Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables
Abstract
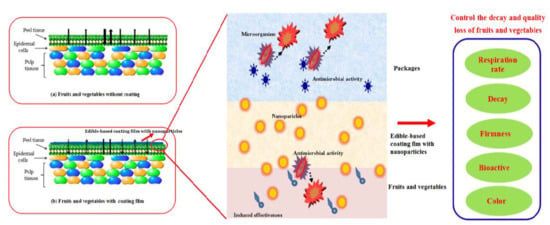
:1. Introduction
2. Preparation Technologies of ECF with Different NPs
2.1. Structure, Composition and Properties of the Main Edible Coating Materials
2.2. Preparation of ECF with NPs
2.2.1. Preparation of Edible Coating Films with TiO2NPs
2.2.2. Preparation of Edible Coating Films with AgNP
2.2.3. Preparation of Edible Coating Films with ZnONP
2.2.4. Preparation of Edible Coating films with Other NPs
2.2.5. Preparation of Edible Coating Films with Mixed NPs
3. Antimicrobial Activity and Mechanism of ECF with NP
3.1. Antimicrobial Activity of ECF with NPs
3.2. Antimicrobial Mechanism of ECF with NPs
4. Physical and Mechanical Properties of ECF with NPs
4.1. Physical Properties of ECF with NPs
4.1.1. Surface Observation of ECF with NPs
4.1.2. Thickness, Contact Angle and Tg Characterization of ECF with NPs
4.1.3. Transparency and Color Characterization of ECF with NPs
4.1.4. Thermal Stability of ECF Film with NPs
4.1.5. Structural Characterization of ECF Film with NPs
4.2. Mechanical Properties of ECF with NPs
4.3. Gas Modification Property of ECF with NPs
5. Applications of ECF with NPs on the Quality of Fruits and Vegetables
5.1. Induction Defense Properties of ECF with NPs
5.2. Metal-Ion Release and Migration Properties of NPs from ECF
5.3. Effect of ECF with NPs on the Quality of Fruits and Vegetables
6. Future Trends of ECF with NPs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xing, Y.; Xu, Q.; Yang, S.X.; Chen, C.; Tang, Y.; Sun, S.; Li, X. Preservation mechanism of chitosan-based coating with cinnamon oil for fruits storage based on sensor data. Sensors 2017, 16, 1111. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, S. Effect of carboxymethyl cellulose and alginate coating combined with brewer yeast on postharvest grape preservation. ISRN Agron. 2013, 2013, 871396. [Google Scholar]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-based coating with antimicrobial agents: Preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016, 2016, 4851730. [Google Scholar] [CrossRef]
- Xu, W.; Xie, W.; Huang, X.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.A.; López, O.V.; Ghilardi, J.; Villar, M.A.; Barbosa, S.E.; García, M.A. Thermoplastic starch/talc bionanocomposites. Influence of particle morphology on final properties. Food Hydrocoll. 2015, 51, 432–440. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Green bionanocomposite based on kefiran and cellulose nanocrystals produced from beer industrial residues. Int. J. Biol. Macromol. 2015, 77, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Darmanin, T.; Nativo, P.; Gilliland, D.; Ceccone, G.; Pascual, C.; Berardis, B.D.; Guittard, F.; Rossi, F. Microwave-assisted synthesis of silver nanoprisms/nanoplates using a “modified polyol process”. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 145–151. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation of UV-protective kefiran/nano-ZnO nanocomposites: Physical and mechanical properties. Int. J. Biol. Macromol. 2015, 72, 41–46. [Google Scholar] [CrossRef]
- Li, J.H.; Hong, R.Y.; Li, M.Y.; Li, H.Z.; Zheng, Y.; Ding, J. Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Prog. Org. Coat. 2009, 64, 504–509. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Li, K. Effect of TiO2 nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog. Org. Coat. 2012, 73, 219–224. [Google Scholar] [CrossRef]
- Carvalho, P.; Sampaio, P.; Azevedo, S.; Vaz, C.; Espinós, J.P.; Teixeira, V.; Carneiro, J.O. Influence of thickness and coatings morphology in the antimicrobial performance of zinc oxide coatings. Appl. Surf. Sci. 2014, 307, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Cano, L.; Pollet, E.; Avérous, L.; Tercjak, A. Effect of TiO2 nanoparticles on the properties of thermoplastic chitosan-based nano-biocomposites obtained by mechanical kneading. Compos. Part A Appl. Sci. Manuf. 2017, 93, 33–40. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.-C. Effect of binder on the physical stability and bactericidal property of titanium dioxide (TiO2) nanocoatings on food contact surfaces. Food Control 2015, 57, 82–88. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wang, L.; Sheng, J.; Xin, Z.; Zhao, L.; Xiao, H.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd). Food Chem. 2009, 114, 547–552. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Kim, S.-C.; Lee, S.-Y.; Kim, J.-W. The facile synthesis of chitosan-based silver nano-biocomposites via a solution plasma process and their potential antimicrobial efficacy. Arch. Biochem. Biophys. 2016, 605, 49–58. [Google Scholar] [CrossRef]
