Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Compounds in Fermented Rice Samples
2.2. Nonvolatile Compounds in Fermented Rice Samples
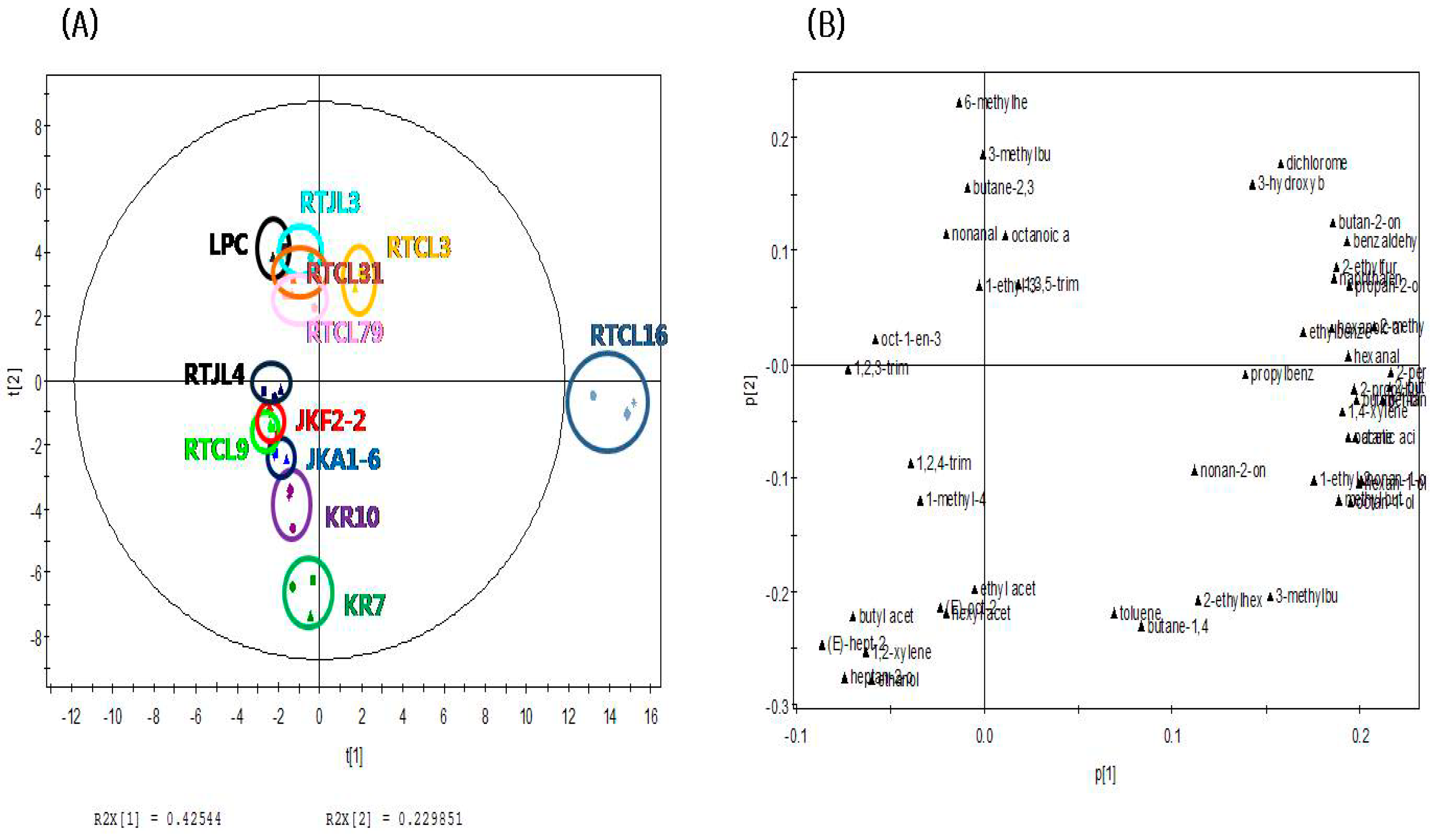
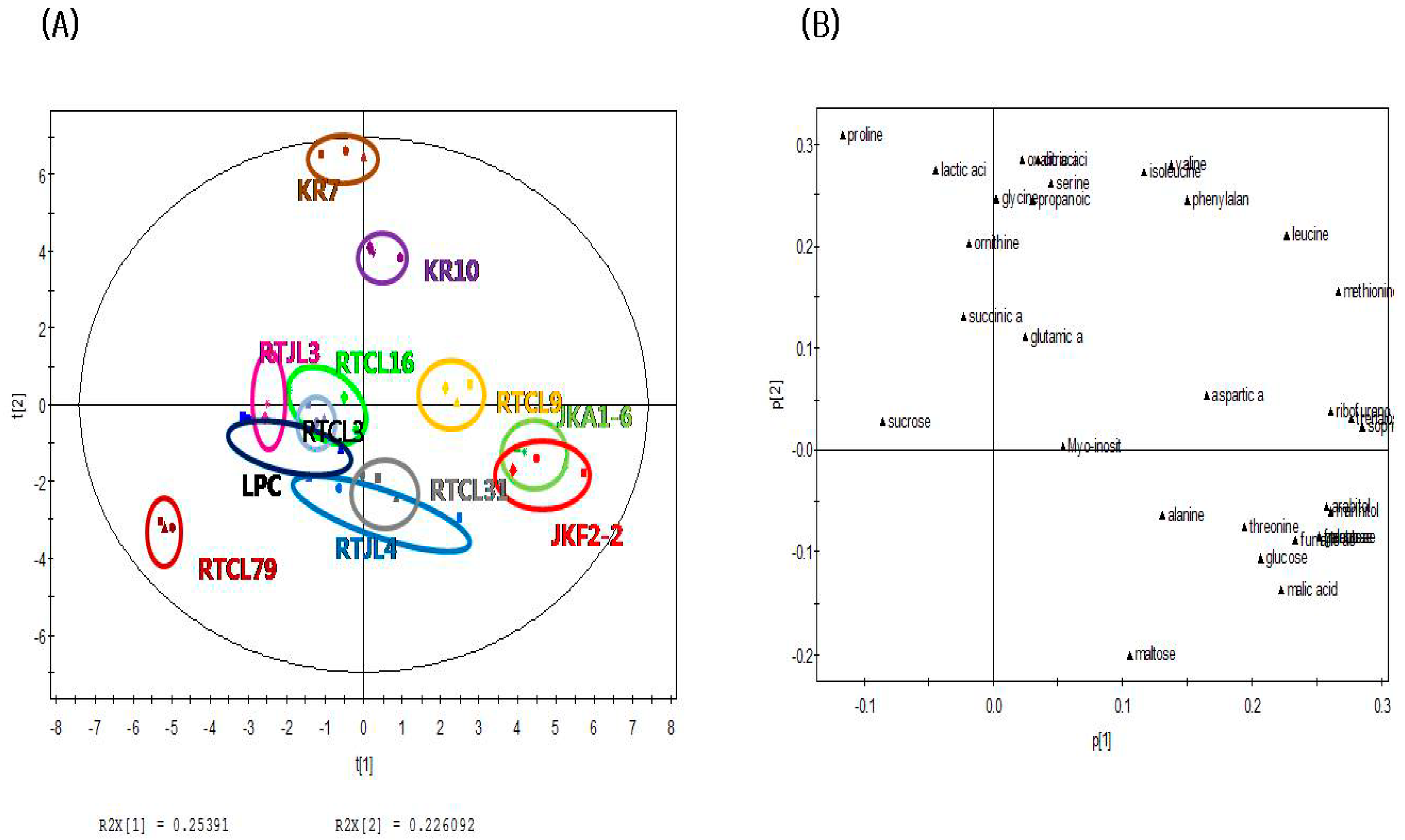
2.3. Principal Component Analysis of Fermented Rice Samples according to Strains of LABs
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Extraction and Analysis of Volatile Compounds by Gas Chromatography-Mass Spectrometry
3.4. Extraction and Analysis of Nonvolatile Compounds by Gas Chromatography-Time of Flight-Mass Spectrometry
3.5. Identification and Semi-Quantification of Volatile and Nonvolatile Compounds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Salmeron, I.; Loeza-Serrano, S.; Perez-Vega, S.; Pandiella, S.S. Headspace gas chromatography (HS-GC) analysis of imperative flavor compounds in lactobacilli-fermented barley and malt substrates. Food Sci. Biotechnol. 2015, 24, 1363–1371. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, J.; Hurh, B.S.; Jeong, G.H.; Shin, Y.G.; Kim, Y.S. Volatile compounds produced by lactobacillus paracasei during oat fermentation. J. Food Sci. 2016, 81, C2915–C2922. [Google Scholar] [CrossRef] [PubMed]
- Nout, M.R. Rich nutrition from the poorest–Cereal fermentations in Africa and Asia. Food Microbiol. 2009, 26, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Gotcheva, V.; Hristozova, T.; Gargova, S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J. Sci. Food Agric. 2005, 85, 2134–2141. [Google Scholar] [CrossRef]
- Kedia, G.; Vazquez, J.A.; Pandiella, S.S. Enzymatic digestion and in vitro fermentation of oat fractions by human lactobacillus strains. Enzyme Microb. Technol. 2008, 43, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Marklinder, I.; Johansson, L. Sour dough fermentation of barley flours with varied content of mixed-linked (1→3),(1→4) β-d-glucans. Food Microbiol. 1995, 12, 363–371. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Albury, M.N.; Pederson, C.S.; Van Veen, A.G.; Steinkraus, K.H. Role of Leuconostoc mesenteroides in leavening the batter of idle, a fermented food of India. Appl. Microbiol. 1965, 13, 227–231. [Google Scholar] [PubMed]
- Ghosh, K.; Maity, C.; Adak, A.; Halder, S.K.; Jana, A.; Das, A.; Parua, S.; Mohapatra, P.K.D.; Pati, B.R.; Mondal, K.C. Ethnic preparation of Haria, a rice-based fermented beverage, in the province of Lateritic West Bengal, India. Ethnobot. Res. Appl. 2014, 12, 39–49. [Google Scholar]
- Ghosh, K.; Ray, M.; Adak, A.; Dey, P.; Halder, S.K.; Das, A.; Jana, A.; Parua, S.; Mohapatra, P.K.D.; Pati, B.R.; et al. Microbial, saccharifying and antioxidant properties of an Indian rice based fermented beverage. Food Chem. 2015, 168, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Salmeron, I.; Fucinos, P.; Charalampopoulos, D.; Pandiella, S.S. Volatile compounds produced by the probiotic strain lactobacillus plantarum NCIMB 8826 in cereal-based substrates. Food Chem. 2009, 117, 265–271. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Drinan, D.F.; Robin, S.; Cogan, T.M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl. Environ. Microbiol. 1976, 31, 481–486. [Google Scholar] [PubMed]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Wache, Y. Biochemistry of lactone formation in yeast and fungi and its utilization for the production of flavor and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Fan, W.; Xu, Y. Changes in volatile compounds of Chinese rice wine wheat Qu during fermentation and storage. J. Inst. Brew. 2009, 115, 300–307. [Google Scholar] [CrossRef]
