 h, then quenched with sat. aqueous NH4Cl (25 mL), the aqueous layer was extracted with CH2Cl2 (3 × 10 mL), and the combined organic layers were dried and concentrated. The residue was purified by MPLC (gradient AcOEt/hexanes 0/100 to 5/95) to afford 15 (1.01 g, 74% brsm, l-erythro-15/d-threo-15 64:36) as colorless oil. 1H NMR (600 MHz, CDCl3) δ (l-erythro-15) −0.07 (s, CH3, 3H), 0.01 (s, CH3, 3H), 0.79 (s, tBu, 9H), 0.85 (t, H-6, J = 7.2 Hz, 3H), 1.21–1.28 (m, H-4a, H-5a, 2H), 1.36–1.47 (m, H-4b, H-5b, 2H), 2.36 (d, OH, J = 2.8 Hz, 1H), 3.17 (dd, H-1a, J = 7.7, 9.4 Hz, 1H), 3.23 (dd, H-1b, J = 4.5, 9.4 Hz, 1H), 3.69 (ddd, H-3, J = 4.1, 4.5, 6.9 Hz, 1H), 3.79-3.83 (m, H-2, 1H), 7.22–7.25 (m, Tr-Hp, 3H), 7.28–7.32 (m, Tr-Hm, 6H), 7.42–7.45 (m, Tr-Ho, 6H); δ (D-threo-15) -0.13 (s, CH3, 3H), 0.02 (s, CH3, 3H), 0.78 (s, tBu, 9H), 0.90 (t, H-6, J = 7.2 Hz, 3H), 1.30-1.35 (m, H-4a, H-5, 3H), 1.58–1.63 (m, H-4b, 1H), 2.37 (d, OH, J = 7.5 Hz, 1H), 3.03 (dd, H-1a, J = 6.1, 9.4 Hz, 1H), 3.22 (dd, H-1b, J = 6.3, 9.4 Hz, 1H), 3.65–3.69 (m, H-2, 1H), 3.79-3.82 (m, H-3, 1H), 7.22–7.25 (m, Tr-Hp, 3H), 7.27–7.31 (m, Tr-Hm, 6H), 7.42–7.45 (m, Tr-Ho, 6H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-15) −4.4 (CH3), −4.3 (CH3), 14.4 (C-6), 18.2 (tBu), 18.3 (C-5), 26.0 (tBu), 34.5 (C-4), 65.1 (C-1), 73.3 (C-3), 73.5 (C-2), 86.9 (Trt-Cq), 127.2 (3 × Tr-Cp), 128.0 (6 × Tr-Cm), 128.8 (6 × Tr-Co), 144.1 (3 × Tr-Cipso); δ (D-threo-15) −4.7 (CH3), −4.1 (CH3), 14.4 (C-6), 18.2 (tBu), 18.7 (C-5), 26.0 (tBu), 36.1 (C-4), 65.3 (C-1), 71.8 (C-2), 71.9 (C-3), 86.8 (Tr-Cq), 127.1 (3 × Tr-Cp), 127.9 (6 × Tr-Cm), 128.8 (6 × Tr-Co), 144.2 (3 × Tr-Cipso); HRMS (ESI) calcd for C31H42O3SiNa+ [M + Na]+: 513.2795, found: 513.2795.