- Andrade, P.F.; de Faria, A.F.; da Silva, D.S.; Bonacin, J.A.; Gonçalves, M.D.C. Structural and morphological investigations of β-cyclodextrin-coated silver nanoparticles. Colloids Surf. B 2014, 118, 289–297. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, P.J.; Jiménez, A.; Muñoz, J.A.; Ibarza, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Banerjee, M.; Mallick, S.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Heightened reactive oxygen species generation in the antimicrobial activity of a three component iodinated chitosan-silver nanoparticle composite. Langmuir 2010, 26, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadehd, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; De Azeredo, H.M.C.; Lorevice, M.V.; de Moura, M.R.; Mattoso, L.H.C.; McHugh, T. Recent advances on edible films based on fruits and vegetables—A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I. Photo-producible and photo-degradable starch/TiO2 bionanocomposite as a food packaging material: Development and characterization. Int. J. Biol. Macromol. 2018, 106, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Almasi, B.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch-CMC-nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Yamashita, F.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E.; Grossmann, M.V.E. Constrained mixture design applied to the development of cassava starch-chitosan blown films. J. Food Eng. 2012, 108, 262–267. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Mu, R.; Wang, Y.; Li, Y.; Wu, D.; Wu, C.; Pang, J. Fabrication of novel Konjac glucomannan/shellac film with advanced functions for food packaging. Int. J. Biol. Macromol. 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Milanese, C.; Licchelli, M. Shellac/nanoparticles dispersions as protective materials for wood. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1058. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Duffy, B.; Jaiswal, A.K.; Stobie, N.; McHale, P. Enhancement of the antibacterial properties of silver nanoparticles using beta-cyclodextrin as a capping agent. Int. J. Antimicrob. Agents 2010, 36, 280–283. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran-whey protein isolate-TiO2 nanocomposite films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Habibi, P. Enhancing structural properties and antioxidant activity of kefiran films by chitosan addition. Food Struct. 2015, 5, 66–71. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran-whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S. Physicochemical, mechanical, optical, microstructural and antimicrobial properties of novel kefiran-carboxymethyl cellulose biocomposite films as influenced by copper oxide nanoparticles (CuONPs). Food Packag. Shelf Life 2018, 17, 196–204. [Google Scholar] [CrossRef]
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Shojaee-Aliabadi, S.; German, J.B.; Ghanati, K. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int. J. Biol. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nano particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Achachlouei, B.F.; Zahedi, Y. Fabrication and characterization of CMC-based nanocomposites reinforced with sodium montmorillonite and TiO2 nanomaterials. Carbohydr. Polym. 2018, 199, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Nafchi, A.M.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar]
- Akbariazam, M.; Ahmadi, M.; Javadian, N.; Nafchi, A.M. Fabrication and characterization of soluble soybean polysaccharide and nanorod-rich ZnO bionanocomposite. Int. J. Biol. Macromol. 2016, 89, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Maghsoudlou, Y.; Khodaiyan, F.; Jafari, S.M.; Ghasemlou, M.; Aalami, M. Soluble soybean polysaccharide: A new carbohydrate to make a biodegradable film for sustainable green packaging. Carbohydr. Polym. 2013, 97, 817–824. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F. Soluble soybean polysaccharide/TiO2 bionanocomposite film for food application. Carbohydr. Polym. 2018, 186, 384–393. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Fan, Z.; Zhang, X.; Zhong, G. Effect of soybean soluble polysaccharide on the pasting, gels, and rheological properties of kudzu and lotus starches. Food Hydrocoll. 2019, 89, 443–452. [Google Scholar] [CrossRef]
