Analysis of Selected Endocrine Disrupters Fraction Including Bisphenols Extracted from Daily Products, Food Packaging and Treated Wastewater Using Optimized Solid-Phase Extraction and Temperature-Dependent Inclusion Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Bisphenols Separation and Selected Validation Issues of the Quantification Protocol
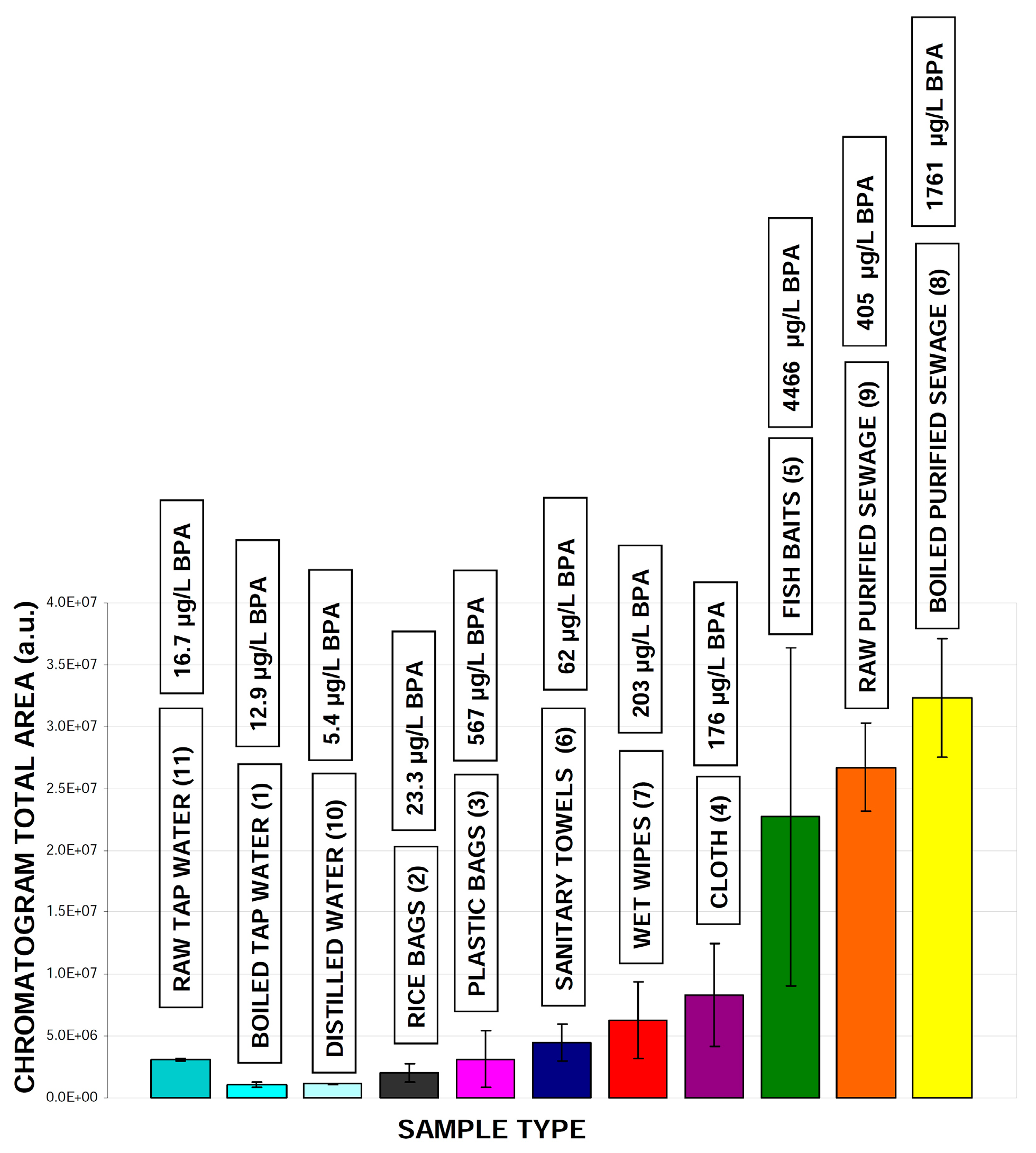
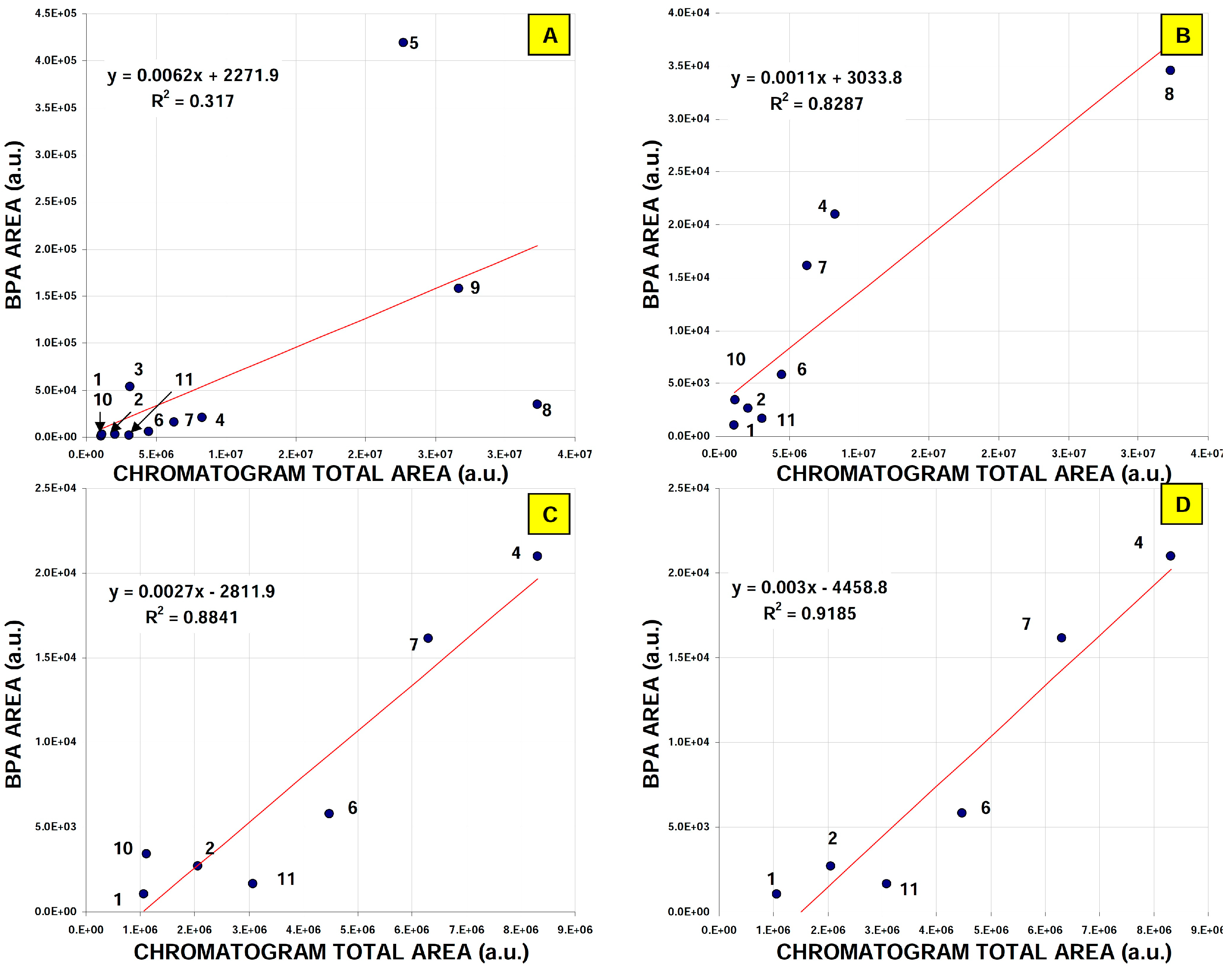
2.2. Real Samples Analysis: Daily Products, Packaging and Treated Wastewater
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Analytical Protocols
3.2.1. Solid-Phase Extraction (SPE)
3.2.2. Chromatography (HPLC)
4. Conclusions
4.1. Detailed Conclusions to Part 2.1. (Optimization of Bisphenols Separation)
4.2. Detailed Conclusions to Part 2.2. (Real Samples Analysis)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wittliff, J.L.; Raffelsberger, W. Mechanisms of signal transduction: Sex hormones, their receptors and clinical utility. J. Clin. Ligand Assay 1995, 18, 211–235. [Google Scholar]
- Aherne, G.W.; Briggs, R. The relevance of the presence of certain synthetic steroids in the aquatic environment. J. Pharm. Pharmacol. 1989, 41, 735–736. [Google Scholar] [CrossRef]
