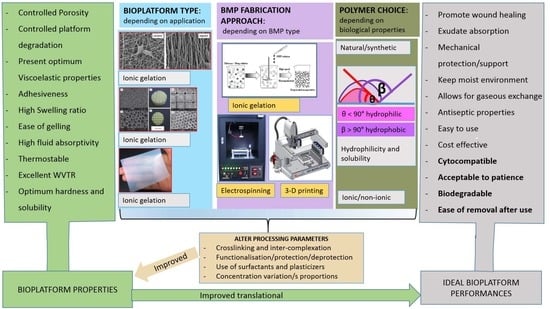
Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings
Abstract
:1. Introduction
2. Polymer Structural Interactions Affecting BMP Property–Performance Factors
3. Effect of Polymer Processing on Physical and Mechanical Properties of BMPs
4. Fabrication Approaches Affecting Physical and Mechanical Properties of Chitosan-Based Interpolymer Complexes
4.1. Scaffold Fabrication Approaches and Their Physical and Physicochemical Properties Impacting BMP Performance
4.1.1. Three-Dimensional Printing of Chitosan-Based IPC BMPs
4.1.2. Ionic Gelation Technique Employed for Fabrication of Chitosan-Based IPC BMPs
4.2. Fibre Physical and Physicochemical Properties Impacting BMP Performance
4.2.1. Electrospun Chitosan-Based IPC BMPs
4.2.2. Ionic Gelation Technique Employed in Fabrication of Chitosan-Based IPC BMPs
4.3. Gels, Hydrogels, and Membranes Physical and Physicochemical Properties Impacting BMP Performance
4.3.1. Ionic Gelation Technique Employed in the Fabrication of Chitosan-Based IPC BMPs
4.3.2. Polymer Coating, Grafting, Solvent Evaporation, and Solvent Casting Approaches in Fabricating Chitosan-Based IPC BMPs
4.4. Sponges Physical and Physicochemical Properties Impacting BMP Performance
4.4.1. Ionic Gelation Technique Employed in Fabrication of Chitosan-Based IPC BMPs
4.4.2. Phase Separation and Grafting Approach for the Fabrication of Chitosan-Based IPC BMPs
5. Standard BMP Properties for Wound Dressing Applications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, K.; Edwards, R. Bacteria and Wound Healing; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; Volume 17. [Google Scholar]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Sweeney, I.; Miraftab, M.; Collyer, G. Absorbent alginate fibres modified with hydrolysed chitosan for wound care dressings–II. Pilot scale development. Carbohydr. Polym. 2014, 102, 920–927. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.; Carvalho, S.; Quintas, M.A.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Ke, G.; Xu, W.; Yu, W. Preparation and properties of drug-loaded chitosan-sodium alginate complex membrane. Int. J. Polym. Mater. 2010, 59, 184–191. [Google Scholar] [CrossRef]
- Costa, R.R.; Costa, A.M.; Caridade, S.G.; Mano, J.o.F. Compact saloplastic membranes of natural polysaccharides for soft tissue engineering. Chem. Mater. 2015, 27, 7490–7502. [Google Scholar] [CrossRef]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-induced changes in the fabrication of multilayers of poly (acrylic acid) and chitosan: Fabrication, properties, and tests as a drug storage and delivery system. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Chuliá-Jordán, R.; Ortega, F.; Rubio, R.G. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly (acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of polymeric biomaterials: A strategy for tissue engineering and medical devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef] [Green Version]
- Matějka, L.; Janata, M.; Pleštil, J.; Zhigunov, A.; Šlouf, M. Self-assembly of POSS-containing block copolymers: Fixing the hierarchical structure in networks. Polymer 2014, 55, 126–136. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Rice, M.A.; Rydholm, A.E.; Salinas, C.N.; Shah, D.N.; Anseth, K.S. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog. Polym. Sci. 2008, 33, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on chitin. VI. Binding of metal cations. J. Appl. Polym. Sci. 1979, 23, 511–515. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Lai, H.L.; Abu’Khalil, A.; Craig, D.Q. The preparation and characterisation of drug-loaded alginate and chitosan sponges. Int. J. Pharm. 2003, 251, 175–181. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.-N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef]
- Wu, P.; Nelson, E.; Reid, W.; Ruckley, C.; Gaylor, J. Water vapour transmission rates in burns and chronic leg ulcers: Influence of wound dressings and comparison with in vitro evaluation. Biomaterials 1996, 17, 1373–1377. [Google Scholar] [CrossRef]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci 2000, 3, 303–311. [Google Scholar]