- Lyu, J.; Nam, P.W.; Lee, S.J.; Lee, K.G. Volatile compounds isolated from rice beers brewed with three medicinal plants. J. Inst. Brew. 2013, 119, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Holland, R.; Liu, S.; Crow, V.; Delabre, M.; Lubbers, M.; Bennett, M. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Laëtitia, G.; Pascal, D.; Yann, D. The citrate metabolism in homo-and heterofermentative LAB: A selective means of becoming dominant over other microorganisms in complex ecosystems. Food Nutr. Sci. 2014, 5, 953. [Google Scholar] [CrossRef]
- Annuk, H.; Shchepetova, J.; Kullisaar, T.; Songisepp, E.; Zilmer, M.; Mikelsaar, M. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 2003, 94, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in lentinusdecadetes. Biosci. Biotechnol. Biochem. 2003, 67, 2280–2282. [Google Scholar] [CrossRef]
- Assaf, S.; Hadar, Y.; Dosoretz, C.G. 1-octen-3-ol and 13-hydroperoxylinoleate are products of distinct pathways in the oxidative breakdown of linoleic acid by pleurotus pulmonarius. Enzyme Microb. Technol. 1997, 21, 484–490. [Google Scholar] [CrossRef]
- Vermeulen, N.; Czerny, M.; Gänzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef]
- Lampi, A.M.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yotsukura, N.; Kajiwara, T. Hydroperoxy-arachidonic acid mediated n-hexanal and (Z)-3-and (E)-2-nonenal formation in laminaria angustata. Phytochemistry 2003, 63, 669–678. [Google Scholar] [CrossRef]
- Filipiak, W.; Sponring, A.; Baur, M.M.; Ager, C.; Filipiak, A.; Wiesenhofer, H. Characterization of volatile metabolites taken up by or released from streptococcus pneumoniae and haemophilus influenzae by using GC-MS. Microbiology 2012, 158, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Schieberle, P. Important aroma compounds in freshly ground wholemeal and white wheat flour identification and quantitative changes during sourdough fermentation. J. Agric. Food Chem. 2002, 50, 6835–6840. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Dürrschmid, K.; Hendriks, K. Comparison of homo-and heterofermentative lactic acid bacteria for implementation of fermented wheat bran in bread. Food Microbiol. 2015, 49, 211–219. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Chu, F.L.; Yaylayan, V.A. Model studies on the oxygen-induced formation of benzaldehyde from phenylacetaldehyde using pyrolysis GC-MS and FTIR. J. Agric. Food Chem. 2008, 56, 10697–10704. [Google Scholar] [CrossRef]
- Ardo, Y.; Thage, B.; Madsen, J. Dynamics of free amino acid composition in cheese ripening. Aust. J. Dairy Technol. 2002, 57, 109. [Google Scholar]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H. Chemical and functional components in different parts of rough rice (oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Sugino, T.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr. Res. 2008, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, G.; Rimaux, T.; Weckx, S.; De Vuyst, L.; Leroy, F. Environmental pH determines citrulline and ornithine release through the arginine deiminase pathway in Lactobacillus fermentum IMDO 130101. Int. J. Food Microbiol. 2009, 135, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quintans, N.; Repizo, G.; Martin, M.; Magni, C.; Lopez, P. Activation of the diacetyl/acetoin pathway in lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 2008, 74, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Cselovszky, J.; Wolf, G.; Hammes, W.P. Production of formate, acetate, and succinate by anaerobic fermentation of lactobacillus pentosus in the presence of citrate. Appl. Microbiol. Biotechnol. 1992, 37, 94–97. [Google Scholar] [CrossRef]
