Hierarchical Structure of the Cocos nucifera (Coconut) Endocarp: Functional Morphology and its Influence on Fracture Toughness
Abstract
1. Introduction
2. Results
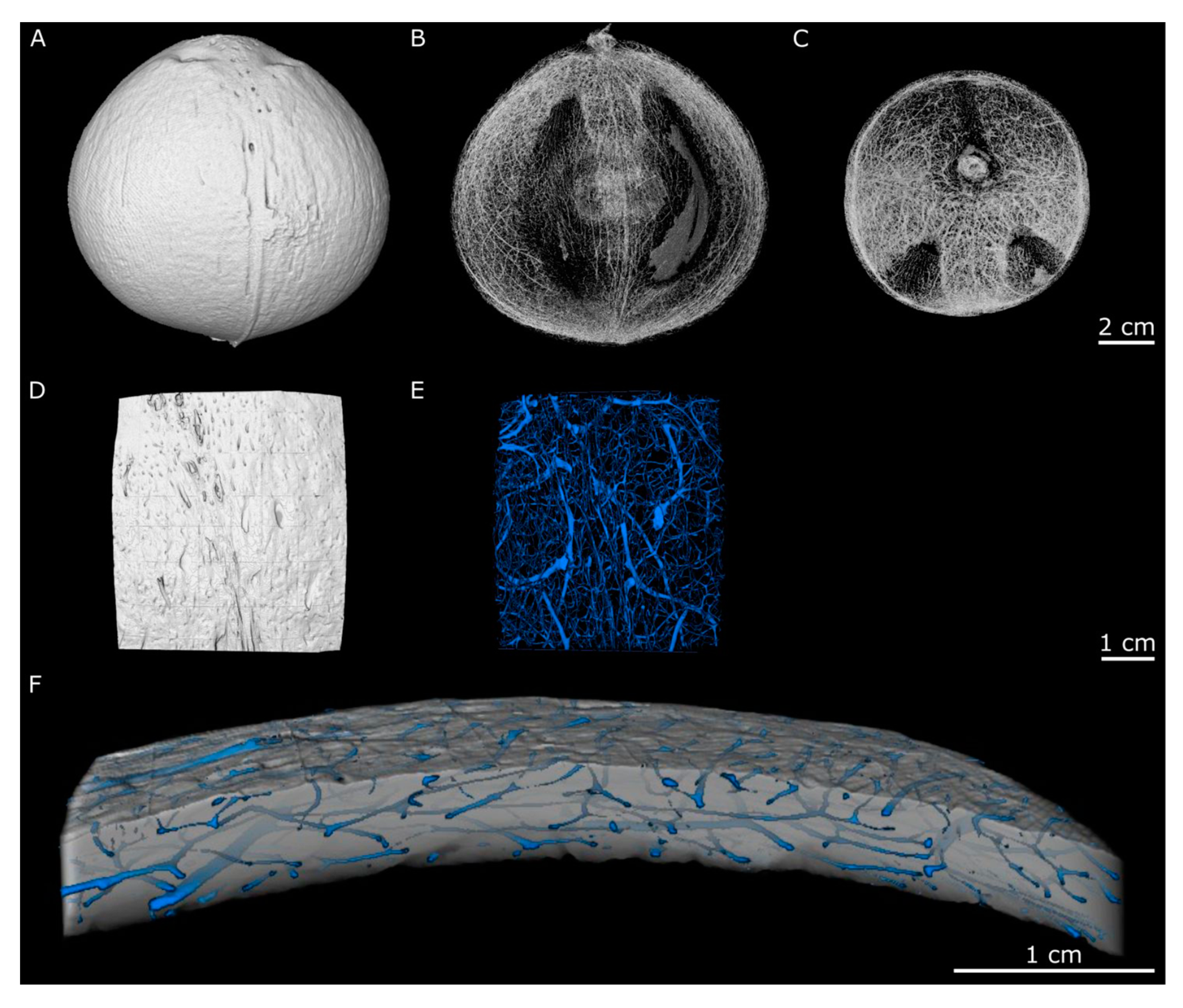
2.1. Hierarchical Level H2—Endocarp
2.2. Hierarchical Level H3
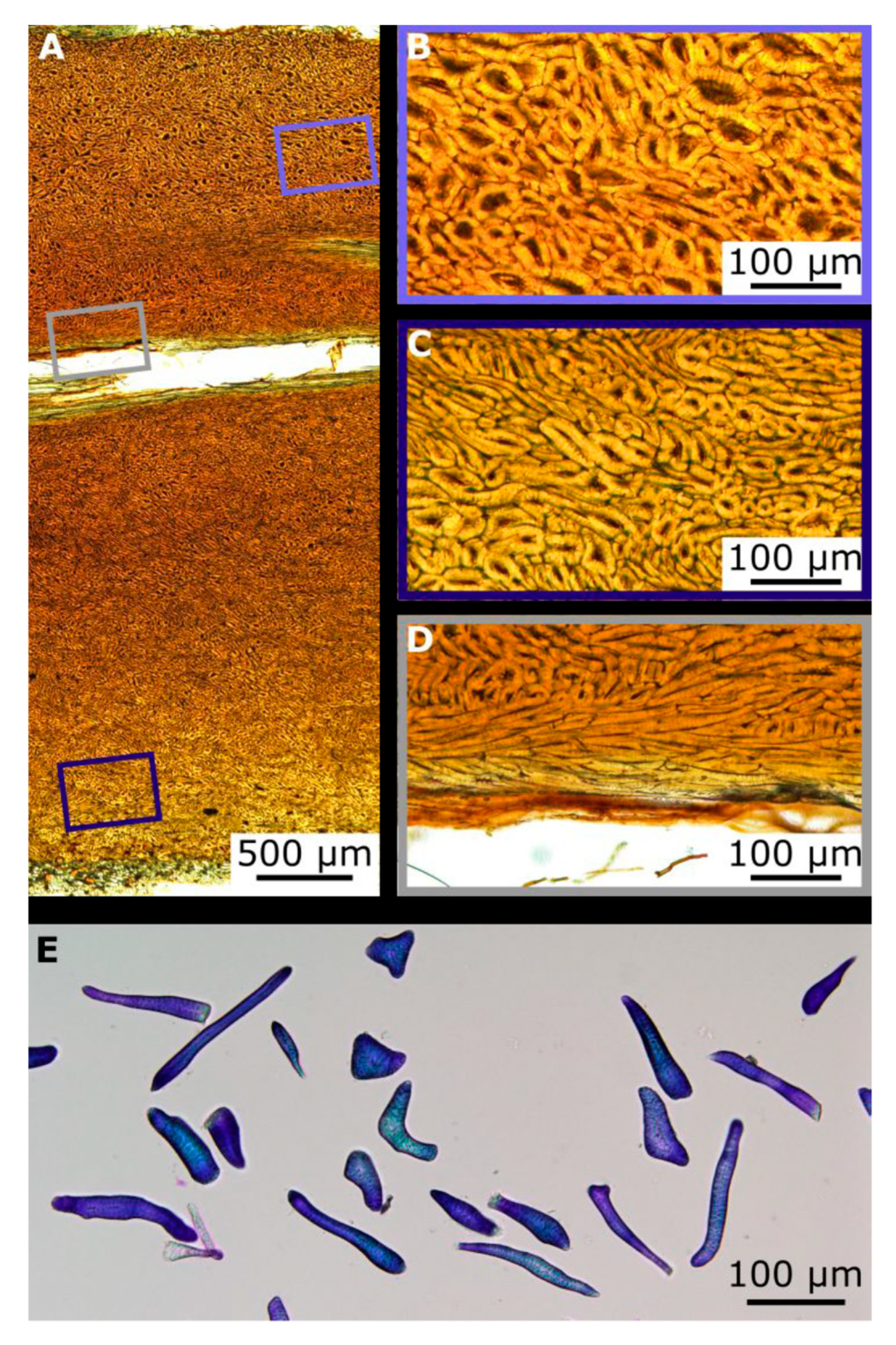
2.3. Hierarchical Level H4
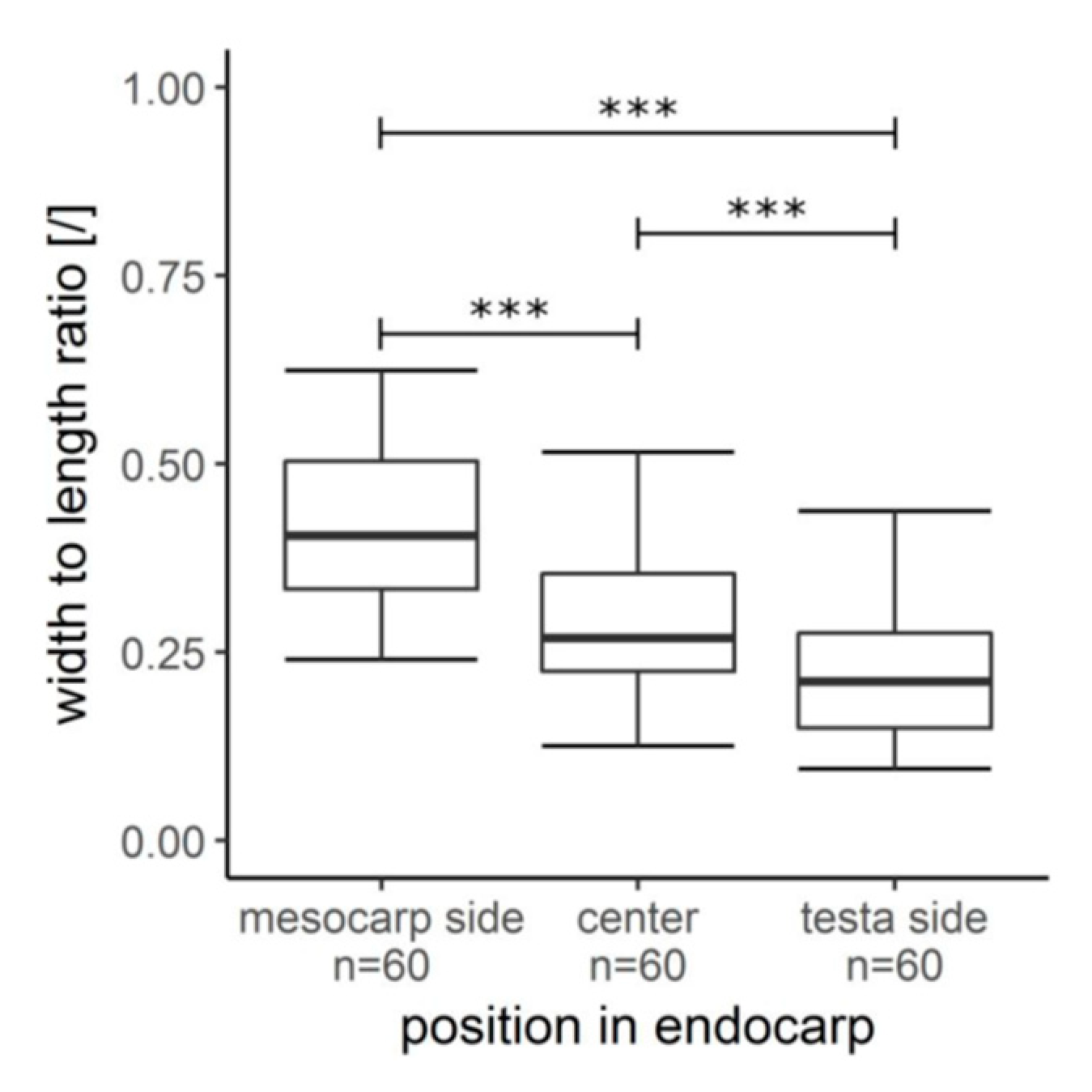
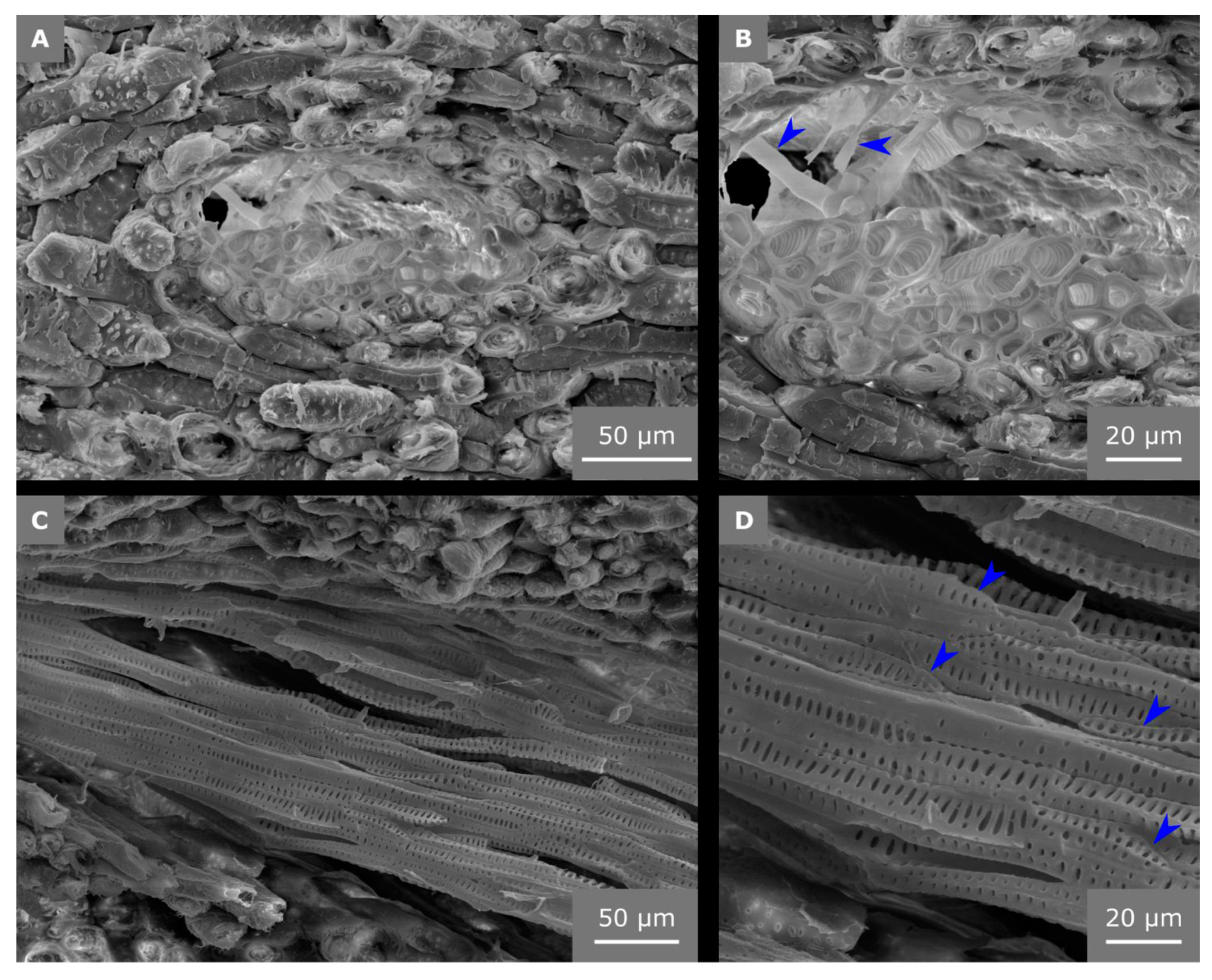
2.4. Hierarchical Level H5
2.5. Hierarchical Level H6