- Stone, R. Environmental estrogens stir debate. Science 1994, 265, 308–310. [Google Scholar] [CrossRef]
- Rivas, A.; Olea, N.; Olea-Serrano, F. Human exposure to endocrine-disrupting chemicals: Assessing the total estrogenic xenobiotic burden. TRAC-Trend. Anal. Chem. 1997, 16, 613–619. [Google Scholar] [CrossRef]
- Clemons, J.H.; Allan, L.M.; Marvin, C.H.; Wu, Z.; McCarry, B.E.; Bryant, D.W.; Zacharewski, T.R. Evidence of estrogen-and TCDD-like activities in crude and fractionated extracts of PM10 air particulate material using in vitro gene expression assays. Environ. Sci. Technol. 1998, 32, 1853–1860. [Google Scholar] [CrossRef]
- Kaleniecka, A.; Zarzycki, P.K. Pharmaceuticals in the acquatic environment: Sources, effects, treatment methods. Arch. Physiother. Glob. Res. 2015, 19, 39–52. [Google Scholar]
- Pivnenko, K.; Pedersen, G.A.; Eriksson, E.; Astrup, T.F. Bisphenol A and its structural analogues in household waste paper. Waste Manag. 2015, 44, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Available online: www https://oceans.taraexpeditions.org/en/m/about-tara/les-expeditions/tara-oceans/ (accessed on 4 August 2018).
