Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC.: An In Vitro Comparative Study between Leaves, Stems, and Flowers
Abstract
:1. Introduction
2. Results and Discussion
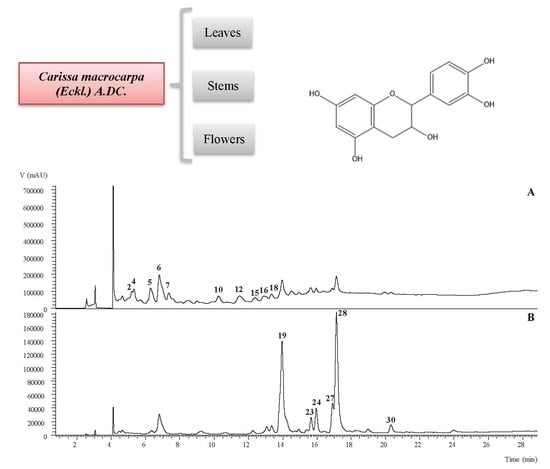
2.1. Phenolic Profile of the Aerial Parts of C. Macrocarpa
2.2. Bioactivities of the Hydroethanolic Extracts of Leaves, Stems, and Flowers
3. Materials and Methods
3.1. Plant Material and Preparation of the Hydroethanolic Extracts
3.2. Phenolic Profile of the Hydroethanolic Extracts of Leaves, Stems, and Flowers
3.3. Bioactivities of the Hydroethanolic Extracts of Leaves, Stems, and Flowers
3.3.1. Antioxidant Activity
3.3.2. Cytotoxic Activity
3.3.3. Anti-Inflammatory Activity
3.3.4. Antibacterial Activity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patel, S. Food, pharmaceutical and industrial potential of Carissa genus: An overview. Rev. Environ. Sci. Biotechnol. 2013, 12, 201–208. [Google Scholar] [CrossRef]
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta 2012, 730, 33–41. [Google Scholar] [CrossRef]
- Khalil, E.; Aljeshi, Y.M.; Saleh, F.A. Authentication of Carissa macrocarpa Cultivated in Saudi Arabia; Botanical, Phytochemical and Genetic Study. J. Pharm. Sci. Res. 2015, 7, 497–508. [Google Scholar]
- Abbas, M.; Rasool, N.; Riaz, M.; Zubair, M.; Abbas, M.; Ul-Haq, N.; Hayat, N. GC-MS profiling, antioxidant, and antimicrobial studies of various parts of Carissa grandiflora. Bulg. Chem. Commun. 2014, 46, 831–839. [Google Scholar]
- Council, N.R. Lost Crops of Africa: Volume III: Fruits; National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Moodley, R.; Chenia, H.; Jonnalagadda, S.B. Antibacterial and anti-adhesion activity of the pentacyclic triterpenoids isolated from the leaves and edible fruits of Carissa macrocarpa. J. Med. Plant Res. 2011, 5, 4851–4858. [Google Scholar]
- Khalil, H.; Mohamed, M.; Morsy, M.; Kandeel, M. Flavonoid and Phenolic Compounds from Carissa macrocarpa: Molecular Docking and Cytotoxicity Studies. Pharmacogn. Mag. 2018, 14, 304. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. The role of phenolic compounds in the fight against cancer—A review. Anticancer. Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crops Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSnidentification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the chlorogenic acids of aster by HPLC–MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal chrysanthemum by LC-MSn. J. Agric. Food Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Heneidak, S.; Grayer, R.J.; Kite, G.C.; Simmonds, M.S.J. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochem. Syst. Ecol. 2006, 34, 575–584. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A bioactive formulation based on Fragaria vesca L. vegetative parts: Chemical characterisation and application in κ-carrageenan gelatin. J. Funct. Foods 2015, 16, 243–255. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, M. Characterization of Polyphenolics in Grape Pomace Extracts Using ESI Q-TOF MS/MS. HSOA J. Food Sci. Nutr. 2015, 1, 1–10. [Google Scholar]