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. CT Measurements
4.3. Light Microscopy
4.4. Scanning Electron Microscopy
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Protocol for Embedding the Endocarp in Acryl
- Transfer the sample to distilled water (duration: 30 min).
- Transfer the sample to 70% EtOH (mixture of distilled water and EtOH (Ethanol 96% denaturated–T171.4, Carl Roth GmbH & Co. KG, Karlsruhe, Germany) at a ratio of three to seven) (duration: Overnight).
- Transfer the sample to 70% EtOH (duration: 2 days).
- Transfer the sample to 80% EtOH (duration: 1 day).
- Transfer the sample to 80% EtOH (duration: 2 days).
- Transfer the sample to 96% EtOH (duration: 1 day).
- Transfer the sample to 96% EtOH (duration: 2 days).
- Transfer the sample to 100% EtOH (duration: 1 day).
- Transfer the sample to 100% EtOH (duration: 2 days).
- Transfer the sample to 100% Xylene (#9713, Carl Roth GmbH & Co. KG, Germany) (duration: 1 day).
- Transfer the sample to 100% Xylene (duration: 2 days).
- Transfer the sample to mixture of Xylene and MMA (Methyl methacrylate, #8.00590.250, Merck KGaA, Darmstadt, Germany) at a ratio of one to one (duration: 7 days).
- Transfer the sample to mixture of 100 mL MMA, 10 mL Di-n-butyl phthalate (Alfa Aesar, Thermo Fisher GmbH, Dreieich, Germany) and 1g Benzoyl peroxide (dried, #8.01641.1000, Merck KGaA, Germany) (duration: 3 days).
- Transfer the sample to mixture of 100 mL MMA, 10 mL Di-n-butyl phthalate and 3g Benzoyl peroxide (dried) (duration: 14 days, until fully hardened).