- Mallegni, N.; Phuong, V.T.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) based tear resistant and biodegradable flexible films by blown film extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, C.H.; Yin, S.W.; Yang, X.Q. Development and characterization of novel antimicrobial bilayer films based on Polylactic acid (PLA)/Pickering emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhuang, Y.; Wu, Y.; Li, L. Quality evaluation of hot peppers stored in biodegradable poly(lactic acid)-based active packaging. Sci. Hortic. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of polylactic acid-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Ind. Crops Prod. 2016, 94, 562–573. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Benedetto, F.D.; Vigliotta, G.; Capodieci, L.; Terzi, R.; Rizzoa, A. Aluminum-doped zinc oxide coatings on polylactic acid films for antimicrobial food packaging. Thin Solid Films 2018, 645, 187–192. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, X.; Ren, S.; Wang, J.; Zhang, H. Physicochemical characterization of a zein prepared using a novel aqueous extraction technology and tensile properties of the zein film. Ind. Crop. Prod. 2019, 130, 57–62. [Google Scholar] [CrossRef]
- Ozcalik, O.; Tihminlioglu, F. Barrier properties of corn zein nanocomposite coated polypropylene films for food packaging applications. J. Food Eng. 2013, 114, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Kadam, D.M.; Thuna, M.; Srinivasan, G.; Wang, S.; Kessler, M.R.; Grewell, D.; Yu, C.; Lamsal, B. Effect of TiO2 nanoparticles on thermo-mechanical properties of cast zein protein films. Food Packag. Shelf Life 2017, 13, 35–43. [Google Scholar] [CrossRef]
- Anderson, T.J.; Ilankovan, P.; Lamsal, B.P. Two fraction extraction of α-zein from DDGS and its characterization. Ind. Crop. Prod. 2012, 37, 466–472. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Yoo, S.; Krochta, J.M. Whey protein-polysaccharide blended edible film formation and barrier, tensile, thermal and transparency properties. J. Sci. Food Agric. 2011, 91, 2628–2636. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Characterization of the new biodegradable WPI/clay nanocomposite films based on kefiran exopolysaccharide. J. Food Sci. Technol. 2015, 52, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.G.; Li, X.H.; Xu, Q.L.; Yun, J.; Lu, Y.Q. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The quality evaluation of postharvest strawberries stored in Nano-Ag packages at refrigeration temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.V.; Kuriakose, S. Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater. Sci. Eng. C 2013, 33, 4409–4415. [Google Scholar] [CrossRef]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel antimicrobial chitosan-cellulose composite films bioconjugated with silver nanoparticles. Ind. Crop. Prod. 2015, 70, 395–403. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef]
- Orsuwan, A.; Shankar, S.; Wang, L.-F.; Sothornvit, R.; Rhim, J.-W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Malini, M.; Thirumavalavan, M.; Yang, W.Y.; Lee, J.F.; Annadurai, G. A versatile chitosan/ZnO nanocomposite with enhanced antimicrobial properties. Int. J. Biol. Macromol. 2015, 80, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Nafchi, A.M.; Alias, A.K.; Mahmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.F.F.; Teofilo, R.F.; Coimbra, J.S.; Vitor, D.M.; Batista, R.A.; Ferreirac, S.O.; de Andrade, N.J.; Medeiros, E.A.A. Physical-mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydr. Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, C.L.; Hao, H.; Dai, Y.; Li, J.R. Preparation and preservation properties of the chitosan coatings modified with the in situ synthesized nano SiOx. Food Hydrocoll. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Peng, J.; Wei, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Li, X.-L. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem. Eng. J. 2010, 160, 378–382. [Google Scholar] [CrossRef]
