Sample Digestion and Combined Preconcentration Methods for the Determination of Ultra-Low Gold Levels in Rocks
Abstract
:1. Introduction
2. Dry Digestion Methods
2.1. Fire Assay
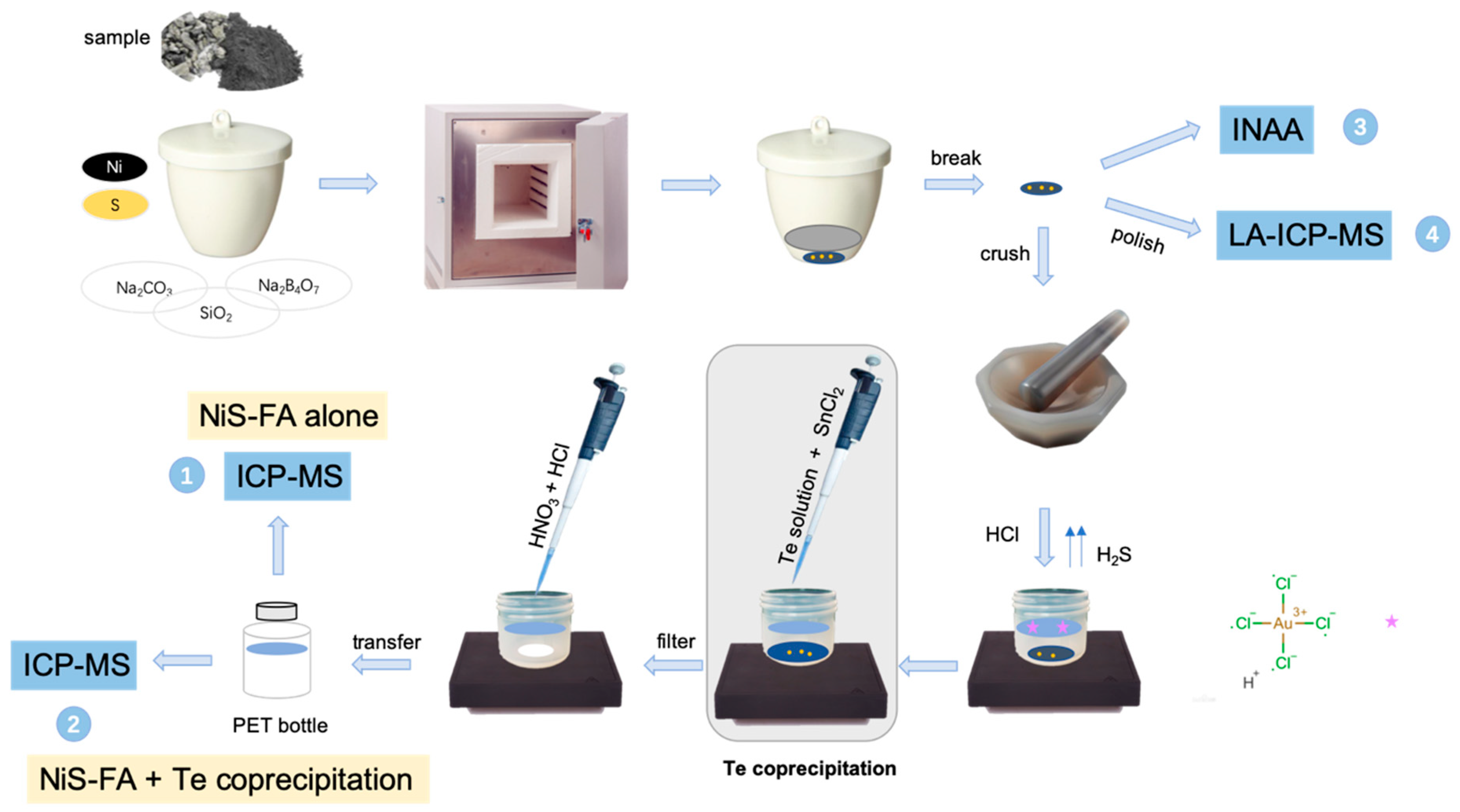
2.1.1. NiS-FA + Te Coprecipitation
2.1.2. FA + INAA or LA-ICPMS
2.2. Fusion
2.2.1. Fusion + Anion Exchange Resin
2.2.2. Fusion + Te Coprecipitation
2.2.3. Fusion of the Residue Formed after Acid Digestion
2.3. Dry Chlorination
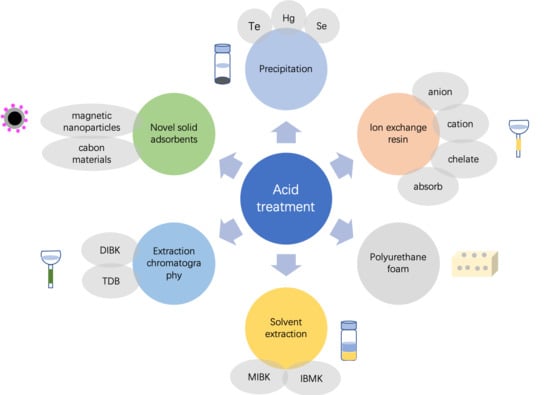
3. Wet Acid Digestion Methods
3.1. Acid Digestion + Precipitation
3.2. Acid Digestion + Ion Exchange Resin
3.3. Acid Digestion + Solvent Extraction/Dispersive Liquid–Liquid Microextraction
3.4. Acid Digestion + PUF
3.5. Acid Digestion + Extraction Chromatography
3.6. Acid Digestion + Novel Solid Adsorbents
4. Direct Determination Methods
5. Conclusions and Perspectives
- In order to eliminate the nugget effect, the sample weight must be large enough. However, it leads to low digestion efficiency and requires the use of complex operating procedures. Therefore, additional studies must be performed to improve the sample representativeness.
- Normally, the results obtained by the methods described above exhibit large deviations, which are often attributed to the nugget effect. However, homogeneous reference materials are required to confirm this conclusion.
- Although FA can attack the entire rock sample, its relatively large reagent blank makes it difficult to determine the low gold content precisely. Wet acid digestion can solve this problem, but aqua regia may partially dissolve the rock samples. The desilication by HF is an effective process; however, it is inconvenient for the use in high-pressure ashers and Carius tubes and may cause a severe interference of 181Ta16O into 197Au determination when ICP-MS is utilized. In addition, the formation of insoluble fluoride compounds may also cause the loss of gold.