- Wittaya-areekul, S.; Prahsarn, C. Development and in vitro evaluation of chitosan–polysaccharides composite wound dressings. Int. J. Pharm. 2006, 313, 123–128. [Google Scholar] [CrossRef]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin cross-linked porous chitosan–collagen–gelatin scaffolds using chitosan–CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef]
- Pierog, M.; Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Effect of ionic crosslinking agents on swelling behavior of chitosan hydrogel membranes. Prog. Chem. Appl. Chitin Deriv. Pol. Chitin Soc. Łódź 2009, 75, 82. [Google Scholar]
- Gonçalves, V.L.; Laranjeira, M.; Fávere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polímeros 2005, 15, 6–12. [Google Scholar] [CrossRef]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.; Choonara, Y.E.; Pillay, V. Development of a Fluid-absorptive Alginate-Chitosan Bioplatform for Potential Application as a Wound Dressing. Carbohydr. Polym. 2019, 222, 114988. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Kweon, D.-K.; Song, S.-B.; Park, Y.-Y. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials 2003, 24, 1595–1601. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Y.; Song, S.; Tong, C.; Shi, X.; Zhao, Y.; Zhang, J.; Hou, M. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Tangsadthakun, C.; Kanokpanont, S.; Sanchavanakit, N.; Banaprasert, T.; Damrongsakkul, S. Properties of collagen/chitosan scaffolds for skin tissue engineering. J. Met. Mater. Miner. 2017, 16, 37–44. [Google Scholar]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.; Anulekha, K.; Nair, S.V.; Nair, S.; Chennazhi, K.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Hong, Y.S. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Bonassar, L.J.; Vacanti, C.A. Tissue engineering: The first decade and beyond. J. Cell. Biochem. 1998, 72, 297–303. [Google Scholar] [CrossRef]
- Atala, A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [Green Version]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yi, J.; Tong, J.; Zhou, X.; Ge, H.; Zou, S.; Wen, H.; Nie, M. Preparation and characterization of Oxidized konjac glucomannan/Carboxymethyl Chitosan/Graphene Oxide hydrogel. Int. J. Biol. Macromol. 2016, 91, 358–367. [Google Scholar] [CrossRef]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Chen, K.; He, X.; Li, N.; Huang, J.; Tang, K.; Li, Y.; Wang, F. Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 2016, 91, 15–22. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.; Park, S.; Kim, G.; Hwang, Y.; Lee, C.; Shin, H.; Lee, J. Development of high efficiency nanofilters made of nanofibers. Curr. Appl. Phys. 2006, 6, 1030–1035. [Google Scholar] [CrossRef]
- Gu, S.-Y.; Wang, Z.-M.; Ren, J.; Zhang, C.-Y. Electrospinning of gelatin and gelatin/poly (l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Park, W.H.; Jeong, L.; Yoo, D.I.; Hudson, S. Effect of chitosan on morphology and conformation of electrospun silk fibroin nanofibers. Polymer 2004, 45, 7151–7157. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Lee, Y.J.; Kim, J.-I.; Kim, C.-H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int. J. Biol. Macromol. 2016, 82, 89–96. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Fang, J.; Wang, H.; Cheng, T.; Wang, X. Using chitosan as a thickener for electrospinning dilute PVA solutions to improve fibre uniformity. Nanotechnology 2006, 17, 3718. [Google Scholar] [CrossRef]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.; Luyt, A. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H. Electrospinning of chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Jancar, J.; Massoud, D.; Fohlerova, Z.; Elhadidy, H.; Spotz, Z.; Hebeish, A. Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int. J. Pharm. 2016, 510, 86–99. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Cuadros, T.R.; Erices, A.A.; Aguilera, J.M. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. J. Mech. Behav. Biomed. Mater. 2015, 46, 331–342. [Google Scholar] [CrossRef]
- Kassem, M.A.; ElMeshad, A.N.; Fares, A.R. Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: Formulation and in vitro evaluation. AAPS PharmSciTech 2015, 16, 537–547. [Google Scholar] [CrossRef]
