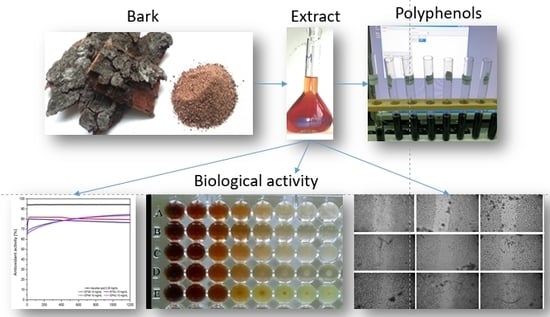
A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity
Abstract
:1. Introduction
2. The Bark of Woody Vascular Plants—Source of Phenolic Compounds
3. Methods Used to Extract Phenolic Compounds from the Bark of Woody Vascular Plants
4. Biological Effects of Extracts Obtained from the Bark of Woody Vascular Plants
4.1. Antioxidant Effect
4.2. Anti-Inflammatory Effect
4.3. Antibacterial Effect
4.4. Other Effects
5. Conclusions
Funding
Conflicts of Interest
References
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Popa, V.I. Wood bark as valuable raw material for compounds with biological activity. Celul. Şi Hârtie 2015, 64, 5–17. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus l.) plants. Ind. Crops Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Valorization of bark for chemicals and materials: A review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Dopico-García, M.; Fique, A.; Guerra, L.; Afonso, J.; Pereira, O.; Valentão, P.; Andrade, P.; Seabra, R. Principal components of phenolics to characterize red vinho verde grapes: Anthocyanins or non-coloured compounds? Talanta 2008, 75, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Oprică, L.; Socaciu, C. Metaboliţi Secundari din Plante: Origine, Structură, Funcţii; Editura Universităţii" Alexandru Ioan Cuza: Iasi, Romania, 2016. [Google Scholar]
- Pereira, D.; Valentão, P.; Pereira, J.; Andrade, P. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Bocalandro, C.; Sanhueza, V.; Gómez-Caravaca, A.M.; González-Álvarez, J.; Fernández, K.; Roeckel, M.; Rodríguez-Estrada, M.T. Comparison of the composition of Pinus radiata bark extracts obtained at bench-and pilot-scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Maldini, M.; Sosa, S.; Montoro, P.; Giangaspero, A.; Balick, M.J.; Pizza, C.; Loggia, R.D. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J. Ethnopharmacol. 2009, 122, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.S.; Dasgupta, D. Quantification of phenolic content from stem-bark and root of Hugonia mystax Linn. using RP-HPLC. J. King Saud Univ.-Sci. 2018, 30, 293–300. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and Eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Brusotti, G.; Andreola, F.; Sferrazza, G.; Grisoli, P.; Merelli, A.; della Cuna, F.R.; Calleri, E.; Nicotera, G.; Pierimarchi, P.; Serafino, A. In vitro evaluation of the wound healing activity of Drypetes klainei stem bark extracts. J. Ethnopharmacol. 2015, 175, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.; Khoo, M.; Ng, S.; Thoo, Y.; Aida, W.W.; Ho, C. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Keshari, A.K.; Kumar, G.; Kushwaha, P.S.; Bhardwaj, M.; Kumar, P.; Rawat, A.; Kumar, D.; Prakash, A.; Ghosh, B.; Saha, S. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino wistar rats. J. Ethnopharmacol. 2016, 181, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD–MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Tamashiro Filho, P.; Olaitan, B.S.; de Almeida, D.A.T.; da Silva Lima, J.C.; Marson-Ascêncio, P.G.; Ascêncio, S.D.; Rios-Santos, F.; de Oliveira Martins, D.T. Evaluation of antiulcer activity and mechanism of action of methanol stem bark extract of Lafoensia pacari (Lytraceae) in experimental animals. J. Ethnopharmacol. 2012, 144, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Marinos, V.A.; Tate, M.E.; Williams, P.J. Lignan and phenylpropanoid glycerol glucosides in wine. Phytochemistry 1992, 31, 4307–4312. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Hofmann, T.; Nebehaj, E.; Stefanovits-Bányai, É.; Albert, L. Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Ind. Crops Prod. 2015, 77, 375–381. [Google Scholar] [CrossRef]
- Hofmann, T.; Tálos-Nebehaj, E.; Albert, L.; Németh, L. Antioxidant efficiency of beech (Fagus sylvatica L.) bark polyphenols assessed by chemometric methods. Ind. Crops Prod. 2017, 108, 26–35. [Google Scholar] [CrossRef]
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crops Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- de Souza Santos, C.C.; Guilhon, C.C.; Moreno, D.S.A.; Alviano, C.S.; dos Santos Estevam, C.; Blank, A.F.; Fernandes, P.D. Anti-inflammatory, antinociceptive and antioxidant properties of Schinopsis brasiliensis bark. J. Ethnopharmacol. 2018, 213, 176–182. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier-El Bouhtoury, F. Green extraction process of tannins obtained from moroccan Acacia mollissima barks by microwave: Modeling and optimization of the process using the response surface methodology RSM. Arab. J. Chem. in press. [CrossRef]