- Shore, L.S.; Gurevitz, M.; Shemesh, M. Estrogen as an environmental pollutant. B. Environ. Contam. Tox. 1993, 51, 361–366. [Google Scholar] [CrossRef]
- Isobe, T.; Serizawa, S.; Horiguchi, T.; Shibata, Y.; Managaki, S.; Takada, H.; Morita, M.; Shiraishi, H. Horizontal distribution of steroid estrogens in surface sediments in Tokyo Bay. Environ. Pollut. 2006, 144, 632–638. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography I. Optimization of separation protocol. J. Chromatogr. A. 2009, 1216, 7602–7611. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography II. Fast screening of free steroids and related low-molecular-mass compounds fraction in the environmental samples derived from surface waters, treated and untreated sewage waters as well as activated sludge material. J. Chromatogr. A 2009, 1216, 7612–7622. [Google Scholar]
- Zarzycki, P.K.; Włodarczyk, E.; Zarzycka, M.B.; Głód, B.K. Optimization of solid-phase extraction protocol for fractionating of selected steroids using retention data from micro thin-layer chromatography. Anal. Sci. 2009, 25, 935–939. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic paper based analytical devices (μPADs) and micro total analysis systems (μTAS): Development, applications and future trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Kaleniecka, A.; Zarzycki, P.K. Advances in the analysis of water and wastewater samples using various sensing protocols and microfluidic devices based on PADs and µTAS systems. J. AOAC Int. 2017, 100, 962–970. [Google Scholar] [CrossRef]
- Chang, H.-S.; Choo, K.-H.; Lee, B.; Choi, S.-J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; Mcdowell, D.; Brauch, H.J.; Brigitte, H.G.; Preuss, G.; William, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater; constituents, effects, and treatment. Crit. Rev. Env. Sci. Tec. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dyes removal from water and wastewater using various physical, chemical and biological processes. J. AOAC Int. 2018, 101, 1–14. [Google Scholar] [CrossRef]
- Kowalkowski, T.; Zbytniewski, R.; Szpejna, J.; Buszewski, B. Application of chemometrics in river water classification. Water Res. 2006, 40, 744–752. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 1–24. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Kulhanek, K.M.; Smith, R. Chromatographic behaviour of selected steroids and their inclusion complexes with β-cyclodextrin on octadecylsilica stationary phases with different carbon loads. J. Chromatogr. A 2002, 955, 71–78. [Google Scholar] [CrossRef]
- Ohta, H.; Włodarczyk, E.; Piaskowski, K.; Kaleniecka, A.; Lewandowska, L.; Baran, M.J.; Wojnicz, M.; Jinno, K.; Saito, Y.; Zarzycki, P.K. Unexpected differences between planar and column liquid chromatographic retention of 1-acenaphthenol enantiomers controlled by supramolecular interactions involving β-cyclodextrin at subambient temperatures. Anal. Bioanal. Chem. 2017, 409, 3695–3706. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Kulhanek, K.M.; Smith, R.; Clifton, V.L. Determination of steroids in human plasma using temperature-dependent inclusion chromatography for metabolomic investigations. J. Chromatogr. A 2006, 1104, 203–208. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Ohta, H.; Saito, Y.; Jinno, K. Interaction of native α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin and their hydroxypropyl derivatives with selected organic low molecular mass compounds at elevated and subambient temperature under RP-HPLC conditions. Anal. Bioanal. Chem. 2008, 391, 2793–2801. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Lamparczyk, H. Evidences for temperature-dependent mechanism of host-guest complexation. Chromatographia 1998, 48, 377–382. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Smith, R. Separation of steroids using temperature-dependent inclusion chromatography. J. Chromatogr. A. 2001, 912, 45–52. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Cauwenberghe, L.V.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Ivar do Sul, J.; Costa, M.J. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Garcia Gimenez, B.C. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Ai, S.; Chen, Q.; Zhu, X.; Liu, X.; Zhu, L. Sensitivity and selectivity determination of BPA in real water samples using PAMAM dendrimer and CoTe quantum dots modified glassy carbon electrode. J. Hazard. Mater. 2010, 174, 236–243. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electro-chemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sensor. Actuator. B 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.Q.; Zhang, K.; Zhou, J.Q. Cd-doped ZnO quantum dots-based immunoassay for the quantitative determination of bisphenol A. Chemosphere 2014, 95, 105–110. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Jiang, G. Nanomaterials for analysis and monitoring of emerging chemical pollutants. TRAC-Trend. Anal. Chem. 2014, 58, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Li, Y.; Wu, D.; Ma, H.; Mao, K.; Fan, D.; Du, B.; Li, H.; Wei, Q. Electrochemical bisphenol A sensor based on N-doped graphene sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free de-tection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Guart, A.; Bono-Blay, F.; Borrell, A.; Lacorte, S. Effect of bottling and storage on the migration of plastic constituents in Spanish bottled waters. Food Chem. 2014, 156, 73–80. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir bar sorptive extraction with EG-Silicone coating for bisphenols determination in personal care products by GC-MS. J. Pharm. Biomed. Anal. 2013, 78–79, 255–260. [Google Scholar] [CrossRef]
- Zhu, R.; Zhao, R.; Zhai, M.; Wei, F.; Cai, Z.; Sheng, N.; Hu, Q. Molecularly imprinted layer-coated silica nanoparticles for selective solid-phase extraction of bisphenol A from chemical cleansing and cosmetics samples. Anal. Chim. Acta 2010, 658, 209–216. [Google Scholar] [CrossRef]
- Nerın, C.; Philo, M.R.; Salafranca, J.; Castle, L. Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction–high-performance liquid chromatography. J. Chromatogr. A 2002, 963, 375–380. [Google Scholar] [CrossRef]