References
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Brulé, V.; Rafsanjani, A.; Pasini, D.; Western, T.L. Hierarchies of plant stiffness. Plant Sci. 2016, 250, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Bührig-Polaczek, A.; Fleck, C.; Speck, T.; Schüler, P.; Fischer, S.F.; Caliaro, M.; Thielen, M. Biomimetic cellular metals—Using hierarchical structuring for energy absorption. Bioinspir. Biomim. 2016, 11, 045002. [Google Scholar] [CrossRef] [PubMed]
- Flores-Johnson, E.A.; Carrillo, J.G.; Zhai, C.; Gamboa, R.A.; Gan, Y.; Shen, L. Microstructure and mechanical properties of hard Acrocomia mexicana fruit shell. Sci. Rep. 2018, 8, 9668. [Google Scholar] [CrossRef] [PubMed]
- Klang, K.; Bauer, G.; Toader, N.; Lauer, C.; Termin, K.; Schmier, S.; Kovaleva, D.; Haase, W.; Berthold, C.; Nickel, K.G.; et al. Plants and animals as source of inspiration for energy dissipation in load bearing systems and facades. In Biomimetic Research for Architecture and Building Construction; Knippers, J., Nickel, K.G., Speck, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 109–133. [Google Scholar]
- Gludovatz, B.; Walsh, F.; Zimmermann, E.A.; Naleway, S.E.; Ritchie, R.O.; Kruzic, J.J. Multiscale structure and damage tolerance of coconut shells. J. Mech. Behav. Biomed. 2017, 76, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Speck, T.; Bold, G.; Masselter, T.; Poppinga, S.; Schmier, S.; Thielen, S.; Speck, O. Biomechanics and Functional Morphology of Plants—Inspiration for Biomimetic Materials and Structures. In Plant Biomechanics; Geitmann, A., Grill, J., Eds.; Springe International Publishing: Cham, Switzerland, 2018; pp. 399–433. [Google Scholar]
- Lauer, C.; Schmier, S.; Speck, T.; Nickel, K.G. Strength-size relationships in two porous biological materials. Acta Biomater. 2018, 77, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Schüler, P.; Meinel, D.; Zaslansky, P.; Currey, J.D. Microstructural features influencing failure in Macadamia nuts. Bioinspir. Biomim. Nanobiomater. 2012, 1, 67–75. [Google Scholar] [CrossRef]
- Schüler, P.; Speck, T.; Bührig-Polaczek, A.; Fleck, C. Structure-function relationships in Macadamia integrifolia seed coats–fundamentals of the hierarchical microstructure. PLoS ONE 2014, 9, e102913. [Google Scholar] [CrossRef] [PubMed]
- Staufenberg, G.; Graupner, N.; Müssig, J. Impact and hardness optimisation of composite materials inspired by the babassu nut (Orbignya speciosa). Bioinspir. Biomim. 2015, 10, 056006. [Google Scholar] [CrossRef] [PubMed]
- Winton, A.L. ART. XXIX.—The Anatomy of the Fruit of Cocos nucifera. Am. J. Sci. 1901, 12, 265–280. [Google Scholar] [CrossRef]
- Reddy, G.N.; Kulkarni, A.R. Contribution to the anatomy of palm fruits—Cocosoid palms. Proc. Plant Sci. 1985, 95, 153–165. [Google Scholar]
- Dransfield, J.; Cooke, D. Plant portraits: 355. Cocos nucifera: Arecaceae. Curtis’s Bot. Mag. 1999, 16, 2–9. [Google Scholar]
- Chan, E.; Elevitch, C.R. Cocos nucifera (coconut). Species Profiles Pac. Isl. Agrofor. 2006, 2, 1–27. [Google Scholar]
- Tomlinson, P.B. Anatomy of the Monocotyledons. II. Palmae; Clarendon Press: Oxford, UK, 1961; pp. 195–198. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 256–260. [Google Scholar]
- Jura-Morawiec, J.; Wiland-Szymańska, J. A novel insight into the structure of amphivasal secondary bundles on the example of Dracaena draco L. stem. Trees 2014, 28, 871–877. [Google Scholar] [CrossRef]
- Van Mier, J.G.; Man, H.K. Some notes on microcracking, softening, localization, and size effects. Int. J. Damage Mech. 2009, 18, 283–309. [Google Scholar] [CrossRef]
- Ritchie, R.O. Mechanisms of fatigue crack propagation in metals, ceramics and composites: Role of crack tip shielding. Mat. Sci. Eng. A 1988, 103, 15–28. [Google Scholar] [CrossRef]