- Koleva, V.; Simeonov, E. Solid liquid extraction of phenolic and flavonoid compounds from Cotinus coggygria and concentration by nanofiltration. Chem. Biochem. Eng. Q. 2014, 28, 545–551. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.-H.; Qi, L.-W.; Cao, J.; Li, P.; Li, C.-Y.; Peng, Y.-B. Advances of modern chromatographic and electrophoretic methods in separation and analysis of flavonoids. Molecules 2008, 13, 2521–2544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, M.; Liu, J.; Zhang, H.; He, G.; Ruan, H. Optimization of ultrasonic-assisted extraction (UAE) of betulin from white birch bark using response surface methodology. Ultrason. Sonochem. 2009, 16, 599–604. [Google Scholar] [CrossRef]
- Paz, J.E.W.; Márquez, D.B.M.; Ávila, G.C.M.; Cerda, R.E.B.; Aguilar, C.N. Ultrasound-assisted extraction of polyphenols from native plants in the mexican desert. Ultrason. Sonochem. 2015, 22, 474–481. [Google Scholar]
- Tatke, P.; Jaiswal, Y. An Overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.; Reynaud, S.; Pizzi, A.; Charrier, B.; Bouhtoury, F.C.-E. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Dubey, K.K.; Goel, N. Evaluation and optimization of downstream process parameters for extraction of betulinic acid from the bark of Ziziphus jujubae L. Sci. World J. 2013, 2013, 469674. [Google Scholar] [CrossRef]
- Seabra, I.; Braga, M.; de Sousa, H. Statistical mixture design investigation of CO2–ethanol–H2O pressurized solvent extractions from tara seed coat. J. Supercrit. Fluids 2012, 64, 9–18. [Google Scholar] [CrossRef]
- Zougagh, M.; Valcárcel, M.; Ríos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Veggi, P.C.; Prado, J.M.; Bataglion, G.A.; Eberlin, M.N.; Meireles, M.A.A. Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J. Supercrit. Fluids 2014, 89, 68–77. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Hu, H.-B.; Liang, H.-P.; Li, H.-M.; Yuan, R.-N.; Sun, J.; Zhang, L.-L.; Han, M.-H.; Wu, Y. Isolation, purification, characterization and antioxidant activity of polysaccharides from the stem barks of Acanthopanax leucorrhizus. Carbohydr. Polym. 2018, 196, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gomes, N.G.; Valentão, P.; Pereira, D.M.; Gil-Izquierdo, A.; Araújo, L.; Silva, T.C.; Andrade, P.B. Leaves and stem bark from Allophylus africanus Beauv.: An approach to anti-inflammatory properties and characterization of their flavonoid profile. Food Chem. Toxicol. 2018, 118, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, Y.; Zhao, L.; Zu, Y.; Gu, H.; Yang, L. Enzymatic hydrolysis and simultaneous extraction for preparation of genipin from bark of Eucommia ulmoides after ultrasound, microwave pretreatment. Molecules 2015, 20, 18717–18731. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Villaverde, J.J.; Freire, C.S.; Domingues, M.R.M.; Neto, C.P.; Silvestre, A.J. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis× E. urophylla) and E. maidenii bark extracts. Ind. Crops Prod. 2012, 39, 120–127. [Google Scholar] [CrossRef]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic Modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, C.; Čop, M.; Kemppainen, K.; Giovando, S.; Pizzi, A.; Laborie, M.-P.; Sernek, M.; Celzard, A. Biobased foams from condensed tannin extracts from norway spruce (Picea abies) bark. Ind. Crops Prod. 2015, 73, 144–153. [Google Scholar] [CrossRef]
- Zhou, Z.; Shao, H.; Han, X.; Wang, K.; Gong, C.; Yang, X. The extraction efficiency enhancement of polyphenols from Ulmus pumila L. barks by trienzyme-assisted extraction. Ind. Crops Prod. 2017, 97, 401–408. [Google Scholar] [CrossRef]
- Ambika, S.; Chauhan, S. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Nat. Prod. Res. 2014, 28, 760–763. [Google Scholar]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical composition of the silver fir (Abies alba) bark extract abigenol and its antioxidant activity. Ind. Crops Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Lee, W.-J.; Lan, W.-C. Properties of resorcinol–tannin–formaldehyde copolymer resins prepared from the bark extracts of taiwan acacia and china fir. Bioresour. Technol. 2006, 97, 257–264. [Google Scholar] [CrossRef]