3.6. (2R,3S)-2-O-Acetyl-3-O-tert-butyldimethylsilyl-1-O-trityl-hexane-1,2,3-triol (l-erythro-16) and (2R,3R)-2-O-acetyl-3-O-tert-butyldimethylsilyl-1-O-trityl-hexane-1,2,3-triol (d-threo-16)
DMAP (771 mg, 6.32 mmol) and Ac2O (0.60 mL, 6.32 mmol) were added to a soluion of triol 15 (1.03 g, 2.11 mmol) in dry CH2Cl2 (30 mL) at room temperature. The reaction mixture was stirred for 30 min and quenched with sat. aqueous NaHCO3 (30 mL). The water phase was extracted with CH2Cl2 (3 × 40 mL), combined organic layers were dried and concentrated. The residue was purified by MPLC (gradient AcOEt/hexanes 0/100 to 5/95) to give 16 (975 mg, 87%, l-erythro-16/d-threo-16 65:35) as colorless oil. 1H NMR (600 MHz, CDCl3) δ (l-erythro-16) -0.10 (s, CH3, 3H), -0.04 (s, CH3, 3H), 0.74 (s, tBu, 9H), 0.85 (t, H-6, J = 7.1 Hz, 3H), 1.22–1.39 (m, H-4, H-5, 4H), 2.10 (s, C(O)CH3, 3H), 3.25–3.29 (m, H-1, 2H), 3.78 (ddd, H-3, J = 3.5, 5.0, 6.7 Hz, 1H), 5.15 (ddd, H-2, J = 3.5, 4.7, 6.8 Hz, 1H), 7.20–7.24 (m, Tr-Hp, 3H), 7.27–7.30 (m, Tr-Hm, 6H), 7.40–7.43 (m, Tr-Ho, 6H); δ (D-threo-16) 0.01 (s, CH3, 3H), 0.02 (s, CH3, 3H), 0.79 (s, tBu, 9H), 0.80 (t, H-6, J = 7.1 Hz, 3H), 1.16–1.30 (m, H-4a, H-5, 3H), 1.33–1.38 (m, H-4b, 1H), 2.15 (s, C(O)CH3, 3H), 3.17 (dd, H-1a, J = 6.8, 10.2 Hz, 1H), 3.25 (dd, H-1b, J = 2.7, 10.2 Hz, 1H), 3.86 (ddd, H-3, J = 4.3, 5.5, 6.8 Hz, 1H), 5.07 (ddd, H-2, J = 2.7, 5.5, 6.8 Hz, 1H), 7.20–7.24 (m, Tr-Hp, 3H), 7.27-7.30 (m, Tr-Hm, 6H), 7.40–7.43 (m, Tr-Ho, 6H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-16) −4.6 (CH3), −4.4 (CH3), 14.3 (C-6), 18.1 (tBu), 18.7 (C-5), 21.4 (C(O)CH3), 25.9 (tBu), 36.1 (C-4), 62.3 (C-1), 72.5 (C-3), 76.0 (C-2), 86.8 (Tr-Cq), 127.1 (3 × Tr-Cp), 127.9 (6 × Tr-Cm), 128.8 (6 × Tr-Co), 144.1 (3 × Tr-Cipso), 170.5 (C(O)CH3); δ (D-threo-16) −4.4 (CH3), −4.4 (CH3), 14.3 (C-6), 18.1 (tBu), 18.5 (C-5), 21.5 (C(O)CH3), 25.9 (tBu), 35.1 (C-4), 62.6 (C-1), 70.9 (C-3), 75.8 (C-2), 86.5 (Tr-Cq), 127.1 (3 × Tr-Cp), 127.9 (6 × Tr-Cm), 128.8 (6 × Tr-Co), 144.1 (3 × Tr-Cipso), 170.7 (C(O)CH3); HRMS (ESI) calcd for C33H44O4SiNa+ [M + Na]+: 555.2901, found: 555.2901.
3.7. (2R,3S)-2-O-Acetyl-3-O-tert-butyldimethylsilyl-hexane-1,2,3-triol (l-erythro-17) and (2R,3R)-2-O-acetyl-3-O-tert-butyldimethylsilyl-hexane-1,2,3-triol (d-threo-17)