- Khatun, M.; Habib, M.R.; Rabbi, M.A.; Amin, R.; Islam, M.F.; Nurujjaman, M.; Karim, M.R.; Rahman, M.H. Antioxidant, cytotoxic and antineoplastic effects of Carissa carandas Linn. leaves. Exp. Toxicol. Pathol. 2017, 69, 469–476. [Google Scholar] [CrossRef]
- Sehar, I.; Pal, H.C.; Shukla, S.; Bhushan, S.; Hamid, A.; Gupta, B.D.; Saxena, A.K. Cytotoxic evaluation and induction of mitochondria-mediated apoptosis in human leukaemia HL-60 cells by Carissa spinarum stem isolate. J. Pharm. Pharmacol. 2011, 63, 1078–1090. [Google Scholar] [CrossRef]
- Nisa, S.; Bibi, Y.; Zia, M.; Waheed, A.; Pharm Sci, P.J.; Fayyaz Chaudhary, M. Anticancer investigations on Carissa opaca and Toona ciliata extracts against human breast carcinoma cell line Biological evaluation and chemical fingerprint of plant-derived materials View project medicinal plant View project Anticancer inv. Pak. J. Pharm. Sci. 2013, 26, 1009–1012. [Google Scholar]
- Begum, S.; Syed, S.A.; Siddiqui, B.S.; Sattar, S.A.; Choudhary, M.I. Carandinol: First isohopane triterpene from the leaves of Carissa carandas L. and its cytotoxicity against cancer cell lines. Phytochem. Lett. 2013, 6, 91–95. [Google Scholar] [CrossRef]
- Wangteeraprasert, R.; Lipipun, V.; Gunaratnam, M.; Neidle, S.; Gibbons, S.; Likhitwitayawuid, K. Bioactive Compounds from Carissa spinarum. Phyther. Res. 2012, 26, 1496–1499. [Google Scholar] [CrossRef]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Gómez-Cordovés, M.C.; Rybarczyk, A.; Amarowicz, R.; Bartolomé, B. Comparative Flavan-3-ol Profile and Antioxidant Capacity of Roasted Peanut, Hazelnut, and Almond Skins. J. Agric. Food Chem. 2009, 57, 10590–10599. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Sarmento, A.; Barros, L.; Fernandes, Â.; Carvalho, A.M.; Ferreira, I.C. Valorization of traditional foods: Nutritional and bioactive properties of Cicer arietinum L. and Lathyrus sativus L. pulses. J. Sci. Food Agric. 2015, 95, 179–185. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Souilem, F.; Fernandes, Â.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem. 2017, 230, 40–48. [Google Scholar] [CrossRef]
- Svobodova, B.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Bittova, M.; Kuban, V.; Ferreira, I.C.F.R. Bioactive properties and phenolic profile of Momordica charantia L. medicinal plant growing wild in Trinidad and Tobago. Ind. Crops Prod. 2017, 95, 365–373. [Google Scholar] [CrossRef]
Sample Availability: The lyophilized samples are available in our laboratory. |

| Peak | Rt (min) | λmax (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 4.65 | 324 | 353 | 191(100),179(45),161(15),135(10) | 3-O-Caffeolyquinic acid |
| 2 | 5.16 | 310 | 337 | 191(100),173(3),161(5) | cis 3-p-Coumaroylquinic acid |
| 3 | 5.21 | 284 | 359 | 239(95),197(100),181(6),153(10),137(5) | Syringic acid hexoside |
| 4 | 5.34 | 290 | 337 | 191(100),173(5),161(5) | trans 3-p-Coumaroylquinic acid |
| 5 | 6.31 | 287 | 353 | 191(32),173(100),161(5),135(5) | cis 4-O-Caffeolyquinic acid |
| 6 | 6.8 | 325 | 353 | 191(75),173(100),161(12),135(5) | trans 4-O-Caffeolyquinic acid |