- Faujdar, S.; Sati, B.; Sharma, S.; Pathak, A.; Paliwal, S.K. Phytochemical evaluation and anti-hemorrhoidal activity of bark of Acacia ferruginea DC. J. Tradit. Complement. Med. 2019, 9, 85–89. [Google Scholar] [CrossRef]
- St-Pierre, F.; Achim, A.; Stevanovic, T. Composition of ethanolic extracts of wood and bark from Acer saccharum and Betula alleghaniensis trees of different vigor classes. Ind. Crops Prod. 2013, 41, 179–187. [Google Scholar] [CrossRef]
- Encarnação, S.; de Mello-Sampayo, C.; Graca, N.A.; Catarino, L.; da Silva, I.B.M.; Lima, B.S.; Silva, O.M.D. Total Phenolic content, antioxidant activity and pre-clinical safety evaluation of an Anacardium occidentale stem bark portuguese hypoglycemic traditional herbal preparation. Ind. Crops Prod. 2016, 82, 171–178. [Google Scholar] [CrossRef]
- Salih, E.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of african savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. South Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Da Silveira, C.; Trevisan, M.; Rios, J.; Erben, G.; Haubner, R.; Pfundstein, B.; Owen, R. Secondary plant substances in various extracts of the leaves, fruits, stem and bark of Caraipa densifolia Mart. Food Chem. Toxicol. 2010, 48, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Ilanhtiraiyan, S.; Ilayaraja, K.; Ashly, A.; Hariharan, S. Influence of ultrasound on avaram bark (Cassia auriculata) tannin extraction and tanning. Chem. Eng. Res. Des. 2014, 92, 1827–1833. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.-J.; Kim, D.H. Anti-Influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of south india. Saudi J. Biol. Sci. 2015, 22, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Bodhankar, S.L.; Singh, V.; Mohan, V.; Thakurdesai, P.A. Anti-asthmatic effects of type-a procyanidine polyphenols from Cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed. Aging Pathol. 2013, 3, 23–30. [Google Scholar] [CrossRef]
- Nunes, L.G.; Gontijo, D.C.; Souza, C.J.; Fietto, L.G.; Carvalho, A.F.; Leite, J.P.V. The mutagenic, DNA-damaging and antioxidative properties of bark and leaf extracts from Coutarea hexandra (Jacq.) K. Schum. Environ. Toxicol. Pharmacol. 2012, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pathania, A.S.; Saxena, A.; Vishwakarma, R.; Ali, A.; Bhushan, S. The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human leukemia HL-60 Cells. Chem. Biol. Interact. 2013, 205, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Baptista, E.A.; Pinto, P.C.; Mota, I.F.; Loureiro, J.M.; Rodrigues, A.E. Ultrafiltration of ethanol/water extract of Eucalyptus globulus bark: Resistance and cake build up analysis. Sep. Purif. Technol. 2015, 144, 256–266. [Google Scholar] [CrossRef]
- Miranda, I.; Lima, L.; Quilhó, T.; Knapic, S.; Pereira, H. The bark of Eucalyptus sideroxylon as a source of phenolic extracts with anti-oxidant properties. Ind. Crops Prod. 2016, 82, 81–87. [Google Scholar] [CrossRef]
- Deutschländer, M.; Lall, N.; Van de Venter, M.; Hussein, A.A. Hypoglycemic evaluation of a new triterpene and other compounds isolated from Euclea undulata Thunb. var. myrtina (Ebenaceae) root bark. J. Ethnopharmacol. 2011, 133, 1091–1095. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Antidiabetic and enzymatic antioxidant properties from methanol extract of Ficus talboti bark on diabetic rats induced by streptozotocin. Asian Pac. J. Reprod. 2014, 3, 97–105. [Google Scholar] [CrossRef]
- Maldini, M.; Di Micco, S.; Montoro, P.; Darra, E.; Mariotto, S.; Bifulco, G.; Pizza, C.; Piacente, S. Flavanocoumarins from Guazuma ulmifolia bark and evaluation of their affinity for STAT1. Phytochemistry 2013, 86, 64–71. [Google Scholar] [CrossRef]
- Iqbal, E.; Salim, K.A.; Lim, L.B. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (airy shaw) from brunei darussalam. J. King Saud Univ.-Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Yong, Y.; Saleem, A.; Guerrero-Analco, J.A.; Haddad, P.S.; Cuerrier, A.; Arnason, J.T.; Harris, C.S.; Johns, T. Larix laricina bark, a traditional medicine used by the cree of eeyou istchee: Antioxidant constituents and in vitro permeability across Caco-2 Cell Monolayers. J. Ethnopharmacol. 2016, 194, 651–657. [Google Scholar] [CrossRef]