Formic acid (12.2 mL) was added to a solution of protected triol 16 (959 mg, 1.80 mmol) in diethyl ether (12.2 mL) at 0 °C. The reaction mixture was stirred for 50 min at room temperature, diluted with diethyl ether (30 mL), and cooled to 0 °C. Sat. aqueous NaHCO3 (equimolar to formic acid, 323 mmol) was added with vigorous stirring to neutralize the reaction mixture (accompanied by the separation of two layers). The water phase was extracted with diethyl ether (3 × 50 mL) and the combined organic layers were dried and concentrated. The residue was purified by MPLC (gradient AcOEt/hexanes 0/100 to 10/90) to afford 346 mg (66%) of yellowish oil as an inseparable mixture of 17 (l-erythro-17/d-threo-17 63:37) and 20 (l-erythro-20/d-threo-20 58:42, the product of the acetyl group migration of 17 taking place even during MPLC) in ratio 82:18. 1H NMR (600 MHz, CDCl3) δ (l-erythro-17) 0.07 (s, CH3, 3H), 0.11 (s, CH3, 3H), 0.90 (s, tBu, 9H), 0.93 (t, H-6, J = 7.3 Hz, 3H), 1.31–1.36 (m, H-5a, 1H), 1.48–1.55 (m, H-4, H-5b, 3H), 2.12 (s, C(O)CH3, 3H), 2.68 (dd, OH, J = 3.7, 7.7 Hz, 1H), 3.81–3.85 (m, H-1a, 1H), 3.90–3.95 (m, H-1b, H-3, 2H), 4.77 (dt, H-2, J = 3.1, 4.9 Hz, 1H); δ (d-threo-17) 0.08 (s, CH3, 3H), 0.12 (s, CH3, 3H), 0.90 (s, tBu, 9H), 0.91 (t, H-6, J = 7.2 Hz, 3H), 1.24–1.33 (m, H-5a, 1H), 1.35–1.45 (m, H-4a, H-5b, 2H), 1.46–1.54 (m, H-4b, 1H), 2.11 (s, C(O)CH3, 3H), 2.13–2.16 (m, OH, 1H), 3.69–3.73 (m, H-1a, 1H), 3.84–3.90 (m, H-1b, H-3, 2H), 4.87 (ddd, H-2, J = 4.1, 4.8, 6.5 Hz, 1H); δ (l-erythro-20) 0.08 (s, CH3, 3H), 0.09 (s, CH3, 3H), 0.90 (s, tBu, 9H), 0.92 (t, H-6, J = 7.1 Hz, 3H), 1.29–1.34 (m, H-4a, 1H), 1.38–1.44 (m, H-5, 2H), 1.55–1.61 (m, H-4b, 1H), 2.10 (s, C(O)CH3, 3H), 2.32 (d, OH, J = 4.7 Hz, 1H), 3.75-3.78 (m, H-3, 1H), 3.78-3.82 (m, H-2, 1H), 4.06 (dd, H-1a, J = 7.7, 11.6 Hz, 1H), 4.23 (dd, H-1b, J = 3.0, 11.6 Hz, 1H); δ (d-threo-20) 0.08 (s, CH3, 3H), 0.10 (s, CH3, 3H), 0.90 (s, tBu, 9H), 0.92 (t, H-6, J = 7.1 Hz, 3H), 1.31-1.44 (m, H-4a, H-5, 3H), 1.64–1.71 (m, H-4a, 1H), 2.09 (s, C(O)CH3, 3H), 2.40 (d, OH, J = 7.8 Hz, 1H), 3.68-3.71 (m, H-3, 1H), 3.72-3.75 (m, H-2, 1H), 4.05 (dd, H-1a, J = 5.1, 11.3 Hz, 1H), 4.08 (dd, H-1b, J = 7.0, 11.3 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (l-erythro-17) −4.5, −4.5 (2 × CH3), 14.3 (C-6), 18.2 (tBu), 18.8 (C-5), 21.4 (C(O)CH3), 25.9 (tBu), 36.8 (C-4), 61.9 (C-1), 73.8 (C-3), 76.6 (C-2), 171.2 (C(O)CH3); δ (d-threo-17) = −4.4 (CH3), −4.4 (CH3), 14.4 (C-6), 18.1 (tBu), 18.9 (C-5), 21.3 (C(O)CH3), 25.9 (tBu), 34.8 (C-4), 62.0 (C-1), 71.5 (C-3), 76.7 (C-2), 171.2 (C(O)CH3); δ (l-erythro-20) −4.5 (CH3), −4.3 (CH3), 14.4 (C-6), 18.2 (tBu), 18.4 (C-5), 21.1 (C(O)CH3), 26.0 (tBu), 34.9 (C-4), 65.8 (C-1), 72.6 (C-2), 73.2 (C-3), 171.5 (C(O)CH3); δ (d-threo-20) −4.7 (CH3),-4.1 (CH3), 14.3 (C-6), 18.2 (tBu), 18.6 (C-5), 21.1 (C(O)CH3), 26.0 (tBu), 35.9 (C-4), 66.2 (C-1), 70.8 (C-2), 71.9 (C-3), 171.2 (C(O)CH3); HRMS (ESI) calcd for C14H30O4SiNa+ [M + Na]+: 313.1806, found: 313.1806.