- Robitzer, M.; Tourrette, A.; Horga, R.; Valentin, R.; Boissière, M.; Devoisselle, J.-M.; Di Renzo, F.; Quignard, F. Nitrogen sorption as a tool for the characterisation of polysaccharide aerogels. Carbohydr. Polym. 2011, 85, 44–53. [Google Scholar] [CrossRef]
- Hoseinpour Najar, M.; Minaiyan, M.; Taheri, A. Preparation and in vivo evaluation of a novel gel-based wound dressing using arginine–alginate surface-modified chitosan nanofibers. J. Biomater. Appl. 2018, 32, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Cegłowski, M.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Molecularly imprinted polymer as drug delivery carrier in alginate dressing. Mater. Lett. 2017, 201, 46–49. [Google Scholar] [CrossRef]
- Mozalewska, W.; Czechowska-Biskup, R.; Olejnik, A.K.; Wach, R.A.; Ulański, P.; Rosiak, J.M. Chitosan-containing hydrogel wound dressings prepared by radiation technique. Radiat. Phys. Chem. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Yang, D.H.; Seo, D.I.; Lee, D.-W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Anjum, S.; Arora, A.; Alam, M.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Xia, G.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Surface fluid-swellable chitosan fiber as the wound dressing material. Carbohydr. Polym. 2016, 136, 860–866. [Google Scholar] [CrossRef]
- Dutra, J.; Carvalho, S.; Zampirolli, A.; Daltoé, R.; Teixeira, R.; Careta, F.; Cotrim, M.; Oréfice, R.; Villanova, J. Papain wound dressings obtained from poly (vinyl alcohol)/calcium alginate blends as new pharmaceutical dosage form: Preparation and preliminary evaluation. Eur. J. Pharm. Biopharm. 2017, 113, 11–23. [Google Scholar] [CrossRef]
- Du, T.; Chen, Z.; Li, H.; Tang, X.; Li, Z.; Guan, J.; Liu, C.; Du, Z.; Wu, J. Modification of collagen–chitosan matrix by the natural crosslinker alginate dialdehyde. Int. J. Biol. Macromol. 2016, 82, 580–588. [Google Scholar] [CrossRef]
- Lu, B.; Wang, T.; Li, Z.; Dai, F.; Lv, L.; Tang, F.; Yu, K.; Liu, J.; Lan, G. Healing of skin wounds with a chitosan–gelatin sponge loaded with tannins and platelet-rich plasma. Int. J. Biol. Macromol. 2016, 82, 884–891. [Google Scholar] [CrossRef]
- Bajpai, M.; Bajpai, S.; Jyotishi, P. Water absorption and moisture permeation properties of chitosan/poly (acrylamide-co-itaconic acid) IPC films. Int. J. Biol. Macromol. 2016, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zou, S.; Ge, H.; Xiao, Y.; Wen, H.; Feng, H.; Liu, M.; Nie, M. Preparation and characterization of hydroxypropyl chitosan modified with collagen peptide. Int. J. Biol. Macromol. 2016, 93, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-W.; Ko, S.-C.; Je, J.-Y.; Kim, Y.-M.; Oh, J.; Jung, W.-K. Fabrication, characterization and determination of biological activities of poly (ε-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int. J. Biol. Macromol. 2016, 93, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified chitosan with poly (2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef]
- Ozseker, E.E.; Akkaya, A. Development of a new antibacterial biomaterial by tetracycline immobilization on calcium-alginate beads. Carbohydr. Polym. 2016, 151, 441–451. [Google Scholar] [CrossRef]
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E.G.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohydr. Polym. 2017, 157, 814–822. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym 2017, 158, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Zorzi Bueno, C.; Maria Moraes, Â. Development of porous lamellar chitosan-alginate membranes: Effect of different surfactants on biomaterial properties. J. Appl. Polym. Sci. 2011, 122, 624–631. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-i.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 1800069. [Google Scholar] [CrossRef]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan–Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Amin, K.A.M. Reinforced materials based on chitosan, TiO2 and Ag composites. Polymers 2012, 4, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Cano, L.; Pollet, E.; Avérous, L.; Tercjak, A. Effect of TiO2 nanoparticles on the properties of thermoplastic chitosan-based nano-biocomposites obtained by mechanical kneading. Compos. Part A Appl. Sci. Manuf. 2017, 93, 33–40. [Google Scholar] [CrossRef]