- Um, M.; Shin, G.-J.; Lee, J.-W. Extraction of total phenolic compounds from yellow poplar hydrolysate and evaluation of their antioxidant activities. Ind. Crops Prod. 2017, 97, 574–581. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A.; Singab, A.N.B.; Sinkkonen, J.; Pihlaja, K. Hypolipidemic and antioxidant effects of Morus alba L. (egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 2006, 78, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Valentín, L.; Kluczek-Turpeinen, B.; Willför, S.; Hemming, J.; Hatakka, A.; Steffen, K.; Tuomela, M. Scots pine (Pinus sylvestris) bark composition and degradation by fungi: Potential substrate for bioremediation. Bioresour. Technol. 2010, 101, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Ucar, M.B.; Ucar, G.; Pizzi, A.; Gonultas, O. Characterization of Pinus brutia bark tannin by MALDI-TOF MS and 13C NMR. Ind. Crops Prod. 2013, 49, 697–704. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of ultrasound on solid–liquid extraction of phenolic compounds from maritime pine sawdust waste. kinetics, optimization and large scale experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Ku, C.; Sathishkumar, M.; Mun, S. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere 2007, 67, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Frevel, M.A.; Pipingas, A.; Grigsby, W.J.; Frampton, C.M.; Gilchrist, N.L. Production, composition and toxicology studies of enzogenol Pinus radiata bark extract. Food Chem. Toxicol. 2012, 50, 4316–4324. [Google Scholar] [CrossRef] [PubMed]
- Tantray, M.A.; Akbar, S.; Khan, R.; Tariq, K.A.; Shawl, A.S. Humarain: A new dimeric gallic acid glycoside from Punica granatum L. Bark. Fitoterapia 2009, 80, 223–225. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry 2013, 92, 113–121. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, É.; Scher, J.; Dicko, A. antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Tewari, R.; Gupta, M.; Ahmad, F.; Rout, P.K.; Misra, L.; Patwardhan, A.; Vasudeva, R. Extraction, quantification and antioxidant activities of flavonoids, polyphenols and pinitol from wild and cultivated Saraca asoca bark using RP-HPLC-PDA-RI Method. Ind. Crops Prod. 2017, 103, 73–80. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Gabaldón-Hernández, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. RP-HPLC–ESI–QTOF/MS2 based strategy for the comprehensive metabolite profiling of Sclerocarya birrea (Marula) bark. Ind. Crops Prod. 2015, 71, 214–234. [Google Scholar] [CrossRef]
- Subramanian, R.; Raj, V.; Manigandan, K.; Elangovan, N. Antioxidant activity of hopeaphenol isolated from Shorea roxburghii stem bark extract. J. Taibah Univ. Sci. 2015, 9, 237–244. [Google Scholar] [CrossRef]
- Shastry Viswanatha, G.L.; Vaidya, S.K.; Ramesh, C.; Krishnadas, N.; Rangappa, S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac. J. Trop. Med. 2010, 3, 965–970. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Sindhe, M.A.; Satyanarayan, N.D.; Pramod, S.N.; Nagaraja, K. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 108–115. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Comparative evaluation of antimicrobial and antioxidant potential of ethanolic extract and its fractions of bark and leaves of Terminalia arjuna from North-Western Himalayas, India. J. Tradit. Complement. Med. 2018, 8, 100–106. [Google Scholar] [CrossRef]
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, Á.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. In vitro multimodal-effect of Trichilia catigua A. Juss.(Meliaceae) bark aqueous extract in CNS targets. J. Ethnopharmacol. 2018, 211, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bolanle, J.D.; Adetoro, K.O.; Balarabe, S.A.; Adeyemi, O.O. Hepatocurative potential of Vitex doniana root bark, stem bark and leaves extracts against CCl4–induced liver damage in rats. Asian Pac. J. Trop. Biomed. 2014, 4, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Diouf, P.N.; Stevanovic, T.; Boutin, Y. The effect of extraction process on polyphenol content, triterpene composition and bioactivity of yellow birch (Betula alleghaniensis Britton) extracts. Ind. Crops Prod. 2009, 30, 297–303. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-J.; Kim, D.-W.; Kim, G.-Y.; Kim, J.-K.; Gebru, Y.A.; Choi, H.-S.; Kim, Y.-H.; Kim, M.-K. Changes of phytochemical components (urushiols, polyphenols, gallotannins) and antioxidant capacity during Fomitella fraxinea – mediated fermentation of Toxicodendron vernicifluum bark. Molecules 2019, 24, 683. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. A review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1, 2-dimethyl hydrazine induced rat colon carcinogenesis. Investig. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Rathinasamy, B.; Lohanathan, B.; Thiyagarajan, V.; Weng, C.-F. Neuroprotective role of phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, Y.-J.; Kwon, S.-H.; Lee, Y.-H.; Choi, S.-Y.; Park, J.-S.; Kwon, H.-J. Inhibitory effects of flavonoids on TNF-α-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 2009, 42, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Cosarca, A.; Man, A.; Miklos, A.; Imre, S. Antibacterial activities of spruce bark (Picea abies L.) extract and its components against human pathogens. Rev. Chim. 2018, 69, 1462–1467. [Google Scholar]
- Tănase, C.; Coşarcă, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. EEMJ 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, M.Y.; Jo, H.; Lim, S.S.; Jung, S.H. Preparative isolation and purification of neuroprotective compounds from Rhus verniciflua by high speed counter-current chromatography. Biol. Pharm. Bull. 2012, 35, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Veerapur, V.; Thippeswamy, B.; Prabhakar, K.; Nagakannan, P.; Shivasharan, B.; Bansal, P.; Sneha, S.; Mishra, B.; Priyadarsini, K.; Unnikrishnan, M. Antioxidant and renoprotective activities of Ficus racemosa Linn. stem bark: Bioactivity guided fractionation study. Biomed. Prev. Nutr. 2011, 1, 273–281. [Google Scholar] [CrossRef]
- Kosalec, I.; Kremer, D.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Randić, M.; Končić, M.Z. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Jitta, S.R.; Daram, P.; Gourishetti, K.; Misra, C.; Polu, P.R.; Shah, A.; Shreedhara, C.; Nampoothiri, M.; Lobo, R. Terminalia tomentosa bark ameliorates inflammation and arthritis in carrageenan induced inflammatory model and freund’s adjuvant-induced arthritis model in rats. J. Toxicol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Source of Bark: Scientific Name (Family)–Commun Name | Extraction | Solvent | Time (min) | Temperature °C | Reference |
|---|---|---|---|---|---|
| Abies alba Mill (Pinaceae)–silver fir | CWBE | ethyl acetate | 120 | 70 | [53] |
| Acacia confuse Merr. (Fabaceae) | CWBE | NaOH 1% | 60 | 100 | [54] |
| Acacia cornigera (L.) Willd. (Fabaceae), bullhorn acacia | CWBE | petroleum ether, chloroform, methanol | 4320 | RT | [12] |
| Acacia ferruginea DC. (Fabaceae)–rusty acacia | SE | methanol 70% | - | - | [55] |
| Acacia mearnsii Wild. (Fabaceae) (Acacia mollissima)–black wattle | MAE | methanol:water 80:20 | - | - | [31] |
| Acacia nilotica L. (Fabaceae)–gum arabic tree | CWBE | methanol:ethanol, acetone:water | 480 | RT | [29] |
| Acanthopanax leucorrhizus (Oliv.) Harms (Araliaceae) | ME | ethanol 90% | 1440 | RT | [44] |
| Acer saccharum Marshal (Sapindaceae)–sugar maple | ME | ethanol 95% | - | - | [56] |
| Allophylus africanus Beauv. (Sapindaceae) | CE | water | 30 | - | [45] |
| Anacardium occidentale L. (Anacardiaceae)–cashew tree | ME | water | 120 | RT | [57] |
| Anogeissus leiocarpa DC. (Combretaceae)–African birch | SE | ethanol | 300 | - | [58] |
| Azadirachta indica A.Juss. (Meliaceae)–nimtree or Indian lilac | CWBE | methanol:ethanol:acetone:water | 480 | RT | [29] |
| Betula alleghaniensis Britt. (Betulaceae)–yellow birch or golden birch | UAE | ethanol 95% | - | - | [56] |
| Betula papyrifera Marshall (Betulaceae)–Paper birch | UAE | ethanol:water 80:20 | 180 | 50 | [35] |
| Byrsonima crassifolia (L.) Kunth (Malpighiaceae)–golden spoon | ME | petroleum ether:chloroform:methanol | 4320 | RT | [12] |
| Caraipa densifolia Mart. (Calophyllaceae) | SE | hexane:methanol | 180 | - | [59] |
| Cassia auriculata (L.) Roxb. (Fabaceae)–matura tea tree | UAE | water | 300 | - | [60] |
| Castanea sativa Mill. (Fagaceae)–sweet chestnut | UAE | methanol | 30 | RT | [18] |
| SE | n-hexane, acetone, ethanol, methanol | 900 | - | [32] | |
| Cayratia pedata Lam. (Vitaceae) | UAE | methanol | 10 | RT | [61] |
| Chloroxylon swietenia DC. (Rutaceae)–East Indian satinwood or buruta | UAE | methanol | 10 | RT | [61] |
| Cinnamon sp. (Lauraceae) | CWBE | ethyl acetate | 600 | 30 | [62] |
| Coutarea hexandra (Jacq.) K. Schum (Rubiaceae) | ME | ethanol 95% | 10,080 | - | [63] |