- It should be noted that gold solutions must be analyzed as soon as possible after separation due to the instability of gold in both the HCl and thiourea media. In addition, the memory effect and instrument damage caused by their usage are normally large. Therefore, future research works may focus on the development of suitable media for gold elution and quantitation.
Funding
Conflicts of Interest
References
- Pitcairn, I.K. Background concentrations of gold in different rock types. Appl. Earth Sci. (Trans. Inst. Min. Metall. B) 2011, 120, 31–38. [Google Scholar] [CrossRef]
- Morgan, J.W.; Wandless, G.A.; Petrie, R.K.; Irving, A.J. Composition of The Earth’s Upper Mantle—I. Siderophile Trace Elements in Ultramafic Nodules. Tectonophysics 1981, 75, 47–67. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Keays, R.R. Abundance and distribution of gold, palladium and iridium in some spine1 and garnet Iberzolites: Implications for the nature and origin of precious metal-rich intergranular components in the upper mantle. Geochim. Cosmochim. Acta 1981, 45, 2425–2442. [Google Scholar] [CrossRef]
- Pattou, L.; Lorand, J.P.; Gros, M. Non-chondritic platinum-group element ratios in the Earth’s mantle. Nature 1996, 379, 712–715. [Google Scholar] [CrossRef]
- Fischer-Gödde, M.; Becker, H.; Wombacher, F. Rhodium, gold and other highly siderophile elements in orogenic peridotites and peridotite xenoliths. Chem. Geol. 2011, 280, 365–383. [Google Scholar] [CrossRef]
- Maier, W.D.; Peltonen, P.; McDonald, I. The concentration of platinum-group elements and gold in southern African and Karelian kimberlite-hosted mantle xenoliths: Implications for the noble metal content of the Earth’s mantle. Chem. Geol. 2012, 302–303, 119–135. [Google Scholar] [CrossRef]
- Lorand, J.P.; Pattou, L.; GROS, M. Fractionation of Platinum-group Elements and Gold in the Upper Mantle: A Detailed Study in Pyrenean Orogenic Lherzolites. J. Petrol. 1999, 40, 957–981. [Google Scholar] [CrossRef]
- Lorand, J.P.; Bodinier, J.L.; Dupuy, C. Abundance and distribution of gold in the orogenic-type spinel peridotites from Ariege (Northeastern Pyrenees, France). Geochim. Cosmochim. Acta 1989, 53, 3085–3090. [Google Scholar] [CrossRef]
- Maier, W.D.; Barnes, S.J.; Campbell, I.H. Progressive mixing of meteoritic veneer into the early Earth’s deep mantle. Nature 2009, 460, 620–623. [Google Scholar] [CrossRef]
- Brenan, J.M.; McDonough, W.F. Core formation and metal–silicate fractionation of osmium and iridium from gold. Nat. Geosci. 2009, 2, 798–801. [Google Scholar] [CrossRef]
- Cameron, E.M. Archean gold: Relation to granulite formation and redox zoning in the crust. Geology 1988, 16, 109–112. [Google Scholar] [CrossRef]
- Cameron, E.M. Scouring of gold from the lower crust. Geology 1989, 17, 26–29. [Google Scholar] [CrossRef]
- Cameron, E.M. Derivation of gold by oxidative metamorphism of a deep ductile shear zone: Part 2. Evidence from the Bamble Belt, south Norway. J. Geochem. Explor. 1989, 31, 149–169. [Google Scholar] [CrossRef]
- Hofmann, A.; Pitcairn, I.K.; Wilson, A. Gold mobility during Palaeoarchaean submarine alteration. Earth. Planet. Sc. Lett. 2017, 462, 47–54. [Google Scholar] [CrossRef]
- Patten, C.G.C.; Pitcairn, I.K.; Teagle, D.A.H. Hydrothermal mobilisation of Au and other metals in supra-subduction oceanic crust: Insights from the Troodos ophiolite. Ore. Geol. Rev. 2017, 86, 487–508. [Google Scholar] [CrossRef] [Green Version]
- Connors, K.A.; Noble, D.C.; Bussey, S.D. Initial gold contents of silicic volcanic rocks: Bearing on the behavior of gold in magmatic systems. Geology 1993, 21, 937–940. [Google Scholar] [CrossRef]
- Webber, A.P.; Roberts, S.; Taylor, R.N.; Pitcairn, I.K. Golden plumes: Substantial gold enrichment of oceanic crust during ridge-plume interaction. Geology 2013, 41, 87–90. [Google Scholar] [CrossRef]
- Pitcairn, I.K. Sources of Metals and Fluids in Orogenic Gold Deposits: Insights from the Otago and Alpine Schists, New Zealand. Econ. Geol. 2006, 101, 1525–1546. [Google Scholar] [CrossRef]
- Fleet, M.E.; Crocket, J.H.; Liu, M.H. Laboratory partitioning of platinum-group elements (PGE) and gold with application to magmatic sulfide–PGE deposits. Lithos 1999, 47, 127–142. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Zhou, Y.; Wang, R. Recycling of metal-fertilized lower continental crust: Origin of non-arc Au-rich porphyry deposits at cratonic edges. Geology 2017, 45, 6–9. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Craw, D.; Teagle, D.A.H. Metabasalts as sources of metals in orogenic gold deposits. Miner. Deposita. 2015, 50, 373–390. [Google Scholar] [CrossRef]
- Patten, C.G.C.; Pitcairn, I.K.; Teagle, D.A.H. Michelle Harris, Mobility of Au and related elements during the hydrothermal alteration of the oceanic crust: Implications for the sources of metals in VMS deposits. Miner. Deposita. 2016, 51, 179–200. [Google Scholar] [CrossRef]
- Boskabadi, A.; Pitcairn, I.K.; Broman, C. Carbonate alteration of ophiolitic rocks in the Arabian–Nubian Shield of Egypt: Sources and compositions of the carbonating fluid and implications for the formation of Au deposits. Int. Geol. Rev. 2017, 4, 391–419. [Google Scholar] [CrossRef]
- Kerr, R.A. Is the World Tottering on the Precipice of Peak Gold? Science 2012, 335, 1038–1039. [Google Scholar] [CrossRef]