- Zaharia, A.; Muşat, V.; Ghisman, V.P.; Baroiu, N. Antimicrobial hybrid biocompatible materials based on acrylic copolymers modified with (Ag)ZnO/chitosan composite nanoparticles. Eur. Polym. J. 2016, 84, 550–564. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, Y. Chitosan-based rechargeable long-term antimicrobial and biofilm-controlling systems. J. Biomed. Mater. Res. A 2010, 89, 960–967. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Mosanchuk, J.D. Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Che, Z.; Li, X.; Li, W. Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits. Food Funct. 2011, 2, 466–474. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Jiang, L.; Cao, D.; Lin, H.; Che, Z.; Ma, Y.; Li, X.; Cai, Y. Effect of different coating materials on the biological characteristics and stability of microencapsulated lactobacillus acidophilus. RSC Adv. 2015, 5, 22825–22837. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y.; Gong, L. Effect of chitosan coating and oil fumigation on the microbiological and quality safety of fresh-cut pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- Soni, S.S.; Dave, G.S.; Henderson, M.J.; Gibaud, A. Visible light induced cell damage of Gram positive bacteria by N-doped TiO2 mesoporous thin films. Thin Solid Films 2013, 531, 559–565. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Tay, S.L.; Gao, W. Photocatalytic TiO2 nanoparticles enhanced polymer antimicrobial coating. Appl. Surf. Sci. 2014, 290, 274–279. [Google Scholar] [CrossRef]
- Magesan, P.; Ganesan, P.; Umapathy, M.J. Ultrasonic-assisted synthesis of doped TiO2 nanocomposites: Characterization and evaluation of photocatalytic and antimicrobial activity. Optik 2016, 127, 5171–5180. [Google Scholar] [CrossRef]
- Huang, S.; Guild, B.; Neethirajan, S.; Therrien, P.; Lim, L.T.; Warriner, K. Antimicrobial coatings for controlling Listeria monocytogenes based on polylactide modified with titanium dioxide and illuminated with UV-A. Food Control 2017, 73, 421–425. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Jiang, H.; Jin, M.; Wang, X.; Huang, J. Silver/chitosan-based Janus particles: Synthesis, characterization, and assessment of antimicrobial activity in vivo and vitro. Food Res. Int. 2015, 78, 433–441. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; El-Shibiny, A.; Salih, E. Photo-induced green synthesis and antimicrobial efficacy of poly (ε-caprolactone)/curcumin/grape leaf extract-silver hybrid nanoparticles. J. Photochem. Photobiol. B 2016, 160, 355–363. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Eslahi, H. Synthesis, characterization and antibacterial properties of a novel nanocomposite based on polyaniline/polyvinyl alcohol/Ag. Arab. J. Chem. 2014, 7, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against postharvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Bakina, O.V.; Glazkova, E.A.; Svarovskaya, N.V.; Rodkevich, N.G.; Lerner, M.I. «Janus»-like Cu-Fe bimetallic nanoparticles with high antibacterial activity. Mater. Lett. 2019, 242, 187–190. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Lungu, M.-V.; Vasile, E.; Lucaci, M.; Pătroi, D.; Mihăilescu, N.; Grigore, F.; Marinescu, V.; Brătulescu, A.; Mitrea, S.; Sobetkii, A.; et al. Investigation of optical, structural, morphological and antimicrobial properties of carboxymethyl cellulose capped Ag-ZnO nanocomposites prepared by chemical and mechanical methods. Mater. Charact. 2016, 120, 69–81. [Google Scholar] [CrossRef]
- Erdural, B.; Bolukbasi, U.; Karakas, G. Photocatalytic antibacterial activity of TiO2-SiO2 thin films: The effect of composition on cell adhesion and antibacterial activity. J. Photochem. Photobiol. A 2014, 283, 29–37. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Cloirec, P.L. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, J.-H.; Wang, Q.; Na, Y.; Gao, L.-P. Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum, and sclerotinia rot of carrot. J. Integr. Agric. 2015, 14, 691–697. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Tran, H.V.; Tran, L.D.; Ba, C.T.; Vu, H.D.; Nguyen, T.N.; Pham, D.G.; Nguyen, P.X. Synthesis, characterization, antibacterial and antiproliferative activities of monodisperse chitosan-based silver nanoparticles. Colloids Surf. A 2010, 360, 32–40. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Gopinath, V.; Velusamy, P. Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochi. Acta Part A 2013, 106, 170–174. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnolgy 2007, 18, 225103–225111. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-R.; Yu, S.-H.; Chao, A.-C.; Wu, J.-Y.; Lin, Y.-F.; Lu, K.-Y.; Mi, F.-L. Preparation and properties of pH-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 9–20. [Google Scholar] [CrossRef]