- Wisselink, H.; Weusthuis, R.; Eggink, G.; Hugenholtz, J.; Grobben, G. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Son, E.Y.; Lee, S.M.; Kim, M.; Seo, J.A.; Kim, Y.S. Comparison of volatile and non-volatile metabolites in rice wine fermented by Koji inoculated with Saccharomycopsis fibuligera and Aspergillus oryzae. Food Res. Int. 2018, 109, 596–605. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |
| No. | Compounds | RI 1 | Relative Peak Area 2 | ID 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTJL3 | LPC | KR10 | KR7 | RTCL16 | RTCL79 | RTJL4 | RTCL3 | RTCL31 | JFK2-2 | JKA1-6 | RTCL | ||||
| ACIDS | |||||||||||||||
| 1 | acetic acid | 1457 | 4.39 ± 0.69 ab 4 | 1.45 ± 0.42 a | 9.99 ± 1.85 ab | 13.42 ± 8.76 ab | 80.20 ± 19.99 c | 11.25 ± 8.77 ab | 8.48 ± 4.02 ab | 14.36 ± 4.06 ab | 1.47 ± 0.71 a | 18.36 ± 8.57 b | 14.87 ± 2.23 ab | 18.39 ± 6.29 b | A |
| 2 | hexanoic acid | 1860 | 0.95 ± 0.06 g | 0.45 ± 0.08 cd | 0.61 ± 0.08 de | N.D. a | 2.17 ± 0.29 h | 0.36 ± 0.14 bc | 0.48 ± 0.10 cd | 0.89 ± 0.12 f | 0.24 ± 0.08 b | 0.78 ± 0.10 efg | 0.59 ± 0.04 de | 0.70 ± 0.06 ef | A |
| 3 | octanoic acid | 2065 | 0.14 ± 0.01 b | 0.34 ± 0.02 d | 0.04 ± 0.01 a | 0.14 ± 0.03 b | 0.17 ± 0.02 bc | 0.16 ± 0.05 bc | 0.22 ± 0.04 c | 0.49 ± 0.12 e | 0.14 ± 0.01 b | 0.05 ± 0.01 a | 0.03 ± 0.01 a | 0.43 ± 0.04 e | A |
| ALDEHYDES | |||||||||||||||
| 4 | 2-methylbutanal | 911 | N.D. 5 a | N.D. a | N.D. a | N.D. a | 0.64 ± 0.08 d | N.D. a | N.D. a | 0.34 ± 0.02 c | 0.07 ± 0.02 b | N.D. a | N.D. a | N.D. a | A |
| 5 | 3-methylbutanal | 915 | 0.42 ± 0.04 c | 0.34 ± 0.05 b | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.58 ± 0.03 d | N.D. a | N.D. a | N.D. a | N.D. a | A |
| 6 | hexanal | 1078 | N.D. a | N.D. a | 0.52 ± 0.34 b | 0.64 ± 0.21 b | 4.08 ± 0.11 d | N.D. a | N.D. a | 3.02 ± 0.10 c | 0.72 ± 0.04 b | 0.51 ± 0.12 b | 0.22 ± 0.01 a | 0.19 ± 0.01 a | A |
| 7 | nonanal | 1397 | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.168 ± 0.018 b | N.D. a | N.D. a | 0.218 ± 0.060 c | N.D. a | N.D. a | N.D. a | A |
| 8 | benzaldehyde | 1530 | 2.68 ± 0.22 c | 2.72 ± 0.02 c | 0.40 ± 0.05 a | 0.52 ± 0.06 a | 6.94 ± 0.54 e | 0.47 ± 0.05 a | 0.24 ± 0.05 a | 3.19 ± 0.04 d | 2.13 ± 0.12 b | 0.28 ± 0.05 a | 0.33 ± 0.03 a | 0.38 ± 0.03 a | A |
| ESTERS | |||||||||||||||
| 9 | ethyl acetate | 885 | 0.33 ± 0.03 a | 0.25 ± 0.06 a | 0.66 ± 0.02 bc | 0.97 ± 0.13 d | 0.72 ± 0.09 c | 0.34 ± 0.12 a | 0.96 ± 0.02 d | 0.37 ± 0.12 a | 0.25 ± 0.03 a | 0.70 ± 0.04 bc | 1.71 ± 0.07 e | 0.56 ± 0.05 b | A |
| 10 | methyl butanoate | 984 | 0.13 ± 0.02 a | 0.13 ± 0.01 a | 0.21 ± 0.02 bc | 0.27 ± 0.02 d | 0.43 ± 0.06 e | 0.19 ± 0.02 bc | 0.19 ± 0.03 bc | 0.23 ± 0.02 cd | 0.18 ± 0.01 ab | 0.18 ± 0.04 ab | 0.22 ± 0.02 bcd | 0.21 ± 0.01 bc | B |
| 11 | butyl acetate | 1070 | N.D. a | N.D. a | 0.09 ± 0.05 b | 0.13 ± 0.01 c | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.13 ± 0.01 c | 0.18 ± 0.02 d | 0.16 ± 0.03 cd | B |
| 12 | hexyl acetate | 1274 | N.D. a | N.D. a | 0.07 ± 0.01 b | 0.12 ± 0.01 c | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | A |
| KETONES | |||||||||||||||
| 13 | propan-2-one | 816 | 2.14 ± 0.43 cd | 1.93 ± 0.15 c | 2.69 ± 0.29 def | N.D. a | 6.90 ± 0.17 g | 2.45 ± 0.26 de | N.D. a | 2.84 ± 0.18 ef | 1.93 ± 0.14 c | 1.00 ± 0.20 b | 1.36 ± 0.06 b | 1.13 ± 0.11 b | A |
| 14 | butan-2-one | 901 | 0.52 ± 0.08 d | 0.55 ± 0.03 d | N.D. a | N.D. a | 1.57 ± 0.05 f | 0.59 ± 0.03 d | N.D. a | 0.69 ± 0.03 e | 0.56 ± 0.02 d | 0.39 ± 0.02 c | 0.38 ± 0.08 c | 0.29 ± 0.07 b | A |