- Regueiro, J.; Breidbach, A.; Wenzl, T. Derivatization of bisphenol A and its analogues with pyridine-3-sulfonyl chloride: Multivariate optimization and fragmentation patterns by liquid chromatography/Orbitrap mass spectrometry. Rapid Commun. Mass Sp. 2015, 29, 1473–1484. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Zhang, J.; Yang, Y.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Portka, J.K. Recent advances in hopanoids analysis: Quantification protocols overview, main research targets and selected problems of complex data exploration. J. Steroid Biochem. 2015, 153, 3–26. [Google Scholar] [CrossRef]
- Bielicka-Daszkiewicz, K.; Voelkel, A.; Rusińska-Roszak, D.; Zarzycki, P.K. Estimation of the breakthrough volume of selected steroids for C-18 solid-phase extraction sorbent using retention data from micro-thin layer chromatography. J. Sep. Sci. 2013, 36, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Gertz, C.; Klostermann, S.; Parkash-Kochhar, S. Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur. J. Lipid Sc. Tech. 2000, 102, 543–551. [Google Scholar] [CrossRef]
- Van Elteren, J.T.; Slejkovec, Z. Ion-exchange separation of eight arsenic compounds by high performance liquid chromatography–UV decomposition–hydride generation–atomic fluorescence spectrometry and stability tests for food treatment procedures. J. Chromatogr. A 1997, 789, 339–348. [Google Scholar] [CrossRef]
- Fromme, H.; Kϋchler, T.; Otto, T.; Pilz, K.; Mϋller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Stachel, B.; Ehrhorn, U.; Heemken, O.P.; Lepom, P.; Reincke, H.; Sawal, G.; Theobald, N. Xenoestrogens in the River Elbe and its tributaries. Environ. Pollut. 2003, 124, 497–507. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; López de Alda, M.J.; Barceló, D. Moni-toring of estrogens, pesticides and bisphenol A in natural waters and drinking water treatment plants by solid-phase extraction–liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1045, 85–92. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Zhang, J.; Shao, B.; Yin, J. Assessment of bisphenol A alternatives in paper products from the Chinese market and their dermal exposure in the general population. Environ. Pollut. 2019, 244, 238–246. [Google Scholar] [CrossRef]
- Michałowicz, J.; Bisphenol, A. Sources, toxicity and biotransformation. Environ. Toxicol. Pharm. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds investigated and collected from environment are available from the authors. |

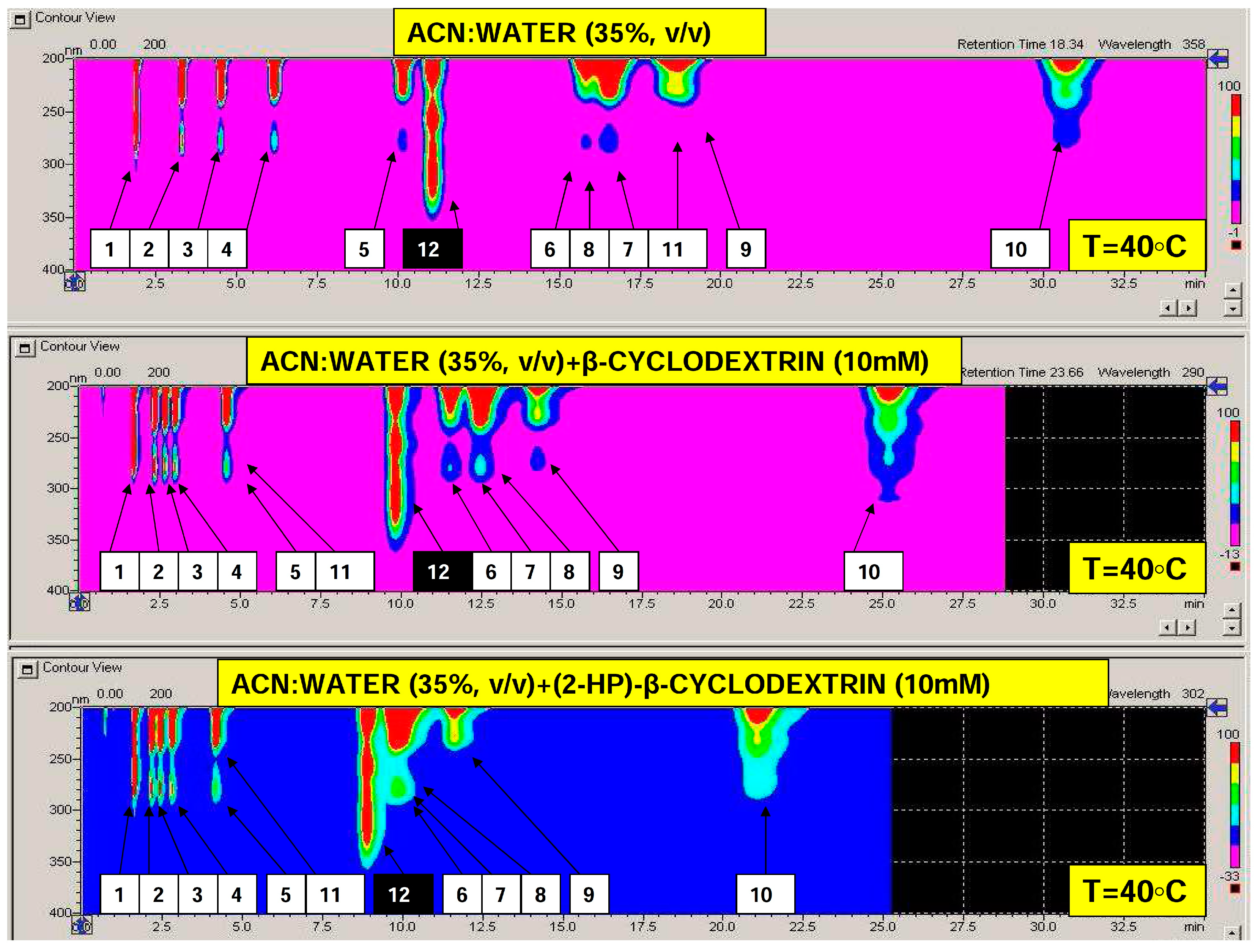
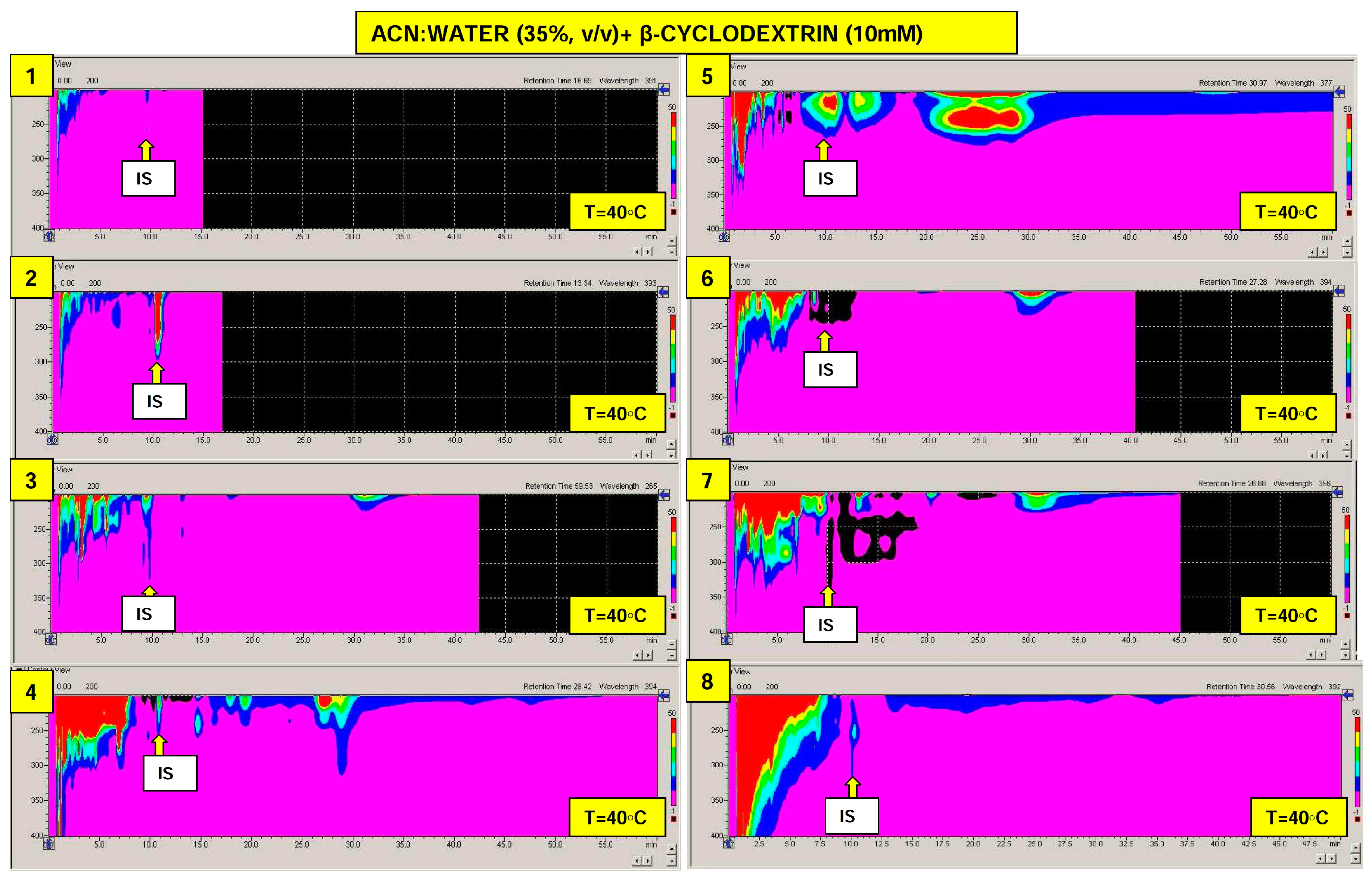


| Analyte | Separation Temperature [°C] | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| k values. | |||||
| A (unmodifed mobile phase) | |||||
| Bisphenol S (1) | 2.863 | 2.162 | 1.959 | 1.629 | 1.394 |
| Bisphenol F (2) | 6.383 | 4.889 | 4.420 | 3.305 | 3.105 |
| Bisphenol E (3) | 9.483 | 7.428 | 6.487 | 5.165 | 4.592 |
| Bisphenol A (4) | 13.837 | 10.665 | 9.398 | 7.365 | 6.523 |
| 7,8-Dimethoxyflavone (12) | 22.674 | 18.213 | 17.051 | 14.287 | 13.077 |
| Bisphenol B (5) | 26.150 | 19.277 | 16.722 | 12.756 | 11.187 |
| Bisphenol C (6) | 40.741 | 29.965 | 26.902 | 20.321 | 17.829 |