3.8. (2R,3S)-2-O-Acetyl-4,5,6-trideoxy-3-O-tert-butyldimethylsilyl- l-erythro-hexose (l-erythro-10) and (2R,3R)-2-O-acetyl-4,5,6-trideoxy-3-O-tert-butyldimethylsilyl- d-threo-hexose (d-threo-10)
Oxalyl chloride (2.0 M in CH2Cl2, 0.85 mL, 1.70 mmol) was added dropwise to a solution of dimethyl sulfoxide (0.18 mL, 2.56 mmol) in dry CH2Cl2 (3.9 mL) at -78 °C. After 30 min of stirring at −78 °C, a solution of alcohol 17 (330 mg, 82:18 mixture with 20, 0.93 mmol of 17) in dry CH2Cl2 (1.9 mL) was added dropwise. The reaction mixture was stirred at −78 °C for 30 min and Et3N (0.63 mL, 4.54 mmol) was added. After 1 h of stirring at −78 °C, the reaction mixture was allowed to reach room temperature slowly (additional 1 h). Subsequently, the reaction mixture was concentrated, dry diethyl ether was added to the residue, and the mixture was filtered through a short silicagel column. The filtrate was concentrated to give the crude aldehyde 10 which was immediately used in the following step without further purification.
3.9. (3R,4S)-3-O-Acetyl-1-(2-acetoxymethyl-3-methoxyphenyl)-4-O-tert-butyldimethylsilyl-hept-1-ene-3,4- diol (18) and (3R,4R)-3-O-acetyl-1-(2-acetoxymethyl-3-methoxyphenyl)-4-O-tert-butyldimethylsilyl-hept-1-ene-3,4-diol (19)
Sulfone 11 (457 mg, 1.14 mmol) in dry DME (10.5 mL) was slowly added to a solution of the crude aldehyde 10 (theor. 0.93 mmol) in dry DME (10.5 mL) and the mixture was cooled to −60 °C. Subsequently, KHMDS (0.5 M in toluene, 3.97 mL, 1.99 mmol) was added dropwise keeping −60 °C and the reaction mixture was allowed to reach room temperature. The reaction mixture was stirred for 40 min, quenched with sat. aqueous NH4Cl (20 mL), and diluted with AcOEt (20 mL). The aqueous layer was extracted with AcOEt (3 × 20 mL) and the combined organic layers were dried and concentrated. The residue (18/19 70:30) was repeatedly purified by MPLC (gradient AcOEt/hexanes 0/100 to 5/95) and preparative TLC to afford 18 (79 mg, 18%), 19 (22 mg, 5%), and the mixture of 18 and 19 (78 mg, 18%) as colorless oils over two steps (41% overall yield).
1H NMR (600 MHz, CDCl3) δ (18) 0.06 (s, CH3, 3H), 0.10 (s, CH3, 3H), 0.90 (t, H-7, J = 7.0 Hz, 3H), 0.91 (s, tBu, 9H), 1.30-1.36 (m, H-6a, 1H), 1.39–1.48 (m, H-5, H-6b, 3H), 2.06 (s, C(O)CH3), 3H), 2.09 (s, C(O)CH3), 3H), 3.84 (s, OCH3, 3H), 3.85–3.88 (m, H-4, 1H), 5.25 (d, CH2OAc, J = 11.8 Hz, 1H), 5.27 (d, CH2OAc, J = 11.8 Hz, 1H), 5.33 (ddd, H-3, J = 1.0, 2.8, 7.6 Hz, 1H), 6.18 (dd, H-2, J = 7.6, 15.9 Hz, 1H), 6.84 (d, H-4′, J = 8.2 Hz, 1H), 6.85 (d, H-1, J = 15.9 Hz, 1H), 7.11 (d, H-6′, J = 7.8 Hz, 1H), 7.30 (t, H-5′, J = 8.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (18) −4.4 (CH3), −4.2 (CH3), 14.3 (C-7), 18.4 (tBu), 18.9 (C-6), 21.1 (C(O)CH3), 21.5 (C(O)CH3), 26.0 (tBu), 36.3 (C-5), 56.0 (OCH3), 57.7 (CH2OAc), 73.8 (C-4), 77.9 (C-3), 110.3 (C-4′), 118.9 (C-6′), 121.5 (C-2′), 127.5 (C-2), 130.0 (C-5′), 131.2 (C-1), 139.0 (C-1′), 158.6 (C-3′), 170.2 (C(O)CH3), 171.2 (C(O)CH3); HRMS (ESI) calcd for C25H40O6SiNa+ [M + Na]+: 487.2486, found: 487.2486; [α] −48.8 (c 1.131 MeOH).