| 15 | butane-2,3-dione | 975 | 67.10 ± 3.89 e | 29.33 ± 1.30 d | 0.08 ± 0.01 a | 0.19 ± 0.04 a | 7.04 ± 0.64 c | 2.66 ± 0.22 ab | N.D. a | 3.77 ± 0.21 b | 1.98 ± 0.02 ab | 0.44 ± 0.03 a | 0.14 ± 0.02 a | 0.09 ± 0.01 a | A |
| 16 | 3-hydroxybutan-2-one | 1284 | 8.80 ± 0.35 c | 3.12 ± 0.33 b | N.D. a | N.D. a | 8.64 ± 0.96 c | 2.88 ± 0.42 b | N.D. a | 2.70 ± 0.35 b | 3.15 ± 0.42 b | N.D. a | N.D. a | N.D. a | A |
| 17 | 6-methylhept-5-en-2-one | 1340 | 0.15 ± 0.03 c | 0.29 ± 0.02 d | N.D. | N.D. a | N.D. a | 0.13 ± 0.01 b | N.D. a | 0.31 ± 0.02 e | 0.11 ± 0.01 b | N.D. a | N.D. a | N.D. a | A |
| 18 | nonan-2-one | 1390 | 0.22 ± 0.01 d | 0.14 ± 0.03 c | 0.34 ± 0.03 e | 0.42 ± 0.05 f | 0.37 ± 0.05 e | 0.16 ± 0.01 a | N.D. a | 0.15 ± 0.01 c | 0.06 ± 0.01 b | N.D. a | N.D. a | N.D. a | A |
| ALCOHOLS | |||||||||||||||
| 19 | ethanol | 938 | 7.28 ± 0.87 a | 7.46 ± 0.42 a | 148.13 ± 6.73 d | 171.54 ± 24.01 e | 20.79 ± 1.95 a | 7.29 ± 0.40 a | 88.28 ± 14.58 bc | 9.52 ± 1.64 a | 7.39 ± 0.46 a | 101.64 ± 7.36 c | 82.42 ± 1.90 b | 85.02 ± 8.11 b | A |
| 20 | butan-1-ol | 1151 | 0.24 ± 0.02 a | 0.18 ± 0.02 a | 0.36 ± 0.02 c | 0.19 ± 0.01 a | 0.90 ± 0.09 d | 0.35 ± 0.03 bc | 0.19 ± 0.03 a | 0.38 ± 0.03 c | 0.21 ± 0.01 a | 0.34 ± 0.02 bc | 0.38 ± 0.01 c | 0.30 ± 0.01 b | A |
| 21 | 3-methylbutan-1-ol | 1212 | 0.60 ± 0.04 ab | 0.49 ± 0.03 a | 1.11 ± 0.03 f | 1.36 ± 0.21 g | 1.78 ± 0.12 | 0.73 ± 0.08 bc | 0.76 ± 0.05 cd | 0.68 ± 0.04 bc | 0.57 ± 0.02 ab | 0.99 ± 0.01 ef | 0.90 ± 0.01 de | 0.93 ± 0.15 e | A |
| 22 | pentan-1-ol | 1255 | 1.32 ± 0.28 ab | 1.47 ± 0.15 abc | 1.42 ± 0.05 ab | 1.73 ± 0.11 c | 4.04 ± 0.13 d | 1.18 ± 0.20 a | 1.36 ± 0.06 ab | 1.56 ± 0.14 bc | 1.55 ± 0.20 bc | 1.36 ± 0.21 ab | 1.29 ± 0.04 ab | 1.23 ± 0.08 a | A |
| 23 | heptan-2-ol | 1325 | N.D. a | N.D. a | 0.44 ± 0.02 d | 0.68 ± 0.10 e | N.D. a | N.D. a | 0.31 ± 0.04 b | N.D. a | N.D. a | 0.39 ± 0.08 cd | 0.41 ± 0.04 d | 0.33 ± 0.06 bc | A |
| 24 | hexan-1-ol | 1358 | 4.18 ± 0.26 a | 4.25 ± 0.19 a | 8.04 ± 0.45 c | 10.17 ± 0.19 d | 20.11 ± 1.36 e | 6.91 ± 0.24 b | 7.51 ± 0.38 bc | 7.55 ± 0.49 bc | 7.58 ± 0.45 bc | 7.22 ± 0.43 bc | 6.92 ± 0.06 b | 7.20 ± 0.05 bc | A |
| 25 | oct-1-en-3-ol | 1454 | 0.76 ± 0.10 b | 0.89 ± 0.03 b | 0.91 ± 0.26 b | 1.27 ± 0.35 c | N.D. a | 0.66 ± 0.22 b | 0.77 ± 0.16 b | 0.82 ± 0.28 b | 0.84 ± 0.03 b | N.D. a | N.D. a | N.D. a | A |
| 26 | 2-ethylhexan-1-ol | 1495 | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 0.20 ± 0.01 cd | 0.44 ± 0.03 e | 0.39 ± 0.07 e | 0.09 ± 0.01 a | 0.12 ± 0.02 ab | 0.24 ± 0.05 d | 0.08 ± 0.01 a | 0.16 ± 0.04 bc | 0.26 ± 0.01 d | 0.21 ± 0.03 cd | A |
| 27 | (E)-hept-2-en-1-ol | 1517 | N.D. a | N.D. a | 0.11 ± 0.01 b | 0.15 ± 0.03 c | N.D. a | N.D. a | 0.15 ± 0.01 c | N.D. a | N.D. a | 0.13 ± 0.01 b | 0.12 ± 0.01 b | 0.1 ± 0.02 b | A |
| 28 | octan-1-ol | 1564 | 0.26 ± 0.03 ab | 0.24 ± 0.02 a | 0.37 ± 0.02 c | 0.45 ± 0.10 d | 0.75 ± 0.05 e | 0.28 ± 0.04 ab | 0.25 ± 0.02 ab | 0.33 ± 0.02 bc | 0.25 ± 0.04 ab | 0.32 ± 0.01 bc | 0.32 ± 0.04 bc | 0.29 ± 0.03 ab | A |
| 29 | (E)-oct-2-en-1-ol | 1616 | N.D. a | N.D. a | 0.05 ± 0.01 b | 0.06 ± 0.01 c | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | A |
| 30 | nonan-1-ol | 1667 | 0.33 ± 0.04 ab | 0.30 ± 0.02 a | 0.40 ± 0.05 bc | 0.59 ± 0.09 d | 1.03 ± 0.07 e | 0.36 ± 0.04 abc | 0.34 ± 0.04 ab | 0.43 ± 0.03 c | 0.31 ± 0.04 a | 0.40 ± 0.01 bc | 0.41 ± 0.02 bc | 0.3 ± 0.03 abc | A |