- Perry, B.J.; Barefoot, R.R.; Van Loon, J.C. Inductively coupled plasma mass spectrometry for the determination of platinum group elements and gold. Trac-Trend. Anal. Chem. 1995, 14, 388–397. [Google Scholar]
- Eller, R.; Alt, F.; Tolg, G. An efficient combined procedure for the extreme trace analysis of gold, platinum, palladium and rhodium with the aid of graphite furnace atomic absorption spectrometry and total-reflection X-ray fluorescence analysis. Fresenius. Z. Anal. Chem. 1989, 334, 723–739. [Google Scholar] [CrossRef]
- Yokoyama, T.; Yokota, T.; Hayashi, S. Determination of trace gold in rock samples by a combination of two-stage solvent extration and graphite furnace atomic absorption spectrometry: The problem of iron interference and its solution. Geochem. J. 1996, 30, 175–181. [Google Scholar] [CrossRef]
- Constantin, M. Determination of Au, Ir and thirty-two other elements in twelve geochemical reference materials by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2006, 267, 407–414. [Google Scholar] [CrossRef]
- Barefoot, R.R. Determination of the precious metals in geological materials by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1998, 13, 1077–1084. [Google Scholar] [CrossRef]
- Barefoot, R.R.; Van Loon, J.C. Recent advances in the determination of the platinum group elements and gold. Talanta 1999, 49, 1–14. [Google Scholar] [CrossRef]
- Balcerzak, M. Sample Digestion Methods for the Determination of Traces of Precious Metals by Spectrometric Techniques. Anal. Sci. 2002, 18, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Recent developments in the determination of gold by atomic spectrometry techniques. Spectrochim. Acta B 2005, 60, 1316–1322. [Google Scholar] [CrossRef]
- Mokhodoeva, O.B.; Myasoedova, G.V.; Kubrakova, I.V. Sorption Preconcentration in Combined Methods for the Determination of Noble Metals. J. Anal. Chem. 2007, 62, 607–622. [Google Scholar] [CrossRef]
- Myasoedova, G.V.; Mokhodoeva, O.B.; Kubrakova, I.V. Trends in Sorption Preconcentration Combined with Noble Metal Determination. Anal. Sci. 2007, 23, 1031–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrzynska, K. Sorbent materials of separation and preconcentration of gold in environmental and geological samples-A review. Anal. Chim. Acta 2012, 741, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pitcairn, I.K.; Warwick, P.E. Method for Ultra-Low-Level Analysis of Gold in Rocks. Anal. Chem. 2006, 78, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Beamish, F.E. An experimental evaluation of neutron activation, wet assay and fire assay methods of determining gold in ores. Talanta 1967, 14, 219–231. [Google Scholar] [CrossRef]
- Hall, G.E.M.; Pelchat, J.C. Analysis of geological materials for gold, platinum and palladium at low ppb levels by fire assay-ICP mass spectrometry. Chem. Geol. 1994, 115, 61–72. [Google Scholar] [CrossRef]
- Hall, G.E.M.; Vaive, J.E.; Coope, J.A. Bias in the analysis of geological materials for gold using current methods. J. Geochem. Explor. 1989, 34, 157–171. [Google Scholar] [CrossRef]
- Juvonen, M.R.; Bartha, A.; Lakomaa, T.M. Comparison of Recoveries by Lead Fire Assay and Nickel Sulfide Fire Assay in the Determination of Gold, Platinum, Palladium and Rhenium in Sulfide Ore Samples. Geostand. Geoanal. Res. 2004, 28, 123–130. [Google Scholar] [CrossRef]
- Plessen, H.G.; Erzinger, J. Determination of the Platinum-Group Elements and Gold in Twenty Rock Reference Materials by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) after Pre-Concentration by Nickel Sulfide Fire Assay. Geostand. Geoanal. Res. 1998, 22, 187–194. [Google Scholar] [CrossRef]
- Cerceau, C.; Carvalho, C.F.; Rabelo, C.S. Recovering lead from cupel waste generated in gold analysis by Pb-Fire assay. J. Environ. Manag. 2016, 183, 771–776. [Google Scholar] [CrossRef]
- Porter, K.A.; Kirk, C.; Fearey, D. Elevated Blood Lead Levels Among Fire Assay Workers and Their Children in Alaska, 2010–2011. Public. Health. Rep. 2015, 130, 440–446. [Google Scholar] [CrossRef]
- Asif, M.; Parry, S.J. Elimination of Reagent Blank Problems in the Fire-assay Pre-concentration of the Platinum Group Elements and Gold with a Nickel Sulphide Bead of Less Than One Gram Mass. Analyst 1989, 114, 1057–1059. [Google Scholar] [CrossRef]
- Lu, C.F.; He, H.L.; Zhou, Z.R.; Zhi, X.X.; Li, B.; Zhang, Q. Determination of platinum-group elements and gold in geochemical exploration samples by nickel sulfide fire assay-ICP-MS, II. Reduction of reagent blank. Rock Miner. Anal. 2002, 21, 7–11. [Google Scholar]
- Jackson, S.; Fryer, B.; Gosse, W.; Healey, D.; Longerich, H.; Strong, D. Determination of the precious metals in geological materials by inductively coupled plasma-mass spectrometry (ICP-MS) with nickel sulphide fire-assay collection and tellurium coprecipitation. Chem. Geol. 1990, 83, 119–132. [Google Scholar] [CrossRef]
- Savard, D.; Barnes, S.J.; Meisel, T. Comparison between Nickel-Sulfur Fire Assay Te Co-precipitation and Isotope Dilution with High-Pressure Asher Acid Digestion for the Determination of Platinum-Group Elements, Rhenium and Gold. Geostand. Geoanal. Res. 2010, 34, 281–291. [Google Scholar] [CrossRef]