| Diotacanthus albiflorus Benth. (Acanthaceae) | UAE | methanol | 10 | RT | [61] |
| Drypetes klainei Pierre ex Pax (Putranjivaceae) | ME | water | 180 | RT | [15] |
| Erythrina suberosa Roxb. (Fabaceae)–Corky coral tree | CWBE | methanol | 1080 | RT | [64] |
| Eucalyptus camaldulensis Dehnh. (Myrtaceae) | UAE | ethanol | - | 40–50 | [36] |
| Eucalyptus globulus Labill (Myrtaceae)–Tasmanian bluegum, blue gum | SE | n-hexane, acetone, ethanol, methanol | 900 | - | [32] |
| CWBE | ethanol:water 80:20 (v/v) | 360 | 82.5 | [65] | |
| Eucalyptus grandis W.Hill ex. Maiden (Myrtaceae)–rose gum | SE | dichloromethane | 360 | - | [47] |
| Eucalyptus maidenii F. Muell (Myrtaceae)–Maiden’s Gum | SE | dichloromethane | 360 | - | [47] |
| Eucalyptus sideroxylon A.Cunn. (Myrtaceae)–mugga, red ironbark | UAE | ethanol:water | 60 | 50 | [66] |
| Eucalyptus urograndis (Myrtaceae)–Hybrid E.grandis x E. urophylla | SE | dichloromethane | 360 | - | [47] |
| Euclea undulata Thunb. (Ebenaceae)–small-leaved guarri, common guarri | CWBE | acetone | 4320 | RT | [67] |
| Eucommia ulmoides Oliv. (Eucommiaceae) | MAE + UAE | water, ethanol | 10–60 | 20–60 | [46] |
| Eugenia jambolana Lam. (Myrtaceae)–Jamun, black plum | CWBE | methanol 80%, ethanol 80%, acetone:water 80:20 | 480 | RT | [29] |
| Fagus sylvatica L. (Fagaceae)–common beech | CWBE | water, methanol:water 80:20, ethanol:water 80:20 | 120, 300, 1440 | RT | [25] |
| MAE | water, methanol:water and ethanol:water (80:20) | 10, 20 | 60, 80, 100, 120 | ||
| UAE | water, methanol:water 80:20, ethanol:water 80:20 | 10, 20, 30 | RT | ||
| Ficus talboti King. (Moraceae)–talbot fig | SE | methanol | - | - | [68] |
| Flourensia cernua DC. (Asteraceae)–American tarwort and tarbush | UAE | ethanol | - | 40–50 | [36] |
| Guazuma ulmifolia Lam. (Malvaceae)–West Indian elm or bay cedar | CWBE | petroleum ether, chloroform, methanol | 4320 | RT | [69] |
| Goniothalamus velutinus Airy Shaw (Annonaceae) | SE | absolute methanol | 600 | - | [70] |
| Hugonia mystax Cav. (Linaceae) | CWBE | distilled water, methanol ethanol | - | RT | [13] |
| Hymenaea courbaril L. (Fabaceae) | SFE | CO2 and water (9:1, v/v) | - | 56.85 | [42] |
| Jatropha dioica Sesse (Euphorbiaceae)–leatherstem | UAE | ethanol | - | 40–50 | [36] |
| Lafoensia pacari A. St.-Hil (Lythraceae) | ME | absolute ethanol | 10,080 | RT | [20] |
| Larix laricina K. Koch (Pinaceae)–tamarack or American larch | CWBE | ethanol 80% | - | - | [71] |
| Liriodendron tulipifera L. (Magnoliaceae)–tulip tree, American tulip tree, tulipwood | CWBE | oxalic acid 0.1 M | 60 | 170 | [72] |
| Malus domestica Miller (Rosaceae)–apple tree | CWBE MAE | ethanol:water, 1:4 ethanol:water, 60:40 v/v | 120 20 | 55 100 | [22] |
| Morus alba L. (Moraceae)–white mulberry | CWBE | methanol:water | - | - | [73] |
| Picea abies L. (Pinaceae)–european spruce | UAE | ethanol:water 50%, 70% (v/v) | 30–60 | 40–60 | [48] |
| CWBE | water | 10–120 | 60–90 | [19] | |
| CWBE | distilled water | 120 | 90 | [50] | |
| UAE | ethanol:water 70% (v/v) | 5–75 | 45–60 | [49] | |
| Picea mariana (Mill.) Britton (Pinaceae)–the black spruce | SE | water | 60 | - | [11] |
| Pinus sylvestris L. (Pinaceae)–Scots pine | CWBE | acetone:water | 3 × 5 | 100 | [74] |
| Pinus brutia Tenore (Pinaceae)–Turkish pine | CWBE | distilled water | 60 | 70 | [75] |
| Pinus pinaster Aiton (Pinaceae)–the maritime pine, cluster pine | MAE | ethanol:water 80:20 | 30 | - | [38] |
| CWBE | distilled water, ethanol, methanol | - | - | [76] | |
| CWBE | water:NaOH:Na2SO3:NaHSO3 | 120 | 70–80 | [77] | |
| Pinus radiata D.Don (Pinaceae)–Monterey pine, insignis pine or radiata pine | CWBE | Water | 60 | 100 | [78] |
| CWBE | deionized water | 30 | 95–99 | [79] | |
| CWBE | ethanol:water, 3:1 (v/v) | 120 | 120 | [10] | |
| Populus nigra L. (Salicaceae)–black poplar | UAE ME | ethanol:water 70:30 ethanol:water 70:30 | 60 - | - RT | [27] |
| Punica granatum L. (Lythraceae)–Pomegranate | CWBE | methanol | - | RT | [80] |
| Prunus domestica L. (Rosaceae)–Plums | UAE | 7 ethanol and HCl 1%, 2,6-di-tety-butyl-4-methylphenol (BHT) | - | - | [81] |
| Quercus robur L. (Fagaceae)–common oak, pedunculate oak | MAE | hydroalcoholic solution of methanol and ethanol | 5–120 | 100 | [82] |
| Rhus verniciflua (Stokes) F.Barkley (Anacardiaceae)–Chinese lacquer tree | SE | ethanol | - | - | [83] |