| Bisphenol AP (7) | 48.169 | 33.228 | 29.417 | 22.246 | 18.394 |
| Bisphenol BP (8) | 50.195 | 34.221 | 29.174 | 21.318 | 18.509 |
| Bisphenol Z (11) | 53.030 | 37.737 | 32.982 | 24.294 | 21.462 |
| Bisphenol AF (9) | 52.820 | 38.002 | 32.990 | 24.858 | 20.700 |
| Bisphenol FL (10) | 100.303 | 67.296 | 57.400 | 41.380 | 34.518 |
| B (β-cyclodextrin in mobile phase) | |||||
| Bisphenol S | 1.770 | 1.585 | 1.412 | 1.295 | 1.188 |
| Bisphenol F | 2.595 | 2.474 | 2.313 | 2.166 | 2.028 |
| Bisphenol E | 2.749 | 2.736 | 2.679 | 2.617 | 2.518 |
| Bisphenol A | 3.048 | 3.041 | 2.980 | 2.854 | 2.722 |
| 7,8-Dimethoxyflavone | 16.462 | 15.147 | 13.772 | 12.419 | 11.084 |
| Bisphenol B | 4.687 | 4.976 | 5.195 | 5.237 | 5.170 |
| Bisphenol C | 18.422 | 17.453 | 16.363 | 14.748 | 13.050 |
| Bisphenol AP | 24.576 | 22.071 | 18.898 | 16.030 | 13.434 |
| Bisphenol BP | 24.504 | 21.709 | 18.851 | 16.048 | 13.433 |
| Bisphenol Z | 4.715 | 5.161 | 5.540 | 5.825 | 5.966 |
| Bisphenol AF | 28.970 | 25.442 | 21.908 | 18.471 | 15.443 |
| Bisphenol FL | 58.406 | 49.536 | 40.997 | 33.466 | 26.676 |
| C (2HP-β-cyclodextrin in mobile phase) | |||||
| Bisphenol S | 1.656 | 1.456 | 1.305 | 1.157 | 1.086 |
| Bisphenol F | 2.289 | 2.129 | 2.010 | 1.908 | 1.793 |
| Bisphenol E | 2.302 | 2.293 | 2.273 | 2.214 | 2.223 |
| Bisphenol A | 2.485 | 2.605 | 2.685 | 2.752 | 2.746 |
| 7,8-Dimethoxyflavone | 14.581 | 13.315 | 12.066 | 10.968 | 9.841 |
| Bisphenol B | 4.208 | 4.413 | 4.526 | 4.634 | 4.511 |
| Bisphenol C | 14.638 | 14.328 | 13.557 | 12.680 | 11.253 |
| Bisphenol AP | 16.392 | 15.127 | 13.562 | 11.920 | 10.716 |
| Bisphenol BP | 16.507 | 15.161 | 13.663 | 11.913 | 10.583 |
| Bisphenol Z | 4.293 | 4.640 | 4.941 | 5.112 | 5.271 |
| Bisphenol AF | 21.881 | 19.333 | 16.793 | 14.742 | 12.395 |
| Bisphenol FL | 46.732 | 39.875 | 33.112 | 27.068 | 22.372 |
| Analyte | Unmodified Mobile Phase | 10mM β-Cyklodextrin | 10mM 2 HP-β-Cyklodekstrin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | r2 | a | b | c | r2 | a | b | c | r2 | |
| Bisphenol S | 0.58 | −2.23 | 1.85 | 0.986 | 0.18 | −0.25 | −0.73 | 0.999 | 0.40 | −1.69 | 1.44 | 0.997 |
| Bisphenol F | 0.77 | −3.45 | 4.38 | 0.974 | −0.39 | 3.17 | −5.35 | 0.999 | −0.0006 | 0.55 | −1.11 | 0.998 |
| Bisphenol E | 0.46 | −1.39 | 1.42 | 0.993 | −0.47 | 3.34 | −4.88 | 0.998 | −0.057 | 0.47 | −0.12 | 0.863 |
| Bisphenol A | 0.44 | −1.25 | 1.45 | 0.990 | −0.59 | 3.64 | −4.5 | 0.994 | −0.55 | 3.39 | −4.24 | 0.994 |
| Bisphenol B | 0.73 | −2.94 | 4.46 | 0.990 | −1.00 | 6.43 | −8.63 | 0.995 | −0.84 | 5.35 | −7.07 | 0.962 |
| Bisphenol C | 0.53 | −1.69 | 2.96 | 0.983 | −1.28 | 9.28 | −13.8 | 0.999 | −1.41 | 9.91 | −14.78 | 0.996 |
| Bisphenol AP | 0.65 | −2.23 | 3.52 | 0.984 | −1.32 | 10.1 | −16.14 | 0.999 | −0.755 | 5.99 | −8.95 | 0.998 |
| Bisphenol BP | 1.31 | −6.42 | 10.2 | 0.988 | −1.21 | 9.40 | −14.9 | 1 | −0.92 | 7.15 | −10.93 | 0.998 |
| Bisphenol Z | 0.95 | −4.26 | 7.1 | 0.985 | −0.75 | 4.42 | −4.72 | 0.999 | −0.59 | 3.50 | −3.45 | 0.999 |
| Bisphenol AF | 0.31 | 0.05 | −0.01 | 0.990 | −1.15 | 9.08 | −14.3 | 1 | −0.90 | 7.28 | −11.35 | 0.999 |