- Mei, L.; Lu, Z.; Zhang, W.; Wu, Z.; Zhang, X.; Wang, Y.; Luo, Y.; Li, C.; Jia, Y. Bioconjugated nanoparticles for attachment and penetration into pathogenic bacteria. Biomaterials 2013, 34, 10328–10337. [Google Scholar] [CrossRef]
- Wang, X.C.; Yu, J.C.; Yip, H.Y.; Wu, L.; Wong, P.K.; Lai, S.Y. A mesoporous Pt/TiO2 nanoarchitecture with catalytic and photocatalytic functions. Chem. Eur. J. 2005, 11, 2997–3004. [Google Scholar] [CrossRef]
- Arellano, U.; Asomoza, M.; Ramirez, F. Antimicrobial activity of Fe-TiO2 thin film photocatalysts. J. Photochem. Photobiol. A 2011, 222, 159–165. [Google Scholar] [CrossRef]
- Thurmana, R.B.; Gerbab, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Manzl, C.; Enrich, J.; Ebner, H.; Dallinger, R.; Krumschnabel, G. Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology 2004, 196, 57–64. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria vibrio fischeri and crus-taceans daphnia magna and thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Chevion, M.; Czapski, G. Roles of copper and O2 in the radiationinduced inactivation of T7 bacteriophage. Radiat. Res. 1984, 99, 562–572. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajeswari, V.; Gomathisankar, P. Optical, electrical, photocatalytic, and bactericidal properties of microwave synthesized nanocrystalline Ag-ZnO and ZnO. Solid State Sci. 2011, 13, 923–928. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediatedreactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and geno-toxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silvernanomaterials and potential implications for human health and the environ-ment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Bala, T.; Armstrong, G.; Laffir, F.; Thornton, R. Titania-silver and alumina-silver composite nanoparticles: Novel, versatile synthesis, reaction mechanism and potential antimicrobial application. J. Colloid Interface Sci. 2011, 356, 395–403. [Google Scholar] [CrossRef]
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Checchetto, R. Gas barrier and optical properties of cellulose nanofiber coatings with dispersed TiO2 nanoparticles. Surf. Coat. Technol. 2018, 343, 131–137. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein chitosan sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Singh, R.K.; Awasthi, S.; Dhayalan, A.; Ferreira, J.M.F.; Kannan, S. Deposition, structure, physical and in vitro characteristics of Ag-doped β-Ca3(PO4)2/chitosan hybrid composite coatings on Titanium metal. Mater. Sci. Eng. C 2016, 62, 692–701. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Amr, A.; Abdel-Mohdy, F.A. Investigation of silver nanoparticles synthesis using aminated-cyclodextrin. Carbohydr. Polym. 2012, 89, 1–6. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwamoto, S.; Kumamoto, Y.; Ohdaira, T.; Suzuki, R.; Isogai, A. Pore size determination of TEMPO-oxidized cellulose nanofibril films by positron annihilation lifetime spectroscopy. Biomacromolecules 2011, 12, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Egger, W.; Koschine, T.; Brusa, R.S.; Checchetto, R. Cellulose nanofibrils films: Molecular diffusion through elongated sub-nano cavities. J. Phys. Chem. C 2017, 121, 15437–15447. [Google Scholar] [CrossRef]