| 7 | 7.35 | 281 | 577 | 425(100),289(13) | Type B (epi)catechin dimer |
| 8 | 8.1 | 325 | 353 | 191(100),179(35),161(5),135(5) | 5-O-Caffeolyquinic acid * |
| 9 | 9.01 | 280 | 577 | 425(100),289(19) | Type B (epi)catechin dimer |
| 10 | 9.45 | 266,347 | 755 | 593(20),285(100) | Kaempherol-O-hexoside-O-rutinoside |
| 11 | 10.26 | 280 | 865 | 451(14),425(16),407(12),289(11) | Type B (epi)catechin trimer |
| 12 | 11.47 | 280 | 1153 | 865(78),577(35),575(43),289(5) | Type B (epi)catechintetramer |
| 13 | 10.77 | 284 | 337 | 191(100),173(5),161(5) | cis 5-p-Coumaroylquinic acid |
| 14 | 11.49 | 280 | 1153 | 865(82),577(24),575(36),289(5) | Type B (epi)catechin tetramer |
| 15 | 12.15 | 310 | 337 | 191(100),173(3),161(3) | trans 5-p-Coumaroylquinic acid |
| 16 | 12.41 | 280 | 865 | 451(15),425(13),407(17),289(7) | Type B (epi)catechin trimer |
| 17 | 12.9 | 280 | 863 | 711(26),573(61),451(12),411(5),289(22) | Type A (epi)catechin trimer |
| 18 | 13.1 | 267,347 | 739 | 593(100),285(25) | Kaempferol-O-deoxyhexoside-O-deoxyhexosyl-hexoside isomer 1 |
| 19 | 13.35 | 280 | 1153 | 865(54),577(23),575(24),289(5) | Type B (epi)catechin tetramer |
| 20 | 13.96 | 265,352 | 755 | 609(100),301(25) | Quercetin-O-deoxyhexoside-O-deoxyhexosyl-hexoside |
| 21 | 14.07 | 266,357 | 739 | 593(100),285(25) | Kaempferol-O-deoxyhexoside-O-deoxyhexosyl-hexoside isomer 2 |
| 22 | 14.7 | 257,352 | 609 | 301(100) | Quercetin-O-deoxyhexosyl-hexoside isomer 1 |
| 23 | 15.62 | 280,339 | 739 | 285(100) | Kaempferol-O-di-deoxyhexoside-hexoside |
| 24 | 15.95 | 271,344 | 575 | 285(100) | Acetylkaempherol-O-malonylhexoside |
| 25 | 16.09 | 266,357 | 739 | 593(100),285(25) | Kaempferol-O-deoxyhexoside-O-deoxyhexosyl-hexoside isomer 3 |
| 26 | 16.39 | 257,348 | 739 | 593(100),285(25) | Kaempferol-O-deoxyhexoside-O-deoxyhexosyl-hexoside isomer 4 |
| 27 | 16.88 | 266,352 | 609 | 301(100) | Quercetin-O-deoxyhexosyl-hexoside isomer 2 |
| 28 | 17.1 | 257,354 | 609 | 301(100) | Quercetin-3-O-rutinoside * |
| 29 | 19.17 | 266,346 | 593 | 285(100) | Kaempherol-O-deoxyhexosyl-hexoside |
| 30 | 20.32 | 266,346 | 593 | 285(100) | Kaempherol-3-O-rutinoside * |
| Peak | Leaves | Stems | Flowers |
|---|---|---|---|
| 1 | nd | 0.49 ± 0.01 | nd |
| 2 | 0.25 ± 0.01 | nd | nd |
| 3 | nd | 0.34 ± 0.01 | nd |
| 4 | 0.34 ± 0.01 b | nd | 0.6 ± 0.01 a |
| 5 | 0.26 ± 0.004 c | 0.61 ± 0.02 b | 3.1 ± 0.1 a |
| 6 | 0.5 ± 0.02 b | 2.28 ± 0.02 a | nd |
| 7 | 2 ± 0.1 b | 6.4 ± 0.2 a | nd |
| 8 | nd | nd | 0.17 ± 0.01 |
| 9 | nd | 2.88 ± 0.01 | nd |
| 10 | nd | nd | 0.48 ± 0.02 |
| 11 | 1.9 ± 0.1 b | 6.09 ± 0.02 a | nd |
| 12 | 2.9 ± 0.1 | nd | nd |
| 13 | nd | 0.97 ± 0.01 b | 1.21 ± 0.02 a |
| 14 | nd | 7.3 ± 0.2 | nd |
| 15 | nd | nd | 0.49 ± 0.02 |
| 16 | 1.9 ± 0.1 b | 3.7 ± 0.1 a | nd |
| 17 | 1.57 ± 0.05 b | 4.9 ± 0.2 a | nd |
| 18 | nd | nd | tr |
| 19 | 2.2 ± 0.1 b | 3.9 ± 0.1 a | nd |
| 20 | 1.79 ± 0.03 | 1.03 ± 0.01 | nd |
| 21 | nd | nd | tr |
| 22 | nd | nd | tr |
| 23 | 1.03 ± 0.01 | nd | nd |
| 24 | 1.1 ± 0.03 | nd | nd |
| 25 | nd | nd | tr |
| 26 | nd | nd | 1.6 ± 0.1 |
| 27 | tr | nd | nd |