- Oguri, K.; Shimoda, G.; Tatsumi, Y. Quantitative determination of gold and the platinum-group elements in geological samples using improved NiS fire-assay and tellurium coprecipitation with inductively coupled plasma-mass spectrometry. Chem. Geol. 1999, 157, 189–197. [Google Scholar] [CrossRef]
- Gros, M.; Lorand, J.P.; Luguet, A. Analysis of platinum group elements and gold in geological materials using NiS fire assay and Te coprecipitation; the NiS dissolution step revisited. Chem. Geol. 2002, 185, 179–190. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, M. Nickel sulfide fire assay improved for pre-concentration of platinum-group elements in geological samples: A practical means of ultra-trace analysis combined with inductively coupled plasma-mass spectrometry. Analyst 2005, 130, 664–669. [Google Scholar] [CrossRef]
- Li, C.S.; Chai, C.F.; Li, X.L. Determination of Platinum-Group Elements and Gold in Two Russian Candidate Reference Materials SCHS-1 and SLg-1 by ICP-MS after Nickel Sulfide Fire Assay Preconcentration. Geostand. Geoanal. Res. 1998, 22, 195–197. [Google Scholar] [CrossRef]
- Juvonen, R.; Lakomaa, T.; Soikkeli, L. Determination of gold and the platinum group elements in geological samples by ICP-MS after nickel sulphide fire assay: Difficulties encountered with different types of geological samples. Talanta 2002, 58, 595–603. [Google Scholar] [CrossRef]
- Asif, M.; Parry, S.J.; Malik, H. Instrumental neutron activation analysis of a nickel sulphide fire assay button to determine the platinum- group elements and gold. Analyst 1992, 117, 1351–1353. [Google Scholar] [CrossRef]
- Bédard, L.P.; Barnes, S.J. A comparison of the capacity of FA-ICP-MS and FA-INAA to determine platinum-group elements and gold in geological samples. J. Radioanal. Nucl. Chem. 2002, 254, 319–329. [Google Scholar] [CrossRef]
- Jarvis, K.E.; Williams, J.G.; Parry, S.J.; Bertalan, E. Quantitative determination of the platinum-group elements and gold using NiS fire assay with laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). Chem. Geol. 1995, 124, 37–46. [Google Scholar] [CrossRef]
- Jorge, A.P.S.; Enzweiler, J.; Shibuya, E.K. Platinum-Group Elements and Gold Determination in NiS Fire Assay Buttons by UV Laser Ablation ICP-MS. Geostand. Geoanal. Res. 1998, 22, 47–55. [Google Scholar] [CrossRef]
- Resano, M.; McIntosh, K.S.; Vanhaecke, F. Laser ablation-inductively coupled plasma-mass spectrometry using a double- focusing sector field mass spectrometer of Mattauch–Herzog geometry and an array detector for the determination of platinum group metals and gold in NiS buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 2012, 27, 165–173. [Google Scholar]
- Resano, M.; Ruiz, E.G.; McIntosh, K.S.; Vanhaecke, F. Laser ablation-inductively coupled plasma-dynamic reaction cell-mass spectrometry for the determination of platinum group metals and gold in NiS buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 2008, 23, 1599–1609. [Google Scholar] [CrossRef]
- Resano, M.; Ruiz, E.G.; McIntosh, K.S.; Hinrichs, J. Comparison of the solid sampling techniques laser ablation-ICP-MS, glow discharge-MS and spark-OES for the determination of platinum group metals in Pb buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 2006, 21, 899–909. [Google Scholar] [CrossRef]
- Vanhaecke, F.; Resano, M.; Koch, J. Femtosecond laser ablation-ICP-mass spectrometry analysis of a heavy metallic matrix: Determination of platinum group metals and gold in lead fire-assay buttons as a case study. J. Anal. At. Spectrom. 2010, 25, 1259–1267. [Google Scholar] [CrossRef]
- Compernolle, S.; Wambeke, D.; Raedt, I.D. Evaluation of a combination of isotope dilution and single standard addition as an alternative calibration method for the determination of precious metals in lead fire assay buttons by laser ablation-inductively coupled plasma-mass spectrometry. Spectrochim. Acta B 2012, 67, 50–56. [Google Scholar] [CrossRef]
- Xue, G. The Analytical Chemistry of Gold; Aerospace Press: Beijing, China, 1990; pp. 74–75. [Google Scholar]
- Enzweiler, J.; Potts, P.J. The separation of platinum, palladium and gold from silicate rocks by the anion exchange separation of chloro complexes after a sodium peroxide fusion: An investigation of low recoveries. Talanta 1995, 42, 1411–1418. [Google Scholar] [CrossRef]
- Dai, X.X.; Koeberl, C.; Fröschl, H. Determination of platinum group elements in impact breccias using neutron activation analysis and ultrasonic nebulization inductively coupled plasma mass spectrometry after anion exchange preconcentration. Anal. Chim. Acta 2001, 436, 79–85. [Google Scholar] [CrossRef]
- Amossé, J. Determination of Platinum-Group Elements and Gold in Geological Matrices by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) after Separation with Selenium and Tellurium Carriers. Geostand. Geoanal. Res. 1998, 22, 93–102. [Google Scholar] [CrossRef]
- Jin, X.D.; Zhu, H.P. Determination of platinum group elements and gold in geological samples with ICP-MS using a sodium peroxide fusion and tellurium co-precipitation. J. Anal. At. Spectrom. 2000, 15, 747–751. [Google Scholar] [CrossRef]