- Hanika, M.; Langowski, H.-C.; Moosheimer, U.; Peukert, W. Inorganic layers on polymeric films-influence of defects and morphology on barrier properties. Chem. Eng. Technol. 2003, 26, 605–614. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Adhikari, B. The effects of ultrasound treatment and nanozinc oxide coating on the physiological activities of fresh-cut kiwi fruit. Food Bioprocess Technol. 2014, 7, 126–132. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sahareen, T. Investigation of cellulosic packets impregnated with silver nanoparticles for enhancing shelf-life of vegetables. LWT-Food Sci. Technol. 2017, 86, 116–122. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Buonocore, G.G.; Nobile, M.A.D. Antimicrobial silver-montmorillonite nanoparticles to prolong the shelf life of fresh fruit salad. Int. J. Food Microbiol. 2011, 148, 164–167. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, L. Effect of chitosan/nano-SiOx complex on quality and physiology of fresh-cut bamboo shoot. Sci. Agric. Sin. 2010, 22, 4694–4700. [Google Scholar]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Qaisar, M.; Jahangir, M.; Abbasi, K.S.; Khan, S.U.; Ali, N.; Liaquat, M. Influence of CMC- and guar gum-based silver nanoparticle coatings combined with low temperature on major aroma volatile components and the sensory quality of kinnow (Citrus reticulata). Int. J. Food Sci. Technol. 2016, 51, 2345–2352. [Google Scholar] [CrossRef]






| NPs | Main Edible Materials | Reference | |
|---|---|---|---|
| TiO2 | Shellac | Yemmireddy and Hung [15] | |
| CS, WPI | Cano et al. [14], Xu et al. [5], Zhang et al. [73] | ||
| Potato starch | Oleyaei et al. [74] | ||
| SSPS | Teymourpour et al. [51] | ||
| WPI/CNFs | Zolfi et al. [44], Alizadeh-Sani et al. [66] | ||
| CMC | Achachlouei et al. [50] | ||
| Ag | CS | Davoodbasha et al. [17], Mathew and Kuriakose [75], Lin et al. [76]; Kumar-Krishnan et al. [77] | |
| Starch, PVA | Cano et al. [78], Usman et al. [79] | ||
| β-CD | Andrade et al. [18] | ||
| Agar, banana powder | Orsuwan et al. [80] | ||
| ZnO | CS | Malini et al. [81] | |
| Sago starch | Nafchi et al. [82] | ||
| FPI, FSG | Arfat et al. [83] | ||
| MC | Espitia et al. [84] | ||
| SSPS | Akbariazam et al. [52] | ||
| Others | Silicon dioxide | CS | Yu et al. [20], Sun et al. [85] |
| Silica | CS | Shi et al. [21], Song et al. [86] | |
| CuO | Kefiran, CMC | Hasheminya et al. [47] | |
| SNP | CS | Shankar et al. [19] | |
| Mixed NPs | Ag-TiO2 | CS | Lin et al. [87] |
| TiO2-Ag | PLA | Chi et al. [57] | |
| Ag-Cu | FSG | Arfat et al. [90] | |
| TiO2-SiO2 | ZP | Kadam et al. [64] | |
| Main Edible Materials and NPs | Inhibited Microorganisms | Reference |
|---|---|---|
| WPI/CNFs, TiO2 | L. monocytogenes, S. aureus, E. coli O157:H7, P. fluorescens, S. enteritidis | Alizadeh-Sani et al. [66] |
| Polylactide, TiO2 | L. monocytogenes, S. Typhimurium, Shiga toxin producing E. coli | Huang et al. [99] |
| SSPS, TiO2 | E. coli, S. aureus | Teymourpour et al. [51] |
| SSPS, TiO2 | S. aureus | Salarbashi et al. [54] |
| CS, Ag | E. coli, S. aureus | Kumar-Krishnan et al. [77] |
| CS, Ag | S. choleraesuis, S. aureus, B. subtilis, E. coli | Jia et al. [101] |
| Poly(ε-caprolactone), curcumin, grape leaf extract, Ag | E. coli, S. aureus, S. enterica, P. aeruginosa, B. subtilis, C. albicans, A. flavus | El-Sherbiny et al. [102] |
| CS, Ag | E. coli, P. aeruginosa, V. parahaemolyticus, V. vulnificus, S. aureus, B. cereus, C. albicans, A. parasiticus | Davoodbasha et al. [17] |
| CS, Ag | S. aureus, E. coli, A. terreus, A. flavus | Mathew and Kuriakose [75] |
| CS, Ag | E. coli, S. aureus, | Lin et al. [76] |
| Starch, PVA, Ag | A. niger, P. expansum, L. innocua, E. coli | Cano et al. [78] |
| CS, ZnO | B. substili, K. planticola | Malini et al. [81] |
| CS, ZnO | S. enterica, E. coli, S. aureus | Al-Naamani et al. [106] |
| FPI, FSG, ZnO, BEO | L. monocytogenes, P. aeruginosa | Arfat et al. [83] |
| Kefiran, CMC, CuO | S. aureus, E. coli | Hasheminya et al. [47] |
| CS, SNP | E. coli, L. monocytogenes | Shankar et al. [108] |
| CS, Ag-TiO2 | E. coli | Lin et al. [87] |
| CS, Ag, ZnO | E. coli, B. subtilis, Aspergillus, Rhizopus, Penicillium, S. aureus, yeast | Li et al. [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.; Bi, X.; Liu, X.; Shui, Y.; Lin, H.; et al. Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables. Molecules 2019, 24, 1695. https://doi.org/10.3390/molecules24091695
Xing Y, Li W, Wang Q, Li X, Xu Q, Guo X, Bi X, Liu X, Shui Y, Lin H, et al. Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables. Molecules. 2019; 24(9):1695. https://doi.org/10.3390/molecules24091695
Chicago/Turabian StyleXing, Yage, Wenxiu Li, Qin Wang, Xuanlin Li, Qinglian Xu, Xunlian Guo, Xiufang Bi, Xiaocui Liu, Yuru Shui, Hongbin Lin, and et al. 2019. "Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables" Molecules 24, no. 9: 1695. https://doi.org/10.3390/molecules24091695