| 28 | 1.86 ± 0.04 b | 2.6 ± 0.1 a | 1.48 ± 0.01 c |
| 29 | nd | nd | tr |
| 30 | tr | nd | 1.73 ± 0.03 |
| TPA | 1.35 ± 0.04 c | 4.68 ± 0.01 b | 5.5 ± 0.1 a |
| TF3O | 12.5 ± 0.2 b | 35.1 ± 0.2 a | nd |
| TF | 5.7 ± 0.1 a | 3.6 ± 0.1 c | 5.3 ± 0.1 b |
| TPC | 19.6 ± 0.3 b | 43.4 ± 0.1 a | 10.8 ± 0.2 c |
| Leaves | Stems | Flowers | Correlation Factor r2 | ||||
|---|---|---|---|---|---|---|---|
| TPA | TF3O | TF | TPC | ||||
| Antioxidant activity EC50 values (μg/mL) A | |||||||
| DPPH scavenging activity | 26 ± 1 b | 281 ± 1 a | 223 ± 6 a | 0.887 | w/n | 0.862 | m |
| Reducing power | 36 ± 1 b | 33 ± 1 a | 279 ± 4 b | w/n | 0.940 | w/n | 0.995 |
| β-carotene bleaching inhibition | 300 ± 1 b | 270 ± 10 b | 1107 ± 47 a | m | 0.781 | 0.719 | m |
| TBARS inhibition | 15.4 ± 0.1 b | 12.1 ± 0.1 c | 92.5 ± 0.1 a | m | 0.794 | 0.718 | m |
| Cytotoxicity GI50 values (μg/mL) B | |||||||
| MCF-7 (breast carcinoma) | 167 ± 2 a | 70.38 ± 0.03 c | 95.25 ± 0.01 b | 0.862 | w/n | 0.846 | m |
| NCI-H460 (non-small cell lung carcinoma) | 120 ± 1 a | 58.7 ± 0.2 c | 68 ± 1 b | 0.911 | w/n | 0.898 | m |
| HeLa (cervical carcinoma) | 101 ± 1 a | 52.1 ± 0.3 c | 75 ± 1 b | 0.721 | m | m | 0.781 |
| HepG2 (hepatocellular carcinoma) | 152 ± 3 a | 89 ± 1 b | >400 | 0.943 | w/n | 0.953 | w/n |
| PLP2 (non-tumour porcine liver primary cells) | >400 | >400 | >400 | - | - | - | - |
| Anti-inflammatory activity IC50 values (μg/mL) C | |||||||
| NO production | 179 ± 6 c | 208 ± 9 a | 196 ± 4 b | m | w/n | w/n | w/n |
| Antibacterial activity MIC values (mg/mL) | |||||||
| Gram-negative bacteria | |||||||
| Escherichia coliD | 5 | 10 | 10 | 0.961 | w/n | 0.952 | w/n |
| Escherichia coli ESBL E | 20 | 20 | 20 | - | - | - | - |
| Klebsiella pneumoniaeD | >20 | >20 | >20 | - | - | - | - |
| Klebsiella pneumoniae ESBL D | >20 | >20 | >20 | - | - | - | - |
| Morganella morganiiD | 10 | 10 | 20 | 0.720 | 0.772 | 0.774 | w/n |
| Pseudomonas aeruginosa | 20 | 20 | 20 | - | - | - | - |
| Gram-positive bacteria | |||||||
| Enterococcus faecalisF | 1.25 | 1.25 | 5 | 0.720 | 0.772 | 0.774 | w/n |
| Listeria monocytogenesG | 2.5 | 0.625 | 20 | m | 0.825 | m | m |
| MRSA *,F | 2.5 | 2.5 | 20 | 0.720 | 0.772 | 0.742 | m |
| MSSA F | 5 | 2.5 | 10 | w/n | 0.938 | m | 0.852 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC.: An In Vitro Comparative Study between Leaves, Stems, and Flowers. Molecules 2019, 24, 1696. https://doi.org/10.3390/molecules24091696
Souilem F, Dias MI, Barros L, Calhelha RC, Alves MJ, Harzallah-Skhiri F, Ferreira ICFR. Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC.: An In Vitro Comparative Study between Leaves, Stems, and Flowers. Molecules. 2019; 24(9):1696. https://doi.org/10.3390/molecules24091696
Chicago/Turabian StyleSouilem, Fedia, Maria Inês Dias, Lillian Barros, Ricardo C. Calhelha, Maria José Alves, Fethia Harzallah-Skhiri, and Isabel C.F.R. Ferreira. 2019. "Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC.: An In Vitro Comparative Study between Leaves, Stems, and Flowers" Molecules 24, no. 9: 1696. https://doi.org/10.3390/molecules24091696