- Qi, L.; Gregoire, D.C.; Zhou, M.F. Determination of Pt, Pd, Ru and Ir in geological samples by ID-ICP-MS using sodium peroxide fusion and Te co-precipitation. Geochem. J. 2003, 37, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Totland, M.M.; Jarvis, I.; Jarvis, K.E. Microwave digestion and alkali fusion procedures for the determination of the platinum-group elements and gold in geological materials by ICP-MS. Chem. Geol. 1995, 124, 21–36. [Google Scholar]
- Jarvis, I.; Totland, M.M.; Jarvis, K.E. Determination of the platinum-group elements in geological materials by ICP-MS using microwave digestion, alkali fusion and cation-exchange chromatography. Chem. Geol. 1997, 143, 27–42. [Google Scholar] [CrossRef]
- Tsimbalist, V.G.; Anoshin, G.N.; Mitkin, V.N.; Razvorotneva, L.L.; Golovanova, N.P. Observations on New Approaches for the Determination of Platinum-Group Elements, Gold and Silver in Different Geochemical Samples from Siberia and the Far East. Geostand. Geoanal. Res. 2000, 12, 171–182. [Google Scholar] [CrossRef]
- Coedo, A.G.; Dorado, M.T.; Padilla, I.; Alguacil, F. Preconcentration and matrix separation of precious metals in geological and related materials using metalfix-chelamine resin prior to inductively coupled plasma mass spectrometry. Anal. Chim. Acta 1997, 340, 31–40. [Google Scholar] [CrossRef]
- Mitkin, V.N.; Galizky, A.A.; Korda, T.M. Some Observations on the Determination of Gold and the Platinum-Group Elements in Black Shales. Geostand. Geoanal. Res. 2000, 24, 227–240. [Google Scholar] [CrossRef]
- Nesbitt, C.C.; Milosavljevic, E.B.; Hendrix, J.L. Determination of the Mechanism of the Chlorination of Gold in Aqueous Solutions. Ind. Eng Chem. Res. 1990, 29, 1696–1700. [Google Scholar] [CrossRef]
- Perry, B.J.; Van Loon, J.C. Dry-chlorination Inductively Coupled Plasma Mass Spectrometric Method for the Determination of Platinum Group Elements in Rocks. J. Anal. At. Spectrom. 1992, 7, 883–888. [Google Scholar] [CrossRef]
- Perry, B.J.; Speller, D.V.; Barefoot, R.R.; Van Loon, J.C. A large sample, dry chlorination, ICP-MS analytical method for the determination of platinum group elements and gold in rocks. Can. J. Appl. Spectrosc. 1993, 38, 131. [Google Scholar]
- Ely, J.C.; Neal, C.R.; O’Neill Jr, J.A.; Jain, J.C. Quantifying the platinum group elements (PGEs) and gold in geological samples using cation exchange pretreatment and ultrasonic nebulization inductively coupled plasma-mass spectrometry (USN-ICP-MS). Chem. Geol. 1999, 157, 219–234. [Google Scholar] [CrossRef]
- Dale, C.W.; Burton, K.W.; Pearson, D.G.; Gannoun, A.; Alard, O.; Argles, T.W.; Parkinson, I.J. Highly siderophile element behaviour accompanying subduction of oceanic crust: Whole rock and mineral-scale insights from a high-pressure terrain. Geochim. Cosmochim. Acta 2009, 73, 1394–1416. [Google Scholar] [CrossRef] [Green Version]
- Dale, C.W.; Macpherson, C.G.; Pearson, D.J.; Hammond, S.J.; Arculus, R.J. Inter-element fractionation of highly siderophile elements in the Tonga Arc due to flux melting of a depleted source. Geochim. Cosmochim. Acta 2012, 89, 202–225. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, P.P.; Liu, J.G. Reassessment of Hydrofluoric Acid Desilicification in the Carius Tube Digestion Technique for Re–Os Isotopic Determination in Geological Samples. Geostand. Geoanal. Res. 2015, 39, 17–30. [Google Scholar] [CrossRef]
- Fryer, B.J.; Kerrich, R. Determination of precious metals at ppb-levels in rocks by a combined wetchemical and flameless atomic absorption method. At. Absorpt. Newsletter. 1978, 17, 4–60. [Google Scholar]
- Sen Gupta, J.G. Determination of trace and ultra-trace amounts of noble metals in geological and related materials by graphite-furnace atomic-absorption spectrometry after separation by ion-exchange or co-precipitation with tellurium. Talanta 1989, 36, 651–656. [Google Scholar] [CrossRef]
- Elson, C.M.; Chatt, A. Determination of Gold in Silicate Rocks and Ores by Coprecipitation with Tellurium and Neutron Activation. Anal. Chim. Acta 1983, 155, 305–310. [Google Scholar] [CrossRef]
- Niskavaara, H.; Kontas, E. Reductive coprecipitation as a separation method for the determination of gold, palladium, platinum, rhodium, silver, selenium and tellurium in geological samples by graphite furnace atomic absorption spectrometry. Anal. Chim. Acta 1990, 231, 273–282. [Google Scholar] [CrossRef]
- Barredo, F.B.; Polo, C.P. The Simultaneous Determination of Gold, Silver and Cadmium at ppb Levels in Silicate Rocks by Atomic Absorption Spectrometry with Electrothermal Atomization. Anal. Chim. Acta 1977, 94, 283–287. [Google Scholar] [CrossRef]
- Sen Gupta, J.G. Determination of noble metals in silicate rocks, ores and metallurgical samples by simultaneous multi-element graphite furnace atomic absorption spectrometry with Zeeman background correction. Talanta 1993, 40, 791–797. [Google Scholar] [CrossRef]
- Meisel, T.; Fellner, N. Moser, J. A simple procedure for the determination of platinum group elements and rhenium (Ru, Rh, Pd, Re, Os, Ir and Pt) using ID-ICP-MS with an inexpensive on-line matrix separation in geological and environmental materials. J. Anal. At. Spectrom. 2003, 18, 720–726. [Google Scholar] [CrossRef]