| 31 | butane-1,4-diol | 1928 | 0.19 ± 0.01 ab | 0.22 ± 0.03 ab | 0.49 ± 0.07 e | 0.77 ± 0.10 f | 0.54 ± 0.04 e | 0.19 ± 0.03 ab | 0.14 ± 0.01 a | 0.31 ± 0.06 cd | 0.18 ± 0.01 a | 0.31 ± 0.05 cd | 0.3 ± 0.04 d | 0.27 ± 0.02 bc | A |
| FURAN DERIVATIVES | |||||||||||||||
| 32 | 2-ethylfuran | 950 | 1.23 ± 0.34 d | 1.16 ± 0.13 cd | 0.57 ± 0.09 ab | 0.90 ± 0.10 bc | 2.40 ± 0.08 f | 1.16 ± 0.11 cd | 0.84 ± 0.08 ab | 1.54 ± 0.38 e | 0.58 ± 0.17 ab | 0.55 ± 0.11 a | 0.56 ± 0.11 a | 0.58 ± 0.07 ab | A |
| 33 | 2-propylfuran | 1030 | 1.59 ± 0.22 e | 1.45 ± 0.11 cde | 1.32 ± 0.08 bcd | 1.88 ± 0.10 f | 3.15 ± 0.19 g | 1.51 ± 0.17 de | 1.32 ± 0.08 bcd | 2.02 ± 0.14 f | 0.86 ± 0.19 a | 1.25 ± 0.09 bc | 1.33 ± 0.08 bcd | 1.10 ± 0.05 b | A |
| 34 | 2-butylfuran | 1130 | 3.48 ± 0.30 b | 3.06 ± 0.09 ab | 3.07 ± 0.06 ab | 4.20 ± 0.47 c | 8.93 ± 0.17 d | 3.40 ± 0.43 b | 3.24 ± 0.08 ab | 4.41 ± 0.16 c | 3.27 ± 0.72 ab | 2.94 ± 0.02 ab | 3.22 ± 0.19 ab | 2.76 ± 0.05 a | A |
| 35 | 2-pentylfuran | 1231 | 16.15 ± 1.55 bc | 13.51 ± 0.78 ab | 12.82 ± 0.46 a | 16.97 ± 1.75 c | 49.08 ± 0.93 e | 16.05 ± 2.59 bc | 14.77 ± 1.06 abc | 20.05 ± 1.59 d | 13.32 ± 2.52 a | 12.93 ± 0.37 a | 14.32 ± 0.82 abc | 12.69 ± 0.59 a | B |
| BENZENE AND BENZENE DERIVATIVES | |||||||||||||||
| 36 | toluene | 1036 | 1.32 ± 0.12 abc | 1.01 ± 0.01 a | 2.06 ± 0.25 de | 2.39 ± 0.10 e | 2.33 ± 0.26 e | 1.11 ± 0.15 a | 2.33 ± 0.18 e | 1.73 ± 0.50 bcd | 1.28 ± 0.16 ab | 1.79 ± 0.18 cd | 1.8 ± 0.09 d | 2.00 ± 0.60 de | A |
| 37 | ethylbenzene | 1120 | 2.23 ± 0.37 ab | 1.93 ± 0.12 ab | 2.10 ± 0.13 ab | 2.47 ± 0.40 ab | 4.51 ± 0.07 d | 2.03 ± 0.19 ab | 2.34 ± 0.32 ab | 2.52 ± 0.05 b | 3.88 ± 0.78 c | 1.93 ± 0.12 ab | 1.96 ± 0.15 ab | 1.90 ± 0.15 a | B |
| 38 | 1,4-xylene | 1136 | 1.63 ± 0.15 ab | 1.45 ± 0.05 a | 1.82 ± 0.13 abc | 2.17 ± 0.41 cd | 3.55 ± 0.20 e | 1.52 ± 0.07 a | 1.69 ± 0.09 ab | 1.97 ± 0.11 bc | 2.49 ± 0.45 d | 1.68 ± 0.15 ab | 1.67 ± 0.08 ab | 1.63 ± 0.08 ab | A |
| 39 | 1,2-xylene | 1179 | N.D. a | N.D. a | 0.80 ± 0.05 b | 1.14 ± 0.11 c | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.61 ± 0.49 b | 0.83 ± 0.04 b | 0.89 ± 0.18 bc | A |
| 40 | propylbenzene | 1205 | N.D. a | 0.04 ± 0.01 ab | 0.05 ± 0.01 bc | 0.04 ± 0.00 ab | 0.15 ± 0.01 e | 0.06 ± 0.01 | 0.10 ± 0.02 d | 0.07 ± 0.01 bcd | 0.09 ± 0.01 cd | N.D. bcd | 0.09 ± 0.06 cd | N.D. a | B |
| 41 | 1-ethyl-2-methylbenzene | 1222 | 0.04 ± 0.01 a | 0.0 ± 0.01 a | 0.14 ± 0.02 d | 0.09 ± 0.02 c | 0.30 ± 0.01 e | 0.04 ± 0.01 a | 0.06 ± 0.01 ab | 0.05 ± 0.01 ab | 0.09 ± 0.01 c | 0.07 ± 0.01 bc | 0.06 ± 0.01 ab | 0.16 ± 0.01 d | B |
| 42 | 1-ethyl-3-methylbenzene | 1223 | 0.13 ± 0.00 bc | 0.16 ± 0.05 c | 0.07 ± 0.00 a | 0.17 ± 0.01 c | 0.13 ± 0.02 bc | 0.10 ± 0.01 ab | 0.16 ± 0.04 c | 0.14 ± 0.02 bc | 0.23 ± 0.06 d | 0.14 ± 0.01 bc | 0.14 ± 0.01 bc | 0.07 ± 0.01 a | A |
| 43 | 1,3,5-trimethylbenzene | 1242 | 0.13 ± 0.00 c | 0.12 ± 0.02 c | 0.19 ± 0.02 d | N.D. a | 0.13 ± 0.01 c | N.D. a | 0.13 ± 0.02 c | 0.13 ± 0.01 c | 0.20 ± 0.04 d | 0.15 ± 0.03 c | 0.07 ± 0.02 b | 0.08 ± 0.02 b | B |
| 44 | 1,2,4-trimethylbenzene | 1280 | N.D. a | 0.53 ± 0.01 ab | N.D. a | 1.18 ± 1.02 b | N.D. a | 0.56 ± 0.05 ab | N.D. a | N.D. a | N.D. a | N.D. b | N.D. a | N.D. a | A |
| 45 | 1,2,3-trimethylbenzene | 1337 | 0.04 ± 0.00 ab | 0.03 ± 0.00 ab | N.D. a | N.D. a | N.D. a | 0.04 ± 0.02 ab | 0.18 ± 0.03 c | 0.05 ± 0.01 ab | 0.07 ± 0.03 b | 0.19 ± 0.05 c | 0.19 ± 0.07 c | N.D. a | A |
| HYDROCARBONS | |||||||||||||||
| 46 | octane | 780 | 0.98 ± 0.17 ab | 0.76 ± 0.04 a | 1.19 ± 0.16 bc | 1.41 ± 0.08 cd | 3.16 ± 0.25 e | 1.06 ± 0.16 ab | 1.62 ± 0.43 d | 1.10 ± 0.19 ab | 0.83 ± 0.16 ab | 1.00 ± 0.18 ab | 0.87 ± 0.08 ab | 0.74 ± 0.11 a | A |