- Temtem, M.; Casimiro, T.; Mano, J.F.; Aguiar-Ricardo, A. Preparation of membranes with polysulfone/polycaprolactone blends using a high pressure cell specially designed for a CO2-assisted phase inversion. J. Supercrit. Fluids 2008, 43, 542–548. [Google Scholar] [CrossRef]
- Song, A.; Rane, A.A.; Christman, K.L. Antibacterial and cell-adhesive polypeptide and poly (ethylene glycol) hydrogel as a potential scaffold for wound healing. Acta Biomater. 2012, 8, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, S.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrod, O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand 1970, 24, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Becker, T.A.; Kipke, D.R.; Brandon, T. Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization. J. Biomed. Mater. Res. Part A 2001, 54, 76–86. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Wu, Y.-P.; Fu, Z.-F.; Tian, Y.; Li, C.-J.; Gao, C.-Y.; Chen, Z.-L.; Feng, X.-Z. Cell adhesive and growth behavior on electrospun nanofibrous scaffolds by designed multifunctional composites. Colloids Surf. B Biointerfaces 2011, 84, 26–34. [Google Scholar] [CrossRef]






| Polymer Composite and Approach | BMP System | Platform Properties | Platform Performance | Ref. |
|---|---|---|---|---|
| Dissolution of CS/Alg crosslinked with TTP and CaCl2 | Gels | Constant pore size, thermal stability, rheology, chemical stability | Biodegradation, cell proliferation, antibacterial activity | [84] |
| Mixing glycerol and molecularly imprinted polymer solutions | Gel | Drug release controlled by diffusion and swelling | Antibacterial activity but no significant data for improved wound healing | [84] |
| Ionic gelation and irradiation | Gel | Decreased crosslinking increased water uptake and platform elasticity | Inhibits Gram-positive bacteria and does not inhibits Gram-negative bacteria | [85] |
| Polymer coating and irradiation | Hydrogel | Increased elasticity, maintained 3-D porosity | Burst and sustained release, accelerated wound healing | [86] |
| Polymer coating | Membrane | Increased tensile strength and reduced porosity | Antibacterial, accelerated wound healing with minimum scar formation | [87] |
| Polymer coating | Scaffold | Increased solubility and water uptake | Antimicrobial and wound healing | [88] |
| Polymer casting | Film | transparent, soft, flexible | increased cell proliferation | [89] |
| Electrospinning and ultra-sonication | Scaffold | Porous, decreased tensile strength | blood clotting efficiency, cell viability and cell infiltration | [90] |
| Ionic gelation | Sponge | Porous, decreased tensile strength | Antibacterial properties, improved healing | [91] |
| Solvent evaporation | Film | pH dependent swelling ration | No platform performance data presented | [92] |
| Ionic gelation and freeze-drying | Scaffold | Porous, high swelling ratio, Slow degradation | Antibacterial activity | [93] |
| Electrospinning | Scaffold | Micro-size porous structure | Aid cell attachment and proliferation | [94] |
| Electrospinning | Bilayer membrane | 3-D porous structure | Antibacterial properties with maintained cell viability | [65] |
| Grafting | - | - | Improved water uptake | [60] |
| Solvent casting | Film | Enhanced tensile strength, decreased flexibility | High water uptake, aid wound healing | [95] |
| Solvent casting | Membrane | Porous, enhanced mechanical properties | Aid cell proliferation and maintains cell viability | [96] |
| Ionic gelation | Hydrogel | Small pore size porous structure, elastic and biodegradable | Cytocompatible, antibacterial properties, aid wound healing, decreased blood loss | [97] |
| Solvent casting | Film | Flexible, moderate drug release | Low cytotoxicity | [98] |
| Free radical polymerisation | Sponge | Porous and flexible | 90% burst release in 4 h | [99] |
| Solvent droplet | Beads | - | Prolonged antibacterial activity | [100] |
| Ionic gelation | Sponge | Porous, high swelling ratio | Antibacterial, aid wound healing | [37] |
| Ionic gelation | Hydrogel | Porous and high swelling ratio | Aid cell growth | [101] |