- Wu, Y.W.; Jiang, Z.C.; Hu, B.; Duan, J.K. Electrothermal vaporization inductively coupled plasma atomic emission spectrometry determination of gold, palladium, and platinum using chelating resin YPA4 as both extractant and chemical modifier. Talanta 2004, 63, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Medved, J.; Bujdoš, M.; Matúš, P.; Kubová, J. Determination of trace amounts of gold in acid-attacked environmental samples by atomic absorption spectrometry with electrothermal atomization after preconcentration. Anal. Bioanal. Chem. 2004, 379, 60–65. [Google Scholar] [CrossRef]
- Terashima, S. Determination of Gold in Sixty Geochemical Reference Samples by Flameless Atomic Absorption Spectrometry. Geostand. Geoanal. Res. 1988, 12, 57–60. [Google Scholar] [CrossRef]
- Terashima, S.; Itoh, S.; Ando, A. Gold in Twenty-Six Janpanese Geochemical Reference Samples. Geostand. Geoanal. Res. 1992, 16, 9–10. [Google Scholar] [CrossRef]
- Ramesh, S.L.; Sunder Raju, P.V.; Anjaiah, K.V.; Mathur, R. Determination of Gold in Rocks, Ores, and Other Geological Materials by Atomic Absorption Techniques. Atom. Spectrosc. 2001, 22, 263–269. [Google Scholar]
- Chattopadhyay, P.; Sahoo, B.N. Modified Decomposition Procedure for the Determination of Gold in Geological Samples by Atomic Absorption Spectrometry. Analyst 1992, 117, 1481–1484. [Google Scholar] [CrossRef]
- Monteiro, M.I.C.; Lavatori, M.P.A.; de Oliveira, N.M.M. Determination of Gold in Ores by Isobutyl Methyl Ketone Extraction and Electrothermal Atomic Absorption Spectrometry: A Stability Study of the Metal in Organic Media. Geostand. Geoanal. Res. 2003, 27, 245–249. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Shamsipur, M.; Ramezani, M. Selective determination of ultra trace amounts of gold by graphite furnace atomic absorption spectrometry after dispersive liquid–liquid microextraction. Talanta 2008, 75, 294–300. [Google Scholar] [CrossRef]
- Kagaya, S.; Takata, D.; Yoshimori, T.; Kanbara, T.; Tohda, K. A sensitive and selective method for determination of gold (III) based on electrothermal atomic absorption spectrometry in combination with dispersive liquid–liquid microextraction using dicyclohexylamine. Talanta 2010, 80, 1364–1370. [Google Scholar] [CrossRef]
- De La Calle, I.; Pena-Pereira, F.; Cabaleiro, N.; Lavilla, I.; Bendicho, C. Ion pair-based dispersive liquid–liquid microextraction for gold determination at ppb level in solid samples after ultrasound-assisted extraction and in waters by electrothermal-atomic absorption spectrometry. Talanta 2011, 84, 109–115. [Google Scholar] [CrossRef]
- Fazelirad, H.; Taher, M.A.; Nasiri-Majd, M. GFAAS determination of gold with ionic liquid, ion pair based and ultrasound-assisted dispersive liquid–liquid microextraction. J. Anal. At. Spectrom. 2014, 29, 2343–2348. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Absorption by Polyurethane Foams: New Method of Separation. J. Chem. Soc. A 1970, 1082–1085. [Google Scholar] [CrossRef]
- Lemos, V.A.; Santos, M.S.; Santos, E.S. Application of polyurethane foam as a sorbent for trace metal pre-concentration—A review. Spectrochim. Acta B 2007, 62, 4–12. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Zolotov, Y.A. Polyurethane foams in chemical analysis: Sorption of various substances and its analytical applications. Russ. Chem. Rev. 2002, 71, 159–174. [Google Scholar] [CrossRef]
- Gesser, H.D. Open-Cell Polyurethane Foam Sponge as a “Solvent Extractor” for Gallium and Iron. Sep. Sci. 1976, 11, 317–327. [Google Scholar] [CrossRef]
- Oren, J.J. The solvent extraction of Fe(III) from acidic chloride solutions by open cell polyurethane foam sponge. Can. J. Chem. 1979, 57, 2023–2036. [Google Scholar] [CrossRef]
- LQ, V.S.K.; Chow, A. Extraction of Tin by Use of Polyurethane Foam. Talanta 1981, 28, 157–164. [Google Scholar]
- Jones, L.; Nel, I.; Koch, K.R. Polyurethane Foams as Selective Sorbents for Noble Metals. Anal. Chim. Acta 1986, 182, 61–70. [Google Scholar] [CrossRef]
- Hamon, R.F. The Cation-Chelation Mechanism of Metal-Ion Sorption by Polyurethanes. Talanta 1982, 29, 313–326. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, Y.H.; Xue, D.S. Synthesis of Amino Polyurethane Foam and Its Application in Trace Gold Enrichment in Geological Samples. Rock. Miner. Anal. 2016, 35, 409–414. [Google Scholar]
- Schiller, P. Determination of Trace Amounts of Gold in Natural Sweet Waters by Non-destructive Activation Analysis after Preconcentration. Anal. Chim. Acta 1971, 54, 364–368. [Google Scholar] [CrossRef]
- Han, J.F. Discussion on the Ability of Different Acid - base Concentration for Foam Plastics to Rich Gold. Anhui. Chem. Ind. 2017, 43, 50–54. [Google Scholar]
- Moawed, E.A. Effect on the Chromatographic Behavior of Gold of the Process Used to Acid-Wash Polyurethane Foam. Chromatographia 2008, 67, 77–84. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, M.J.; Xiong, Y.X. Determination of Trace Gold in Geological Samples Combining Foam Adsorption Inductively Coupled Plasma-Mass Spectrometry with Closed Water Bath Dissolution. J. Central China Normal. Univ. 2017, 51, 626–637. [Google Scholar]
- Zheng, L. Determination of Gold in Geochemical Samples by Foam Adsorption-Graphite Furnace Atomic Absorption Spectrometry. Low Carbon World. 2016, 3, 218–219. [Google Scholar]
- Hu, X.C. Determination of Trace Gold in Geochemical Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS X-2): Foam Adsorption Separation. Technol. Outlook 2015, 23, 140. [Google Scholar]