| TERPENES | |||||||||||||||
| 47 | 1-methyl-4-prop-1-en-2-ylcyclohexene (limonene) | 1194 | N.D. a | N.D. a | 0.33 ± 0.06 c | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.15 ± 0.03 b | A |
| No. | Compounds | Relative Peak Area 1 | ID 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTJL3 | LPC | KR10 | KR7 | RTCL16 | RTCL79 | RTJL4 | RTCL3 | RTCL31 | JFK2-2 | JKA1-6 | RTCL | |||
| AMINO ACIDS | ||||||||||||||
| 1 | alanine | 0.43 ± 0.05 f 3 | 0.36 ± 0.02 e | 0.16 ± 0.02 b | 0.22 ± 0.02 c | 0.55 ± 0.01 cg | 0.08 ± 0.02 a | 0.24 ± 0.03 c | 0.05 ± 0.01 a | 0.44 ± 0.03 f | 0.38 ± 0.02 e | 0.47 ± 0.02 f | 0.31 ± 0.04 d | A |
| 2 | asparagine | N.D. 4 a | N.D. a | 0.03 ± 0.00 d | 0.03 ± 0.01 cd | N.D. a | 0.01 ± 0.00 b | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.01 ± 0.00 b | 0.02 ± 0.01 c | 0.02 ± 0.00 b | A |
| 3 | citrulline | 0.03 ± 0.00 b | 0.03 ± 0.00 b | N.D. a | N.D. a | 0.04 ± 0.01 c | 0.04 ± 0.00 c | N.D. a | N.D. a | 0.07 ± 0.01 e | 0.05 ± 0.01 d | 0.05 ± 0.01 d | N.D. a | A |
| 4 | γ-aminobutyric acid | 0.05 ± 0.01 a | 0.09 ± 0.00 d | 0.09 ± 0.00 d | 0.64 ± 0.02 g | 0.09 ± 0.00 de | 0.07 ± 0.01 b | 0.16 ± 0.01 f | 0.08 ± 0.00 cd | 0.08 ± 0.01 cd | 0.09 ± 0.00 d | 0.08 ± 0.01 cd | 0.08 ± 0.00 cd | A |
| 5 | tryptophan | 0.01 ± 0.00 b | N.D. a | 0.03 ± 0.01 c | 0.02 ± 0.00 c | 0.01 ± 0.00 b | N.D. a | N.D. a | 0.02 ± 0.00 c | N.D. a | 0.02 ± 0.01 c | 0.02 ± 0.00 c | 0.02 ± 0.00 c | A |
| 6 | valine | 0.21 ± 0.02 e | 0.11 ± 0.00 b | 0.29 ± 0.01 b | 0.25 ± 0.01 g | 0.23 ± 0.00 f | 0.01 ± 0.00 a | 0.11 ± 0.00 b | 0.19 ± 0.00 d | 0.17 ± 0.00 c | 0.16 ± 0.00 c | 0.18 ± 0.01 d | 0.23 ± 0.01 f | A |
| 7 | leucine | 0.20 ± 0.02 c | 0.14 ± 0.01 b | 0.50 ± 0.01 j | 0.40 ± 0.01 g | 0.43 ± 0.01 i | 0.01 ± 0.01 a | 0.20 ± 0.00 c | 0.35 ± 0.01 e | 0.27 ± 0.00 d | 0.37 ± 0.01 f | 0.40 ± 0.00 gh | 0.42 ± 0.03 hi | A |
| 8 | isoleucine | 0.03 ± 0.01 c | N.D. a | 0.12 ± 0.00 g | 0.08 ± 0.00 ef | 0.08 ± 0.00 e | N.D. a | N.D. a | 0.02 ± 0.01 b | 0.02 ± 0.00 b | 0.03 ± 0.00 c | 0.05 ± 0.00 d | 0.09 ± 0.00 f | A |
| 9 | proline | 0.36 ± 0.03 e | 0.31 ± 0.01 d | 0.46 ± 0.01 g | 0.44 ± 0.01 f | 0.27 ± 0.01 c | 0.27 ± 0.01 c | 0.23 ± 0.01 b | 0.27 ± 0.01 c | 0.25 ± 0.01 c | 0.22 ± 0.01 ab | 0.21 ± 0.01 a | 0.22 ± 0.002 ab | A |
| 10 | glycine | 0.13 ± 0.05 b | 0.23 ± 0.02 c | 0.20 ± 0.01 c | 0.19 ± 0.02 c | 0.10 ± 0.08 b | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.11 ± 0.01 b | 0.09 ± 0.00 b | 0.11 ± 0.00 b | 0.11 ± 0.01 b | 0.10 ± 0.01 b | A |
| 11 | serine | 0.24 ± 0.07 efg | 0.20 ± 0.02 cde | 0.26 ± 0.01 g | 0.26 ± 0.02 fg | 0.15 ± 0.01 ab | 0.15 ± 0.01 ab | 0.16 ± 0.02 abc | 0.12 ± 0.01 a | 0.17 ± 0.02 bc | 0.18 ± 0.01 bcd | 0.20 ± 0.02 cde | 0.22 ± 0.012 def | A |
| 12 | threonine | 0.04 ± 0.01 def | 0.01 ± 0.00 a | 0.03 ± 0.00 cd | 0.02 ± 0.00 ab | 0.04 ± 0.00 gh | 0.02 ± 0.01 bc | 0.02 ± 0.01 ab | 0.04 ± 0.01 efg | 0.03 ± 0.00 de | 0.05 ± 0.00 h | 0.04 ± 0.00 fgh | 0.05 ± 0.00 h | A |
| 13 | methionine | 0.03 ± 0.00 bc | 0.03 ± 0.01 bc | 0.04 ± 0.00 e | 0.03 ± 0.00 cd | 0.03 ± 0.00 bc | N.D. a | 0.03 ± 0.00 bc | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.04 ± 0.01 e | 0.04 ± 0.01 de | 0.04 ± 0.00 cd | A |
| 14 | aspartic acid | 0.16 ± 0.02 e | 0.12 ± 0.02 d | 0.09 ± 0.00 c | 0.06 ± 0.01 b | 0.08 ± 0.004 c | N.D. a | N.D. a | 0.10 ± 0.00 c | 0.06 ± 0.00 b | 0.15 ± 0.01 e | 0.13 ± 0.00 d | 0.15 ± 0.01 e | A |