| Salix alba L. (Salicaceae)–white willow | ME | ethanol:water 70:30 | 1440 | - | [84] |
| Saraca asoca (Roxb.) Willd (Fabaceae)–the ashoka tree | CWBE | methanol | 1440 | RT | [85] |
| Schinopsis brasiliensis Engl. (Anacardiaceae)–baraúna | ME | ethanol 90% | 7200 | RT | [47] |
| Sclerocarya birrea (A. Rich.) Hochst. (Anacardiaceae)–marula | ME | distilled water | 2880 | RT | [86] |
| Shorea roxburghii D.Don (Dipterocarpaceae) | CWBE | acetone:methanol | - | - | [87] |
| Strychnos minor Dennst. (Loganiaceae) | UAE | methanol | 10 | RT | [61] |
| Strychnos nux-vomica Dennst. (Loganiaceae)–the strychnine tree, nux vomica, poison nut | UAE | methanol | 10 | RT | |
| Sweetia panamensis Yakovlev (Fabaceae) | CWBE | petroleum ether:chloroform:methanol | 4320 | RT | [12] |
| Terminalia brownie Fresen (Combretaceae) | SE | absolute ethanol | 300 | - | [58] |
| Terminalia arjuna Wight & Arn (Combretaceae)–arjun tree | SE | petroleum ether:ethanol | - | 60–80 | [88] |
| MAE | distilled water | 5 | - | [89] | |
| ME | ethanol | 7200 | - | [90] | |
| CWBE | methanol:ethanol:acetone:water | 480 | RT | [29] | |
| Terminalia laxiflora Engl. & Diels (Combretaceae) | SE | absolute ethanol | 300 | - | [58] |
| Trichilia catigua A.Juss. (Meliaceae) | CWBE | distilled water | 20 | 100 | [91] |
| Turnera diffusa Willd (Passifloraceae)–Damiana | UAE | ethanol | - | 40–50 | [36] |
| Ulmus pumila L. (Ulmaceae)–the Siberian elm | EAE | cellulose, pectinase, β-glucosidase | 60–90 | 40–60 | [51] |
| UAE | ethanol 50% | 10–90 | 52 | ||
| CWBE | ethanol 50% | 10–90 | 52 | ||
| Vitex doniana L. (Lamiaceae)–Black plum | ME | distilled water | 2880 | RT | [92] |
| Ziziphus jujuba Mill. (Rhamnaceae)–Jujube | CWBE | ethanol, methanol, hexane, acetone | 160 | 70 | [39] |
| UAE | methanol | 20–60 | RT | ||
| SE | methanol | 40–140 | 68 | ||
| MAE | methanol | 4 | - |
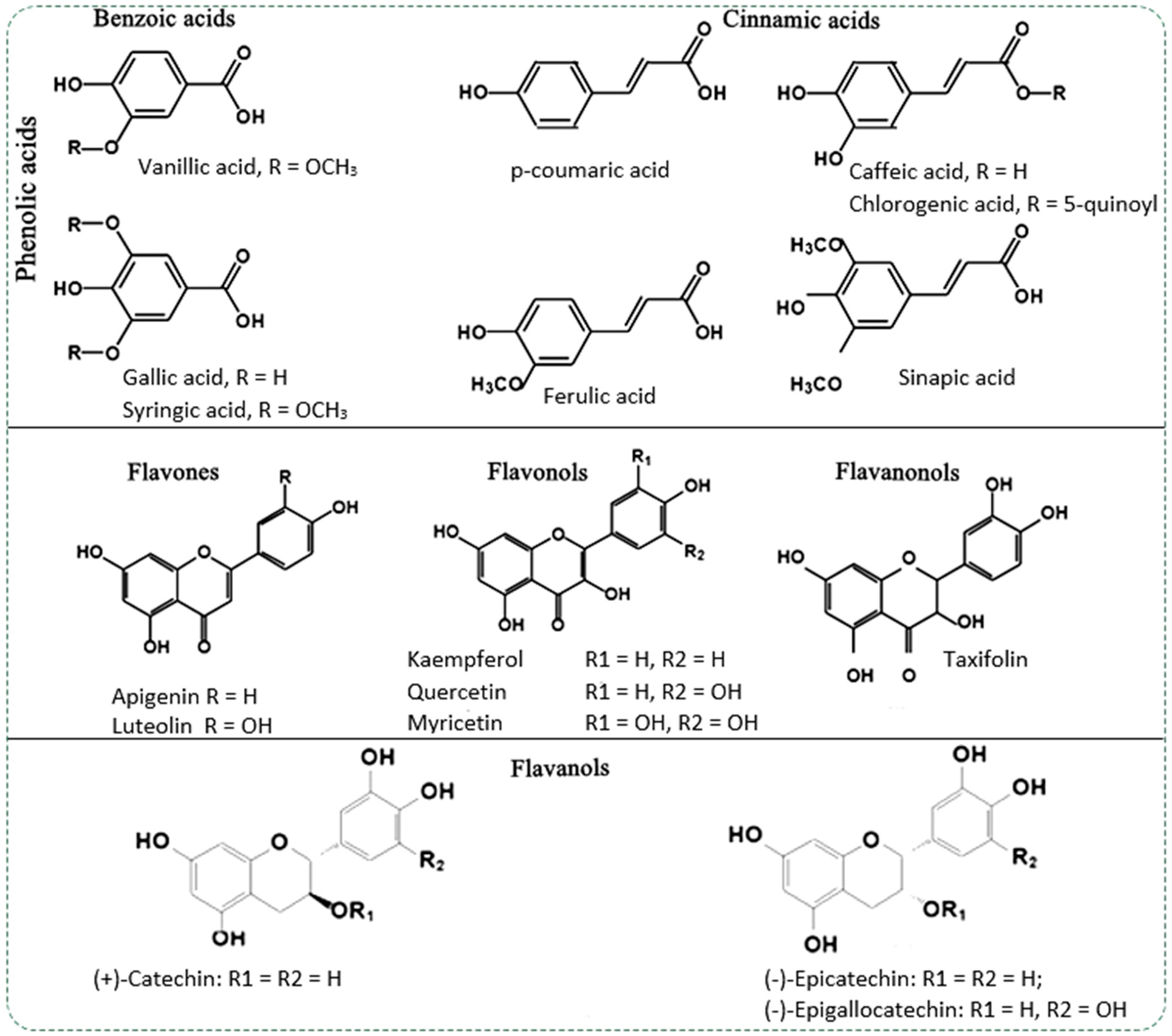
| Source of Bark: Scientific Name (Family)–Commun Name | Composition of Extract | Action/Application | Reference |
|---|---|---|---|
| Acacia cornigera (L.) Willd. (Fabaceae), bullhorn acacia | - | anti-inflammatory topical | [12] |
| Allophylus africanus Beauv. (Sapindaceae) | Apigenin, Luteolin, Vitexin, Apigetrin, Cymaroside | anti-inflammatory | [45] |
| Anogeissus leiocarpa DC. (Combretaceae)–African birch | Gallic acid, ellagitannin, Ampelopsin, Gallotannin, Epigallocatechin gallate, Ellagic acid derivative | antibacterial | [58] |
| Byrsonima crassifolia (L.) Kunth (Malpighiaceae)–golden spoon | - | anti-inflammatory topical | [12] |
| Caraipa densifolia Mart. (Calophyllaceae) | procaynidin dimer B2, procyanidin trimer C1, epicatechin, lupeol, betulinic acid | cancer prevention chemoprevention | [59] |
| Cayratia pedata Lam. (Vitaceae) | quercetin, o-coumaric acid, gallic acid | Antioxidant antiviral, cytotoxic | [61] |
| Chloroxylon swietenia DC. (Rutaceae)–East Indian satinwood or buruta | quercetin, ferulic acid, gallic acid | Antioxidant antiviral, cytotoxic | |
| Diotacanthus albiflorus Benth. (Acanthaceae) | quercetin, o-coumaric acid, ferulic acid, gallic acid | Antioxidant antiviral, cytotoxic | |
| Erythrina suberosa Roxb. (Fabaceae)–Corky coral tree | α-Hydroxyerysotrine, 4′-Methoxy licoflavanone (MLF), Alpinumisoflavone, (AIF), Wighteone | antitumoral, cytotoxic effect on HL-60 cells | [64] |
| Eucalyptus grandis W.Hill ex. Maiden (Myrtaceae)–rose gum | quinic acid, gallic acid, protocatechuic acid, catechin, ellagic acid, ellagic, acid-rhamnoside | antioxidant | [47] |
| Eucalyptus maidenii F. Muell (Myrtaceae)–Maiden’s Gum | quinic acid, gallic acid, protocatechuic acid, catechin, chlorogenic acid, ellagic acid, taxifolin, quercetin, mearnsetin, naringenin, ellagic acid-rhamnoside | antioxidant | |
| Eucalyptus sideroxylon A.Cunn. (Myrtaceae)–mugga, red ironbark | Monosaccharides, glucose, xylose, galactose, arabinose, mannose, rhamnose | antioxidant | [66] |
| Eucalyptus urograndis (Myrtaceae)–Hybrid E.grandis x E. urophylla | quinic acid, gallic acid, protocatechuic acid, catechin, ellagic acid, ellagic, acid-rhamnoside | antioxidant | [47] |
| Fagus sylvatica L. (Fagaceae)–common beech | Procyanidin, Epicatechin, Coumaric acid, Coniferin, Quercetin, Taxifolin-O-hexoside, Coumaric, acid-di-O-hexoside, Syringic acid-di-O-hexoside, Coniferyl alcohol-O-hexoside-O-pentoside | antioxidant | [26] |