- Saeed, M.M.; Ghaffar, A. Adsorption Syntax of Au (III) on Unloaded Polyurethane Foam. J. Radioanal. Nucl. Chem. 1998, 232, 171–177. [Google Scholar] [CrossRef]
- Liu, X.L.; Wen, T.Y.; Sun, W.J. Determination of Au and Pt in Geological Samples by Graphite Furnace Atomic Absorption Spectrometry with Concentrate and Extraction by Foam Plastics and Thiourea. Rock. Miner. Anal. 2013, 32, 576–580. [Google Scholar]
- He, Z.L. Determination of Trace Gold in Geochemical Samples by Graphite Furnace Atomic Absorption Spectrometry with Thiourea Adsorbed by Foam. Xinjiang Nonferrous Metal. 2016, 6, 49–50. [Google Scholar]
- Chen, K. Determination of Gold in Geological Samples by Foam Adsorption Atomic Absorption Spectrometry. Chem. Manag. 2018, 2, 208–209. [Google Scholar]
- Peng, Z.J. Determination of Trace Gold by Graphite Furnace Atomic Absorption Spectrometry with Foam Absorption. Guangdong Chem. 2015, 15, 222–223. [Google Scholar]
- Han, K.Y. Study on Determination of Trace Gold in Carbonate Rocks. Acad. BBS 2017, 272. [Google Scholar]
- Xiong, Z.C. Spectrometric Determination of Trace Gold in Rocks and Soils with Polyurethane Foam. Rock. Miner. Anal. 1984, 4, 364–367. [Google Scholar]
- Gu, T.X.; Zhang, Z.; Wang, C.S.; Yan, W.D. Preparation and Certification of High-Grade Gold Ore Reference Materials (GAu 19-22). Geostand. Geoanal. Res. 2001, 25, 153–158. [Google Scholar] [CrossRef]
- Braun, T.; Farag, A.B. Chemical Enrichment and Separation of Gold in the Tributylphosphate-Thiourea-Perchloric acid system. Anal. Chim. Acta 1973, 65, 115–126. [Google Scholar] [CrossRef]
- Braun, T.; Huszar, E. Separation of Trace Amounts of Cobalt from Nickel in the Tri-n-Octylamine-Hydrochloric Acid System. Anal. Chim. Acta 1973, 64, 77–84. [Google Scholar] [CrossRef]
- Yan, M.C.; Wang, C.S.; Cao, Q.X. Eleven Gold Geochemical Reference Samples (GAu 8-18). Geostand. Geoanal. Res. 1995, 19, 125–133. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Bashammakh, A.S.; Al-Sibaai, A.A. Solid phase preconcentration and determination of trace concentrations of total gold (I) and/or (III) in sea and wastewater by ion pairing impregnated polyurethane foam packed column prior flame atomic absorption spectrometry. Int. J. Miner. Process. 2011, 100, 110–115. [Google Scholar] [CrossRef]
- Bashammakh, A.S.; Bahhafi, S.O.; Al-Shareef, F.M.; El-Shahawi, M.S. Development of an Analytical Method for Trace Gold in Aqueous Solution Using Polyurethane Foam Sorbents: Kinetic and Thermodynamic Characteristic of Gold (III) Sorption. Anal. Sci. 2009, 25, 413–418. [Google Scholar] [CrossRef]
- El-Shahat, M.F.; Moawed, E.A.; Farag, A.B. Chemical enrichment and separation of uranyl ions in aqueous media using novel polyurethane foam chemically grafted with different basic dyestuff sorbents. Talanta 2007, 71, 236–241. [Google Scholar] [CrossRef]
- Xue, D.S.; Wang, H.Y.; Liu, Y.H. Cytosine-functionalized polyurethane foam and its use as a sorbent for the determination of gold in geological samples. Anal. Methods. 2016, 8, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Moawed, E.A.; Zaid, M.A.A.; El-Shahat, M.F. The chromatographic behavior of group (IIB) metal ions on polyurethane foam functionalized with 8-hydroxyquinoline. Anal. Bioanal. Chem. 2004, 378, 470–478. [Google Scholar]
- Moawed, E.A.; Moawed, M.F. Synthesis, characterization of low density polyhydroxy polyurethane foam and its application for separation and determination of gold in water and ores samples. Anal. Chim. Acta 2013, 788, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Pohlandt, C.; Steele, T.W. Separation of the non-volatile noble metals by reversed-phase extraction chromatography. Talanta 1972, 19, 839–850. [Google Scholar] [CrossRef]
- Pohlandt, C.; Steele, T.W. Chromatographic determination of matte-leach separation and noble metals in residues. Talanta 1974, 21, 919–925. [Google Scholar] [CrossRef]
- Bao, G.M. Determination of Trace Gold in Ores by Extraction Layer-Atom Absorption Method. Chin. J. Anal. Chem. 1977, 6, 428–431. [Google Scholar]
- Hassan, J.; Shamsipur, M.; Karbasi, M.H. Single granular activated carbon microextraction and graphite furnace atomic absorption spectrometry determination for trace amount of gold in aqueous and geological samples. Microchem. J. 2011, 99, 93–96. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Mróz, A.; Dąbrowska, M.; Olszański, P. Solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry for gold determination in geological samples after preconcentration onto carbon nanotubes. Spectrochim. Acta B 2017, 132, 13–18. [Google Scholar] [CrossRef]
- Xue, D.S.; Wang, H.Y.; Liu, Y.H.; Shen, P. Multicolumn solid phase extraction with hybrid adsorbent and rapid determination of Au, Pd and Pt in geological samples by GF-AAS. Miner. Eng. 2015, 81, 149–151. [Google Scholar] [CrossRef]
- Ye, J.J.; Liu, S.X.; Tian, M.M. Preparation and characterization of magnetic nanoparticles for the on-line determination of gold, palladium, and platinum in mine samples based on flow injection micro-column preconcentration coupled with graphite furnace atomic absorption spectrometry. Talanta 2014, 118, 231–237. [Google Scholar] [CrossRef]