| 15 | glutamic acid | 0.08 ± 0.00 c | 0.08 ± 0.00 c | 0.13 ± 0.00 d | 0.02 ± 0.00 ab | 0.07 ± 0.01 c | N.D. a | N.D. a | 0.07 ± 0.01 c | 0.06 ± 0.00 c | 0.03 ± 0.05 b | 0.05 ± 0.00 c | 0.08 ± 0.01 c | A |
| 16 | phenylalanine | 0.05 ± 0.00 b | 0.04 ± 0.00 b | 0.11 ± 0.00 e | 0.10 ± 0.01 de | 0.08 ± 0.01 cd | N.D. a | 0.05 ± 0.01 b | 0.08 ± 0.01 cd | N.D. a | 0.06 ± 0.05 bc | 0.08 ± 0.00 cde | 0.08 ± 0.00 cde | A |
| 17 | ornithine | N.D. a | N.D. a | 0.44 ± 0.03 d | 0.44 ± 0.01 a | 0.01 ± 0.01 a | N.D. d | 0.33 ± 0.02 d | 0.35 ± 0.00 a | 0.27 ± 0.02 a | N.D. c | N.D. c | N.D. b | A |
| ORGANIC ACIDS | ||||||||||||||
| 18 | lactic acid | 122.46 ± 3.81 cd | 151.00 ± 10.23 d | 107.17 ± 6.14 cd | 991.52 ± 92.63 e | 39.93 ± 0.69 a | 146.83 ± 9.76 d | 71.80 ± 4.81 abc | 110.65 ± 8.23 cd | 53.92 ± 2.68 ab | 92.52 ± 8.17 bc | 70.19 ± 3.22 abc | 72.45 ± 5.00 abc | A |
| 19 | citric acid | 0.09 ± 0.01 b | N.D. a | 0.24 ± 0.00 cd | 2.05 ± 0.06 f | N.D. a | N.D. a | 0.21 ± 0.02 c | 0.22 ± 0.01 c | N.D. a | 0.27 ± 0.04 de | 0.30 ± 0.02 e | 0.26 ± 0.01 de | A |
| 20 | propanoic acid | 0.06 ± 0.01 cd | 0.05 ± 0.01 cd | 0.03 ± 0.00 ab | 0.27 ± 0.03 f | 0.04 ± 0.01 abc | 0.03 ± 0.01 ab | 0.04 ± 0.01 bcd | 0.02 ± 0.00 a | 0.02 ± 0.01 a | 0.09 ± 0.02 e | 0.06 ± 0.01 d | 0.03 ± 0.01 ab | A |
| 18 | lactic acid | 122.46 ± 3.81 cd | 151.00 ± 10.23 d | 107.17 ± 6.14 cd | 991.52 ± 92.63 e | 39.93 ± 0.69 a | 146.83 ± 9.76 d | 71.80 ± 4.81 abc | 110.65 ± 8.23 cd | 53.92 ± 2.68 ab | 92.52 ± 8.17 bc | 70.19 ± 3.22 abc | 72.45 ± 5.00 abc | A |
| 19 | citric acid | 0.09 ± 0.01 b | N.D. a | 0.24 ± 0.00 cd | 2.05 ± 0.06 f | N.D. a | N.D. a | 0.21 ± 0.02 c | 0.22 ± 0.01 c | N.D. a | 0.27 ± 0.04 de | 0.30 ± 0.02 e | 0.26 ± 0.01 de | A |
| 20 | propanoic acid | 0.06 ± 0.01 cd | 0.05 ± 0.01 cd | 0.03 ± 0.00 ab | 0.27 ± 0.03 f | 0.04 ± 0.01 abc | 0.03 ± 0.01 ab | 0.04 ± 0.01 bcd | 0.02 ± 0.00 a | 0.02 ± 0.01 a | 0.09 ± 0.02 e | 0.06 ± 0.01 d | 0.03 ± 0.01 ab | A |
| 21 | oxalic acid | 0.05 ± 0.00 bc | 0.05 ± 0.00 b | 0.05 ± 0.01 b | 0.43 ± 0.03 d | 0.05 ± 0.01 b | N.D. a | 0.06 ± 0.01 bc | 0.05 ± 0.00 b | 0.05 ± 0.00 bc | 0.06 ± 0.01 bc | 0.07 ± 0.00 c | 0.06 ± 0.01 bc | A |
| 22 | succinic acid | 0.07 ± 0.01 ab | 0.22 ± 0.12 c | 0.03 ± 0.00 a | 0.24 ± 0.02 c | 0.06 ± 0.06 ab | 0.07 ± 0.01 ab | 0.10 ± 0.01 b | 0.05 ± 0.01 ab | 0.03 ± 0.01 ab | 0.05 ± 0.01 ab | 0.03 ± 0.00 ab | 0.30 ± 0.01 d | A |
| 23 | fumaric acid | N.D. a | N.D. a | N.D. a | N.D. a | 0.03 ± 0.00 b | N.D. a | N.D. a | N.D. a | N.D. a | 0.06 ± 0.00 c | 0.06 ± 0.01 d | N.D. a | A |
| 24 | malic acid | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | N.D. a | 0.28 ± 0.02 b | N.D. a | N.D. a | 0.27 ± 0.03 b | 0.32 ± 0.02 c | N.D. a | A |
| CARBOHYDRATES | ||||||||||||||
| 25 | ribofuranose | N.D. a | N.D. a | 1.88 ± 0.33 cd | 1.32 ± 0.13 b | 1.70 ± 0.40 bcd | N.D. a | 1.84 ± 0.38 cd | 1.35 ± 0.10 b | 1.89 ± 0.33 cd | 2.03 ± 0.29 e | 1.60 ± 0.09 bc | 1.63 ± 0.08 bcd | B |
| 26 | arabitol | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.05 ± 0.00 bc | 0.05 ± 0.00 bcd | 0.03 ± 0.00 a | 0.04 ± 0.01 b | 0.06 ± 0.01 e | 0.05 ± 0.00 bcd | 0.06 ± 0.00 de | 0.07 ± 0.00 f | 0.07 ± 0.01 f | 0.05 ± 0.01 cde | A |
| 27 | xylose | 0.07 ± 0.00 | 0.10 ± 0.04 | 0.14 ± 0.03 | 0.09 ± 0.01 | 0.13 ± 0.04 | 0.01 ± 0.00 | 0.13 ± 0.02 | 0.09 ± 0.01 | 0.14 ± 0.04 | 0.21 ± 0.07 | 0.12 ± 0.01 | 0.13 ± 0.00 | A |
| 28 | myo-inositol | 0.37 ± 0.09 b | 0.41 ± 0.01 bcd | 0.48 ± 0.01 d | 0.44 ± 0.04 cd | 0.45 ± 0.03 cd | 0.43 ± 0.01 bcd | 0.44 ± 0.06 cd | 0.40 ± 0.01 bc | 0.46 ± 0.017 cd | 0.46 ± 0.03 cd | 0.44 ± 0.00 cd | 0.31 ± 0.00 a | A |