| Bisphenol FL | 0.92 | −3.7 | 6.16 | 0.988 | −1.36 | 10.8 | −17.15 | 1 | −1.00 | 8.36 | −13.15 | 0.999 |
| Analyte | Separation Temperature °C | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| A: k0mMCD/k10mMCD ratio values for β-cyclodextrin | |||||
| Bisphenol A | 5.02 | 3.86 | 3.07 | 2.51 | 2.11 |
| Bisphenol AF | 1.78 | 1.57 | 1.45 | 1.37 | 1.33 |
| Bisphenol AP | 1.89 | 1.64 | 1.49 | 1.40 | 1.36 |
| Bisphenol B | 5.44 | 4.03 | 3.13 | 2.53 | 2.11 |
| Bisphenol BP | 1.96 | 1.68 | 1.50 | 1.39 | 1.33 |
| Bisphenol C | 2.16 | 1.80 | 1.58 | 1.43 | 1.34 |
| Bisphenol E | 3.40 | 2.80 | 2.36 | 2.04 | 1.79 |
| Bisphenol F | 2.41 | 2.08 | 1.83 | 1.64 | 1.48 |
| Bisphenol FL | 1.65 | 1.46 | 1.34 | 1.28 | 1.26 |
| Bisphenol S | 1.57 | 1.45 | 1.35 | 1.26 | 1.18 |
| Bisphenol Z | 10.89 | 7.73 | 5.72 | 4.39 | 3.48 |
| B: k0mMCD/k10mMCD ratio values for hydroxypropyl β-cyclodextrin | |||||
| Bisphenol A | 5.47 | 4.24 | 3.38 | 2.78 | 2.33 |
| Bisphenol AF | 2.36 | 2.07 | 1.87 | 1.74 | 1.65 |
| Bisphenol AP | 2.82 | 2.39 | 2.09 | 1.87 | 1.71 |
| Bisphenol B | 6.07 | 4.56 | 3.56 | 2.89 | 2.41 |
| Bisphenol BP | 2.90 | 2.42 | 2.08 | 1.85 | 1.69 |
| Bisphenol C | 2.72 | 2.20 | 1.88 | 1.68 | 1.55 |
| Bisphenol E | 4.04 | 3.34 | 2.80 | 2.38 | 2.04 |
| Bisphenol F | 2.74 | 2.39 | 2.11 | 1.87 | 1.67 |
| Bisphenol FL | 2.06 | 1.82 | 1.66 | 1.57 | 1.51 |
| Bisphenol S | 1.67 | 1.58 | 1.48 | 1.38 | 1.29 |
| Bisphenol Z | 11.93 | 8.60 | 6.43 | 4.97 | 3.95 |
| Analyte | Average Recovery (%) | STD | CV% |
|---|---|---|---|
| BPS | 28.91 | 5.57 | 19.28 |
| BPF | 95.22 | 4.07 | 4.27 |
| BPE | 97.27 | 0.91 | 0.93 |
| BPA | 213.64 | 21.10 | 9.88 |
| BPB | 96.14 | 3.38 | 3.52 |
| BPZ | 101.14 | 8.44 | 8.35 |
| BPC | 93.02 | 11.38 | 12.24 |
| BPAP + BPBP | 77.69 | 12.33 | 15.87 |
| BPAF | 159.13 | 72.01 | 45.25 |
| BPFL | 92.95 | 3.44 | 3.70 |
| 7,8-DMF | 94.35 | 6.96 | 7.37 |
| No | Samples and Materials |
|---|---|
| 1 | Tap water boiled (250 mL) |
| 2 | Rice bags (2 g) |
| 3 | Plastic bags (2 g) |
| 4 | Cloth (2 g) |
| 5 | Fish baits (2 g) |
| 6 | Sanitary towels (2 g) |
| 7 | Wet wipes (2 g) |
| 8 | Boiled purified sewage (250 mL) |
| 9 | Raw purified sewage (250 mL) |
| 10 | Distilled water (250 mL) |
| 11 | Raw tap water (250 mL) |
| Sample Source | BPA (Peak Heights; a.u.) | IS (Peak Heights; a.u.) | BPA/IS Ratio {Concentration µg/L} |
|---|---|---|---|
| Sample Type: Boiled Tap Water (1) | |||
| (A) (n = 2) | 865.0 | 4053.0 | 0.21 |
| (B) (n = 2) | 1307.5 | 2749.5 | 0.47 |
| (C) (n = 2) | 1039.0 | 4118.5 | 0.25 |
| AVG | 1070.5 | 3640.3 | 0.31 {12.9} |
| STD | 222.9 | 772.2 | |
| CV% | 20.8 | 21.2 | |
| Sample Type: Rice Bags (2) | |||
| (A) (n = 2) | 1604.5 | 4225.0 | 0.38 |
| (B) (n = 2) | 1761.0 | 4190.5 | 0.42 |
| (C) (n = 2) | 4657.5 | 5290.0 | 0.88 |
| AVG | 2674.3 | 4568.5 | 0.56 {23.3} |
| STD | 1719.3 | 625.1 | |
| CV% | 64.3 | 13.7 | |
| Sample Type: Plastic Bags (3) | |||
| (A) (n = 2) | 257.0 | 1627.0 | 0.16 |
| (B) (n = 2) | 155957.0 | 7043.0 | 22.14 |
| (C) (n = 2) | 3730.5 | 3068.5 | 1.22 |
| AVG | 53314.8 | 3912.8 | 13.63 {567,5} |
| STD | 88907.7 | 2805.0 | |
| CV% | 166.8 | 71.7 | |
| Sample Type: Cloth (4) | |||