| Padding and ionotropic gelation | Gauze and nanoparticles | Moderate water uptake | Poor antimicrobial properties | [102] |
| Electrospining | Scaffold | Nano size | Antibacterial, antioxidant and accelerated wound healing | [46] |
| Dissolution | Sponges | Porous thereby facilitating high fluid absorption | Cytocompatible and antibacterial activity | [103] |
| Solution casting | Membranes | Porous, high swelling ratio | Cytocompatible | [38] |
| Electrospinning | Nanofiber | Porous and High swelling ratio | Antimicrobial properties | [75] |
| Ionic gelation | Sponge | Porous, low tensile strength and high swelling ratio | Antibacterial properties with inflammation induction in cells | [104] |
| Ionic gelation | Gel | Porous structures, high drug loading, and swelling | - | [59] |
| Needle punching process | Gauze | Porous thereby facilitating high fluid absorption | Aid blood clotting, and blood absorption | [105] |
| Polymer coating | Membrane microfiber | Porous structure | Enhanced wound healing | [87] |
| Dissolution with wet-dry-spinning | Mats | Maintained thermal stability with improved tensile strength | Enhanced wound closure and cell attachment | [78] |
| Ionic gelation and droplet extrusion | Hydrogel | Lowered swelling ratios, stable mechanical properties (G′ and G″) | Effective antibacterial properties | [36] |
| Ionic gelation | Membranes | Higher tensile strength, acceptable fluid uptake, improved polymer dispersion and porosity | no antibacterial properties | [106] |
| Ionic gelation | Hydrogel sheets | High fluid uptake | Stimulated wound healing | [107] |
| Ionic gelation approach | Hydrogel | Optimum mechanical properties, porous, and high fluid uptake | Fast re-epithelialisation and formation of granulation tissues rate | [108] |
| Ionic gelation | Hydrogel | Optimum mechanical properties | Enhanced wound healing rate | [42] |
| Ionic gelation | Fibres | Optimum fluid uptake and mechanical properties | Cytocompatible, Improved wound healing and EGF expression | [48] |
| Ionic gelation | Nanofibers | Nano size and high viscosity | Enhanced wound healing rate | [83] |
| Chitosan Interactive Compounds (Polymers, Lipids, and Proteins) | Interactive Bioactives | Crosslinkers | Ref. |
|---|---|---|---|
| Silk fibroin | - | - | [67] |
| Gelatine | Fe3O4 | - | [68] |
| Gelatine | - | Glutaraldehyde | [71] |
| Alginate | AgNPs | CaCl2 | [75] |
| Methoxy poly(ethylene glycol) | VEGF-PDGF-BB | Visible light irradiation and glycidyl methacrylate | [86] |
| Partially oxidised Bletilla striatapolysaccharide | AgNPs | Genipin | [109] |
| Collagen | - | Alginate | [90] |
| Collagen | - | Tannin acid | [91] |
| - | Ag–ZnO | - | [37] |
| Glutamic acid and Hyaluronic acid | Ag | - | [104] |
| Alginate | - | CaCl2 | [59] |
| Collagen | TiO2 | - | [60] |
| Polyethylene glycol | - | - | [87] |
| Collagen | - | Transglutaminase biocatalyst | [93] |
| - | TiO2 | - | [96,110,111] |
| PVA and cyclodextrins | Ibuprofen | - | [38,112] |
| Polyacrylamide | - | Itaconic acid | [92] |
| - | - | Succinic anhydride | [88] |
| - | Silver sulfadiazine | - | [103] |
| Glucan | - | - | [78] |
| Gelatine | - | Glutaraldehyde | [58] |
| Gelatine | - | - | [44] |
| Alginate | - | CaCl2 as crosslinker, Pluronic F68 and Tween-80 as surfactants | [106] |
| Alginate | Fucoidan | CaCl2 and ethylene glycol diglycidyl ether | [107] |
| Alginate | Epidermal growth factor | CaCl2 and epidermal growth factor | [108] |
| Alginate | D-glucono-δ-lactone | - | [42] |
| Alginate and collagen | - | - | [48] |
| Alginate | Arginine | - | [83] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mndlovu, H.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings. Molecules 2020, 25, 222. https://doi.org/10.3390/molecules25010222
Mndlovu H, du Toit LC, Kumar P, Choonara YE, Marimuthu T, Kondiah PPD, Pillay V. Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings. Molecules. 2020; 25(1):222. https://doi.org/10.3390/molecules25010222
Chicago/Turabian StyleMndlovu, Hillary, Lisa C. du Toit, Pradeep Kumar, Yahya E. Choonara, Thashree Marimuthu, Pierre P. D. Kondiah, and Viness Pillay. 2020. "Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings" Molecules 25, no. 1: 222. https://doi.org/10.3390/molecules25010222