- Constantin, M. Trace Element Data for Gold, Iridium and Silver in Seventy Geochemical Reference Materials. Geostand. Geoanal. Res. 2009, 33, 115–132. [Google Scholar] [CrossRef]



| Sample Weight/g | Collector/Flux | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|
| 10–30 | Pb/Na2CO3, Na2B4O7, SiO2, flour(C) | ICP-MS | 2 | [38] | |
| 50 | NiS/Na2CO3, Na2B4O7, CaF2 | ICP-MS | 0.023 | [41] | |
| 15 | NiS/Na2CO3, Na2B4O7, SiO2 | Te coprecipitation | ICP-MS | 1.69 | [46] |
| 15 | NiS/Na2CO3, Na2B4O7, SiO2 | Te coprecipitation | ICP-MS | 0.484 | [47] |
| 20 | NiS/Na2CO3, Na2B4O7, SiO2 | duplicate NiS-FA and Te coprecipitation | ICP-MS | 0.053 | [48] |
| 20 | NiS/Na2CO3, Li2B4O7, SiO2 | Te coprecipitation | ICP-MS | 0.33 | [49] |
| 10 | NiS/Na2CO3, Na2B4O7 | INAA | 2 | [53] | |
| 10–15 50 | NiS/Na2CO3, Na2B4O7 Pb/Na2CO3, NaOH, Na2B4O7, SiO2, C | UV-LA-ICP-MS FS-LA-ICP-MS | 1.7 4 | [54,60] |
| Sample Weight/g | Crucible | Flux | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|---|
| 0.5 | zirconium | Na2O2 | Anion resin | GFAAS | - | [63] |
| 1.0 | graphite | Na2O2 | Anion resin | USN-ICP-MS+NAA | ppt/- | [64] |
| 1–20 | zirconium | Na2O2/NaKCO3/KOH | Se-Te coprecipitation | ICP-MS | 0.58 | [65] |
| 1–20 2 | Corundum Corundum | Na2O2 Na2O2 | Te coprecipitation Te coprecipitation | ICP-MS ICP-MS | 0.007 0.32 | [66,67] |
| Sample Weight/g | Digestion | Dissolution | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|---|
| 2–5 | Teflon beaker | HF + aq.reg. | Te coprecipitation | GFAAS | - | [81] |
| 0.3–1.3 | Teflon beaker | HNO3 + HF + HClO4 + HCl | Te coprecipitation | INAA | 0.7 | [82] |
| 0.5–1.5 | PTFE bomb | HNO3 + HF + aq.reg. + HClO4 + HCl | Se coprecipitation | GFAAS/TXRF | 0.2/1.2 | [26] |
| 0.5 | Borosilicate tube | HCl + HNO3 | Hg coprecipitation | GFAAS | 0.3 | [83] |
| Sample Weight/g | Digestion | Dissolution | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|---|
| 2 | - | HNO3 + HCl + HClO4 + HF | Anion exchange resin | GFAAS | 0.2 | [84] |
| 5 | Teflon beaker | HF + aq.reg. + HNO3 + HCl | Cation exchange resin | GFAAS | - | [85] |
| 2 | Carius tube/HPA-s | HCl + HNO3 | Cation exchange resin | ICP-MS | - | [5] |
| 0.25 | Microwave digestion | aq.reg. + HF + HClO4 | Chelating resin | FI-ICP-MS | 1.2 | [71] |
| 0.05–1.5 | Mild heating | HNO3 + HClO4 + HF | Chelating resin | ETV-ICP-AES | 0.075 | [87] |
| 5–10 | - | aq.reg. | Chelating sorbent | GFAAS | 0.5 | [88] |
| Sample Weight/g | Digestion | Dissolution | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|---|
| 0.1–2.0 | Borosilicate beaker | HNO3 + HCl | MIBK | GFAAS | - | [89] |
| 0.5–2.0 | Teflon beaker | aq.reg. + HF | MIBK | GFAAS | - | [90] |
| 1 | PFTE beaker | HClO4 + HF + aq.reg. | diethyl ether + MIBK | GFAAS | 0.13 | [27] |
| 10 | Glass beaker | aq.reg. | MIBK | GFAAS | 0.1 | [91] |
| 5–10 | Borosilicate beaker | HBr + Br2 | IBMK | GFAAS | 15 | [92] |
| 10 | Erlenmeyer flask | HCl + HNO3 | IBMK | GFAAS | 0.2 | [93] |
| 0.2 | - | aq.reg. | DLLME | GFAAS | 0.005 | [95] |
| 0.003–0.03 | Eppednorf vail | HNO3 + HCl | DLLME | GFAAS | 1.5 | [97] |
| 0.02 | - | HNO3 + HCl + HF | DLLME | GFAAS | 0.002 | [98] |
| Sample Weight/g | Digestion | Dissolution | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|---|
| 10 | Polypropylene beaker | aq.reg. | PUF | GFAAS | 0.23 | [115] |
| 10–20 | - | aq.reg. | MIBK-loaded PUF | GFAAS | - | [124] |
| 4 | Teflon pot | HNO3 + HF + HCl + aq.reg. | DIBK-loaded CG71 resin | ICP-MS | 0.002 | [36] |
| 0.2 | Microwave vessel | aq.reg. | Single granular carbon | GFAAS | 0.9 | [134] |
| 0.5 | Microwave vessel | aq.reg. | Modified carbon nanotubes | SS-HR-CS-GFAAS | 0.002 | [135] |
| 10 | PFA vessel | aq.reg. | hybrid adsorbent | GFAAS | 0.008 | [136] |
| 5–10 | Hot-plate | aq.reg. | magnetic nanoparticles | FI-column-GFAAS | 0.16 | [137] |
| Sample Weight/g | Dissolution | Separation Technique | Detection Technique | LOD/ppb | Reference |
|---|---|---|---|---|---|
| 15 | NiS/Na2CO3, Na2B4O7, SiO2 | Te coprecipitation | ICP-MS | 0.484 | [47] |
| 1–20 | Na2O2 | Te coprecipitation | ICP-MS | 0.007 | [66] |
| 0.5–1.5 | HNO3 + HF + aq.reg. + HClO4 + HCl | Se coprecipitation | GFAAS/TXRF | 0.2/1.2 | [26] |
| 2 | HCl + HNO3 | Cation exchange resin | ICP-MS | - | [5] |
| 0.02 | HNO3 + HCl + HF | DLLME | GFAAS | 0.002 | [98] |
| 10 | aq.reg. | PUF | GFAAS | 0.23 | [115] |
| 4 | HNO3 + HF + HCl + aq.reg. | DIBK-loaded CG71 resin | ICP-MS | 0.002 | [36] |
| 0.2 | aq.reg. | Single granular carbon | GFAAS | 0.9 | [134] |
| 5–10 | aq.reg. | magnetic nanoparticles | FI-column-GFAAS | 0.16 | [137] |
| 1–3 | - | - | INAA | ~0.1 | [138] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-h.; Wan, B.; Xue, D.-s. Sample Digestion and Combined Preconcentration Methods for the Determination of Ultra-Low Gold Levels in Rocks. Molecules 2019, 24, 1778. https://doi.org/10.3390/molecules24091778
Liu Y-h, Wan B, Xue D-s. Sample Digestion and Combined Preconcentration Methods for the Determination of Ultra-Low Gold Levels in Rocks. Molecules. 2019; 24(9):1778. https://doi.org/10.3390/molecules24091778
Chicago/Turabian StyleLiu, Yan-hong, Bo Wan, and Ding-shuai Xue. 2019. "Sample Digestion and Combined Preconcentration Methods for the Determination of Ultra-Low Gold Levels in Rocks" Molecules 24, no. 9: 1778. https://doi.org/10.3390/molecules24091778