1H NMR (600 MHz, CDCl3) δ (19) 0.08 (s, CH3, 3H), 0.11 (s, CH3, 3H), 0.89 (t, H-7, J = 7.0 Hz, 3H), 0.90 (s, tBu, 9H), 1.30–1.37 (m, H-6a, 1H), 1.38-1.50 (m, H-5, H-6b, 3H), 2.06 (s, C(O)CH3), 3H), 2.11 (s, C(O)CH3), 3H), 3.80 (ddd, H-4, J = 3.6, 6.0, 7.2 Hz, 1H), 3.84 (s, OCH3, 3H), 5.26 (s, CH2OAc, 2H), 5.38 (ddd, H-3, J = 1.4, 6.1, 6.1 Hz, 1H), 6.11 (dd, H-2, J = 6.2, 15.9 Hz, 1H), 6.83 (d, H-4′, J = 8.3 Hz, 1H), 6.84 (dd, H-1, J = 1.3, 15.9 Hz, 1H), 7.09 (d, H-6′, J = 7.8 Hz, 1H), 7.29 (t, H-5′, J = 8.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (19) −4.4 (CH3), −4.2 (CH3), 14.5 (C-7), 18.2 (tBu), 18.5 (C-6), 21.1 (C(O)CH3), 21.4 (C(O)CH3), 26.0 (tBu), 35.3 (C-5), 56.0 (OCH3), 57.7 (CH2OAc), 73.0 (C-4), 76.8 (C-3), 110.2 (C-4′), 118.8 (C-6′), 121.4 (C-2′), 128.1 (C-2), 129.5 (C-1), 130.0 (C-5′), 139.0 (C-1′), 158.6 (C-3′), 170.2 (C(O)CH3), 171.2 (C(O)CH3); HRMS (ESI) calcd for C25H40O6SiNa+ [M + Na]+: 487.2486, found: 487.2486; [α] +5.5 (c 0.431 MeOH).
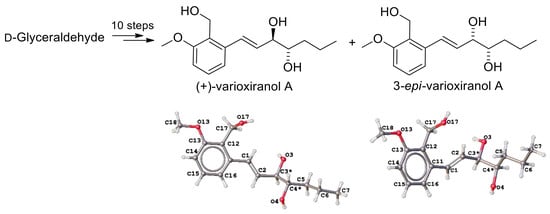
3.10. Varioxiranol A (4)
Compound 18 (21.1 mg, 0.045 mmol) was dissolved in methanol (2 mL) and K2CO3 (12.6 mg, 0.091 mol) was added. The reaction mixture was stirred at room temperature for 2.5 h, diluted with AcOEt (4 mL) and with water (4 mL). The water phase was extracted with AcOEt (4 × 2 mL) and combined organic layers were dried and concentrated. The residue was diluted in THF (0.5 mL), the solution was cooled to 0 °C, and TBAF × 3H2O in THF (1.0 M solution, 46 µL, 0.046 mmol) was added. The reaction mixture was stirred at room temperature for 4.5 h and quenched with sat. aqueous NH4Cl (5 mL), the aqueous layer was extracted with CH2Cl2 (3 × 5 mL), and the combined organic layers were dried and concentrated. The residue was purified by FLC (isocratic acetone/CH2Cl2 20/80 then 50/50) to afford varioxiranol A (4, 11.1 mg, 92% over two steps) as colorless crystalline solid that was subsequently recrystallized from AcOEt-hexanes. 