| Ficus racemosa L. (Moraceae)–cluster fig tree, Indian fig tree or goolar (gular) | Kaempferol, Quercetin, Naringenin, Baicalein | normalizes glycogenol levels and hepatic glycogen, normalizes blood glucose levels | [17] |
| - | Antioxidant renoprotective activity | [103] | |
| Ficus talboti King. (Moraceae)–talbot fig | Gallic acid, Caffeic acid, Rutin, Ellagic acid, Quercetin, Kaempferol | hypocolesterolemiant, antidiabetic—increases the insulin sensitivity of pancreatic β cells, normalizes blood glucose level, antioxidant | [68] |
| Guazuma ulmifolia Lam. (Malvaceae)–West Indian elm or bay cedar | Flavanocoumarin epiphyllocoumarin, Epiphyllocoumarin-[4β→8]-(−)-epicatechin | anti-inflammatory, antioxidant | [69] |
| Hugonia mystax Cav. (Linaceae) | Gallic acid, catechol, caffeic acid, vanillin, p-coumaric acid, ferulic acid | anti-inflammatory, antioxidant, antirheumatic | [13] |
| Larix laricina K. Koch (Pinaceae)–tamarack or American larch | Rhaponticin, Rhapontigenin, Piceatannol, Taxifolin | antioxidant | [71] |
| Lafoensia pacari A. St.-Hil (Lythraceae) | Ellagic acid | anti-ulcerative-gastric hypopoietic, gastroprotector effect | [20] |
| Liriodendron tulipifera L. (Magnoliaceae)–tulip tree, American tulip tree, tulipwood | Furan-2-carboxylic acid, Mannose, β-d-glucopyranose, 3,5-dimethoxyphenol, 3,4-dimethoxy-mandelic acid, 2-Amino-3-hydroxybenzoic acid | antioxidant | [72] |
| Malus domestica Miller (Rosaceae)–apple tree | Gallic acid, Chlorogenic acid, Vanillic acid, Caffeic acid, Syringic acid, Ferulic acid, Sinapic acid, Resveratrol, Myricetin, Quercetin, Cinnamic Acid | antioxidant in food, cosmetics and pharmaceutical industry | [12] |
| Picea mariana (Mill.) Britton (Pinaceae)–the black spruce | Neolignans, Lignans: pinoresinol, Secoisolariciresinol, isolariciresinol, Epi-pinoresinol. Phenolic acids: trans-p-coumaric acid, vanillic acid, protocatechuic acid. Stilbenes: transresveratrol. Flavonoids: Kaempferol, quercetin, taxifolin, epitaxifolin, pallasiin, mearnsetin. Other phenolic compounds: p-vanillin, dihydroconiferyl alcohol | antiproliferative, antioxidant, anti-inflammatory | [11] |
| Pinus radiata D.Don (Pinaceae)–Monterey pine, insignis pine or radiata pine | Dihydroxybenzoic acid, 3,4-Dihydroxyphenylacetic acid, p-Hydroxybenzoic acid, Proanthocyanidin B2, Catechin, Epicatechin, Syringic acid, Taxifolin, Quercetin, Homovanillic acid, Epigallocatechin | antioxidant | [10] |
| Rhamnus alaternus L. (Rhamnaceae)–Italian buckthorn | Emodin, Chrysophanol, Physcion | Antioxidant antimicrobial | [104] |
| Schinopsis brasiliensis Engl. (Anacardiaceae)–baraúna | Gallic acid | Analgesic anti-inflammatory topical | [28] |
| Solidago canadensis L. (Asteraceae)–Canada goldenrod | - | Antioxidant antimicrobial | [94] |
| Strychnos minor Dennst. (Loganiaceae) | quercetin, coumaric acid, ferulic acid, gallic acid | antioxidant, antiviral, cytotoxic | [61] |
| Strychnos nux-vomica Dennst. (Loganiaceae)–the strychnine tree, nux vomica, poison nut | quercetin, ferulic acid, gallic acid | antioxidant, antiviral, cytotoxic | |
| Sweetia panamensis Yakovlev (Fabaceae) | - | anti-inflammatory topic | [12] |
| Terminalia arjuna Wight & Arn (Combretaceae)–arjun tree | - | Antioxidant antimutagenic | [88] |
| Terminalia brownie Fresen (Combretaceae) | Gallic acid, Ellagitannin, Punicalagin, Gallotannin, Corilagin | antibacterial | [58] |
| Terminalia laxiflora Engl. & Diels (Combretaceae) | Gallic acid, EllagitanninEllagic acid glucuronide, GallotanninMethylellagic acid glucuronide, Methyl-(S)-flavogallonate and its isomer | antibacterial | |
| Terminalia tomentosa Wight & Arn (Combretaceae)–Asan, Indian Laurel, Silver grey wood | - | anti-inflammatory | [105] |
| Trichilia catigua A.Juss. (Meliaceae) | Catechin, Procyanidin, Epicatechin, Apocynin E, Cinchonain I, 3-Methoxybenzoylquinic acid | antioxidant, anti-inflammatory, antidepressant, neuroprotective | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. https://doi.org/10.3390/molecules24061182
Tanase C, Coșarcă S, Muntean D-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules. 2019; 24(6):1182. https://doi.org/10.3390/molecules24061182
Chicago/Turabian StyleTanase, Corneliu, Sanda Coșarcă, and Daniela-Lucia Muntean. 2019. "A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity" Molecules 24, no. 6: 1182. https://doi.org/10.3390/molecules24061182