| (A) (n = 2) | 54927.5 | 4747.0 | 11.57 |
| (B) (n = 2) | 4265.5 | 7043.0 | 0.61 |
| (C) (n = 2) | 3730.5 | 3068.5 | 1.22 |
| AVG | 20974.5 | 4952.8 | 4.23 {176.8} |
| STD | 29405.4 | 1995.2 | |
| CV% | 140.2 | 40.3 | |
| Sample Type: Fish Baits (5) | |||
| (A) (n = 2) | 5953.5 | 2362.0 | 2.52 |
| (B) (n = 2) | 248460.5 | 4492.5 | 55.31 |
| (C) (n = 2) | 1003649.5 | 4882.0 | 205.58 |
| AVG | 419354.5 | 3912.2 | 107.19 {4466.2} |
| STD | 520339.2 | 1356.5 | |
| CV% | 124.1 | 34.7 | |
| Sample Type: Sanitary Towels (6) | |||
| (A) (n = 2) | 7370.0 | 522.5 | 14.11 |
| (B) (n = 2) | 705.5 | 6736.5 | 0.10 |
| (C) (n = 2) | 9342.0 | 4496.5 | 2.08 |
| AVG | 5805.8 | 3918.5 | 1.48 {61.7} |
| STD | 4525.7 | 3147.1 | |
| CV% | 78.0 | 80.3 | |
| Sample Type: Wet Wipes (7) | |||
| (A) (n = 2) | 13330.5 | 4124.0 | 3.23 |
| (B) (n = 2) | 11834.5 | 2527.5 | 4.68 |
| (C) (n = 2) | 23285.5 | 3303.5 | 7.05 |
| AVG | 16150.2 | 3318.3 | 4.87 {203} |
| STD | 6224.5 | 798.4 | |
| CV% | 38.5 | 24.1 | |
| Sample Type: Boiled Purified Sewage (8) | |||
| (A) (n = 2) | 61888.5 | 4598.0 | 13.46 |
| (B) (n = 2) | 362477.0 | 3326.5 | 108.97 |
| (C) (n = 2) | 50848.0 | 3326.5 | 15.29 |
| AVG | 158404.5 | 3750.3 | 42.24 {1760.8} |
| STD | 176818.2 | 734.1 | |
| CV% | 111.6 | 19.6 | |
| Sample Type: Raw Purified Sewage (9) | |||
| (A) (n = 2) | 35839.0 | 2907.5 | 12.33 |
| (B) (n = 2) | 37057.0 | 4032.0 | 9.19 |
| (C) (n = 2) | 30623.0 | 3700.0 | 8.28 |
| AVG | 34506.3 | 3546.5 | 9.73 {405.4} |
| STD | 3417.8 | 577.8 | |
| CV% | 9.9 | 16.3 | |
| Sample Type: Distilled Water (10) | |||
| (A) (n = 2) | 3233.0 | 24947.0 | 0.13 |
| (B) (n = 2) | 3640.0 | 27988.0 | 0.13 |
| (C) (n = 2) | 3325.0 | 26221.0 | 0.13 |
| AVG | 3399.3 | 26385.3 | 0.13 {5.41} |
| STD | 213.4 | 1527.1 | |
| CV% | 9.9 | 16.3 | |
| Sample Type: Raw Tap Water (11) | |||
| (A) (n=2) | 1896.0 | 4193.0 | 0.45 |
| (B) (n=2) | 1695.0 | 3974.0 | 0.43 |
| (C) (n=2) | 1400.0 | 4257.0 | 0.33 |
| AVG | 1663.7 | 4141.3 | 0.40 {16.7} |
| STD | 249.5 | 148.4 | |
| CV% | 15.0 | 3.6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleniecka, A.; Zarzycki, P.K. Analysis of Selected Endocrine Disrupters Fraction Including Bisphenols Extracted from Daily Products, Food Packaging and Treated Wastewater Using Optimized Solid-Phase Extraction and Temperature-Dependent Inclusion Chromatography. Molecules 2019, 24, 1285. https://doi.org/10.3390/molecules24071285
Kaleniecka A, Zarzycki PK. Analysis of Selected Endocrine Disrupters Fraction Including Bisphenols Extracted from Daily Products, Food Packaging and Treated Wastewater Using Optimized Solid-Phase Extraction and Temperature-Dependent Inclusion Chromatography. Molecules. 2019; 24(7):1285. https://doi.org/10.3390/molecules24071285
Chicago/Turabian StyleKaleniecka, Aleksandra, and Paweł K. Zarzycki. 2019. "Analysis of Selected Endocrine Disrupters Fraction Including Bisphenols Extracted from Daily Products, Food Packaging and Treated Wastewater Using Optimized Solid-Phase Extraction and Temperature-Dependent Inclusion Chromatography" Molecules 24, no. 7: 1285. https://doi.org/10.3390/molecules24071285