1H NMR (600 MHz, CDCl3) δ (4) 0.94 (t, H-7, J = 7.2 Hz, 3H), 1.36-1.43 (m, H-6a, 1H), 1.43–1.48 (m, H-5, 2H), 1.51–1.57 (m, H-6b, 1H), 2.09 (d, OH, J = 4.3 Hz, 1H), 2.20 (t, OH, J = 5.8 Hz, 1H), 2.22 (d, OH, J = 4.6 Hz, 1H), 3.78–3.82 (m, H-4, 1H), 3.87 (s, OCH3, 3H), 4.26–4.30 (m, H-3, 1H), 4.76–4.83 (m, CH2OH, 2H), 6.19 (dd, H-2, J = 6.9, 15.8 Hz, 1H), 6.84 (d, H-4′, J = 8.2 Hz, 1H), 7.02 (d, H-1, J = 15.8 Hz, 1H), 7.10 (d, H-6′, J = 7.9 Hz, 1H), 7.25 (t, H-5′, J = 8.2 Hz, 1H); 13C NMR (150 MHz, acetone-d6) δ (4) 14.5 (C-7), 19.8 (C-6), 35.7 (C-5), 55.5 (CH2OH), 56.1 (OCH3), 75.0 (C-4), 76.7 (C-3), 110.4 (C-4′), 119.4 (C-6′), 127.9 (C-2′), 129.2 (C-1), 129.3 (C-5′), 133.7 (C-2), 139.6 (C-1′), 158.9 (C-3′); HRMS (ESI) calcd for C15H22O4Na+ [M+Na]+: 289.1410, found: 289.1410; [α] +2.5 (c 0.207 MeOH); mp 120–121 °C.
3.11. 4-epi-Varioxiranol A (9)
Compound 19 (20.8 mg, 0.045 mmol) was dissolved in methanol (2 mL) and K2CO3 (12.4 mg, 0.090 mmol) was added. The reaction mixture was stirred at room temperature for 2.5 h and diluted with AcOEt (4 mL) and with water (4 mL). The water phase was extracted with AcOEt (4 × 2 mL) and combined organic layers were dried and concentrated. The residue was diluted in THF (0.5 mL), the solution was cooled to 0 °C, and TBAF × 3H2O in THF (1.0 M solution, 45 µL, 0.045 mmol) was added. The reaction mixture was stirred at room temperature for 4.5 h and quenched with sat. aqueous NH4Cl (5 mL). The aqueous layer was extracted with CH2Cl2 (3 × 5 mL) and the combined organic layers were dried and concentrated. The residue was purified by FLC (isocratic acetone/CH2Cl2 30/70) to afford 4-epi-varioxiranol A (9, 6.8 mg, 57% over two steps) that was subsequently recrystallized from CH2Cl2-hexanes yielding colorless crystalline solid. 1H NMR (600 MHz, CDCl3) δ (9) 0.94 (t, H-7, J = 7.1 Hz, 3H), 1.39–1.45 (m, H-6a, 1H), 1.46–1.56 (m, H-5, H-6b, 3H), 2.20 (t, OH, J = 6.3 Hz, 1H), 2.28 (d, OH, J = 4.3 Hz, 1H), 2.40 (d, OH, J = 4.3 Hz, 1H), 3.56–3.61 (m, H-4, 1H), 3.87 (s, OCH3, 3H), 4.10–4.14 (m, H-3, 1H), 4.78 (dd, CH2OH, J = 6.3, 12.1 Hz, 1H), 4.81 (dd, CH2OH, J = 6.3, 12.1 Hz, 1H), 6.12 (dd, H-2, J = 6.8, 15.8 Hz, 1H), 6.84 (d, H-4′, J = 8.2 Hz, 1H), 7.04 (d, H-1, J = 15.8 Hz, 1H), 7.08 (d, H-6′, J = 7.8 Hz, 1H), 7.25 (t, H-5′, J = 8.0 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ (9) 14.2 (C-7), 19.0 (C-6), 35.4 (C-5), 55.8 (OCH3), 56.6 (CH2OH), 74.5 (C-4), 76.3 (C-3), 110.0 (C-4′), 119.4 (C-6′), 126.4 (C-2′), 129.1 (C-5′), 129.8 (C-1), 132.6 (C-2), 137.7 (C-1′), 158.2 (C-3′); HRMS (ESI) calcd for C15H22O4Na+ [M + Na]+: 289.1410, found: 289.1410; [α] +45.0 (c 0.094 MeOH); mp 112–113 °C.