| 29 | sucrose | 0.41 ± 0.03 bc | 0.32 ± 0.03 a | 0.55 ± 0.01 ef | 0.54 ± 0.05 ef | 0.45 ± 0.01 cd | 0.51 ± 0.03 de | 0.59 ± 0.08 f | 0.59 ± 0.02 f | 0.69 ± 0.04 g | 0.31 ± 0.03 a | 0.38 ± 0.02 ab | 0.37 ± 0.07 ab | A |
| 30 | maltose | 20.47 ± 1.12 b | 15.34 ± 1.44 a | 12.24 ± 0.82 a | 21.79 ± 1.49 bc | 22.45 ± 3.81 bc | 24.67 ± 0.81 cd | 29.39 ± 3.90 ef | 24.92 ± 0.96 cd | 32.78 ± 1.05 f | 24.93 ± 1.60 cd | 27.01 ± 3.96 de | 25.28 ± 0.83 cd | A |
| 31 | trehalose | 4.07 ± 0.47 b | 2.67 ± 0.48 a | 7.69 ± 0.25 e | 7.75 ± 0.67 e | 4.99 ± 0.18 c | 5.70 ± 0.30 c | 6.75 ± 0.89 d | 6.74 ± 0.19 d | 7.67 ± 0.22 e | 9.86 ± 0.47 f | 9.54 ± 0.09 f | 10.01 ± 0.37 f | B |
| 32 | sophorose | 4.99 ± 0.28 b | 3.94 ± 0.658 a | 7.34 ± 0.161 e | 7.15 ± 0.56 de | 5.60 ± 0.26 bc | 5.81 ± 0.193 c | 6.64 ± 0.96 de | 6.40 ± 0.18 cd | 7.32 ± 0.298 e | 8.94 ± 0.55 f | 8.56 ± 0.22 f | 9.10 ± 0.39 f | B |
| 33 | mannose | 82.23 ± 0.87 ab | 93.91 ± 11.22 cd | 85.02 ± 2.92 abcd | 83.09 ± 3.32 abc | 79.09 ± 5.55 a | 76.00 ± 1.81 a | 91.48 ± 12.40 bcd | 83.10 ± 1.78 abc | 93.37 ± 3.57 cd | 94.44 ± 4.12 d | 94.39 ± 4.28 d | 93.65 ± 1.41 cd | A |
| 34 | fructose | 23.30 ± 0.25 ab | 26.61 ± 3.18 cd | 24.09 ± 0.83 abcd | 23.54 ± 0.94 abc | 22.41 ± 1.57 a | 21.53 ± 0.51 a | 25.92 ± 3.51 bcd | 23.54 ± 0.50 abc | 26.46 ± 1.01 cd | 26.76 ± 1.17 d | 26.74 ± 1.21 d | 26.53 ± 0.40 cd | A |
| 35 | galactose | 17.82 ± 0.19 ab | 20.35 ± 2.43 cd | 18.42 ± 0.63 abcd | 18.00 ± 0.72 abc | 17.14 ± 1.20 a | 16.47 ± 0.39 a | 19.82 ± 2.69 bcd | 18.00 ± 0.39 abc | 20.23 ± 0.77 cd | 20.46 ± 0.89 d | 20.45 ± 0.93 d | 20.30 ± 0.30 cd | A |
| 36 | glucose | 60.56 ± 2.79 abc | 70.09 ± 5.14 d | 59.22 ± 2.69 ab | 59.22 ± 2.05 ab | 56.34 ± 3.70 a | 54.54 ± 1.02 a | 66.37 ± 8.78 bcd | 59.43 ± 1.37 ab | 67.10 ± 3.12 cd | 66.68 ± 2.75 bcd | 67.26 ± 4.68 cd | 66.54 ± 3.10 bcd | A |
| 37 | mannitol | N.D. a | N.D. a | 0.39 ± 0.04 b | 0.45 ± 0.26 b | N.D. a | N.D. a | 0.76 ± 0.11 c | N.D. a | N.D. a | 3.13 ± 0.16 e | 2.09 ± 0.04 d | N.D. a | A |
| Samples Abbreviation | Lactic Acid Bacteria | Food Source from which Isolated |
|---|---|---|
| LPC | Lactobacillus paracasei (1) b | Korean traditional rice wine (makgeolli) |
| RTJL3 | Lactobacillus paracasei (2) b | traditional rice wine (Myeoncheon Dugyeonju) |
| RTCL16 | Lactobacillus sakeib | traditional fermented barley paste |
| RTCL79 | Lactobacillus pentosusb | traditional fermented barley paste |
| KR10 | Lactobacillus brevis (1) c | radish kimchi |
| KR7 | Lactobacillus brevis (2) c | kimchi |
| RTJL4 | Lactobacillus hilgardiic | traditional rice wine (Myeoncheon Dugyeonju) |
| RTCL3 | Pediococcus pentoseceusa | traditional fermented barley paste |
| RTCL31 | Pediococcus loliia | traditional fermented barley paste |
| JKA1-6 | Leuconostoc mesenteroides (1) c | kimchi |
| JKF2-2 | Leuconostoc mesenteroides (2) c | radish kimchi |
| RTCL9 | Weissella cibariac | traditional fermented barley paste |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Hwang, Y.R.; Kim, M.S.; Chung, M.S.; Kim, Y.-S. Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria. Molecules 2019, 24, 1183. https://doi.org/10.3390/molecules24061183
Lee SM, Hwang YR, Kim MS, Chung MS, Kim Y-S. Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria. Molecules. 2019; 24(6):1183. https://doi.org/10.3390/molecules24061183
Chicago/Turabian StyleLee, Sang Mi, Young Rim Hwang, Moon Seok Kim, Myung Sub Chung, and Young-Suk Kim. 2019. "Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria" Molecules 24, no. 6: 1183. https://doi.org/10.3390/molecules24061183