- Schmier, S.; Lauer, C.; Schäfer, I.; Klang, K.; Bauer, G.; Thielen, M.; Termin, K.; Berthold, C.; Schmauder, S.; Speck, T.; et al. Developing the experimental basis for an evaluation of scaling properties of brittle and ‘quasi-brittle’ biological materials. In Biomimetic Research for Architecture and Building Construction; Knippers, J., Nickel, K.G., Speck, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 277–294. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; De Zonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |







| Hierarchical Level | Structure | n | Length | Width | Thickness | Others | Evaluated Data |
|---|---|---|---|---|---|---|---|
| H0 | fruit | 161 | up to 25 [cm] 1 | up to 20 [cm] 1 | − | “ellipsoidal to broadly ovoid, indistinctly 3-angled” [Dransfield and Cooke, 1999] | |
| H1 | − | − | − | − | − | “almost always 1 only, very large, with a narrow layer of homogeneous endosperm, and a large central cavity partially filled with fluid; embryo basal, opposite one of the endocarp pores” [Dransfield and Cooke, 1999] | |
| pericarp | − | − | − | − | − | ||
| H2 | exocarp | − | − | − | 0.015 [mm] 2 | “smooth” [Dransfield and Cooke, 1999]; “a smooth, tough coat, of a brownish or grayish color” [Winton, 1901] | |
| mesocarp | − | − | − | 3–4 [cm] 2 | “very thick and fibrous, dry” [Dransfield and Cooke, 1999]; “consists of a hard outer coat, but a few mm thick and a soft portion usually 3–4 cm thick on the sides and much thicker on the base. Imbedded in the mesocarp are numerous longitudinally arranged fibers, varying in size from slender hairs to large […] forms, 2–3 mm broad.” [Winton, 1901] | ||
| endocarp | 10 | 116.5 ± 9.2 [mm] | 92.8 ± 5.2 [mm] | thin: 2.5 ± 0.5 thick: 4.7 ± 0.6 [mm] | Shell with a prolate, spheroidal shape and varying thickness | digital calliper | |
| H3-1 | vb | − | − | − | − | diameter: 0.22 ± 0.14 [mm] | CT data (res: 42 µm) + CT Analyser |
| H3-2 | scm | porosity: 0.033 | CT data (res: 42 µm) + CT Analyser | ||||
| H4-1 | t | − | 424.3 ± 263.3 [µm] (n = 6) | 11.5 ± 3.6 [µm] (n = 80) | − | − | LM images (macerated cells) + ImageJ |
| H4-2 | sc-ms | 60 | 100.3 ± 21.5 [µm] | 40.7 ± 9.2 [µm] | − | w:l ratio 0.42 ± 0.10 | LM images (macerated cells) + ImageJ |
| sc-c | 60 | 115.6 ± 29.4 [µm] | 31.3 ± 6.5 [µm] | − | w:l ratio 0.29 ± 0.09 | LM images (macerated cells) + ImageJ | |
| sc-ts | 60 | 125.2 ± 38.8 [µm] | 25.4 ± 5.4 [µm] | − | w:l ratio 0.22 ± 0.09 | LM images (macerated cells) + ImageJ | |
| H5-1 | cw (t) | 30 | − | − | 1.7 ± 0.4 [µm] | scalariform pitting | SEM images + ImageJ |
| H5-2 | cw (sc) | 30 | − | − | 13.8 ± 2.9 [µm] | thickness in relation to cell radius 89.2 ± 4.3 [%] | SEM images + ImageJ |
| H6-2 | l1 (sc) | 24 | − | − | 2.0 ± 0.5 [µm] 3 | − | SEM images + ImageJ |
| l2 (sc) | 145 | − | − | 253 ± 74 [nm] | − | SEM images + ImageJ | |
| p (sc) | 414 | 2.5 ± 0.6 [µm] | 1.8 ± 0.4 [µm] | − | bordered, ramiform pits | SEM images + ImageJ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmier, S.; Hosoda, N.; Speck, T. Hierarchical Structure of the Cocos nucifera (Coconut) Endocarp: Functional Morphology and its Influence on Fracture Toughness. Molecules 2020, 25, 223. https://doi.org/10.3390/molecules25010223
Schmier S, Hosoda N, Speck T. Hierarchical Structure of the Cocos nucifera (Coconut) Endocarp: Functional Morphology and its Influence on Fracture Toughness. Molecules. 2020; 25(1):223. https://doi.org/10.3390/molecules25010223
Chicago/Turabian StyleSchmier, Stefanie, Naoe Hosoda, and Thomas Speck. 2020. "Hierarchical Structure of the Cocos nucifera (Coconut) Endocarp: Functional Morphology and its Influence on Fracture Toughness" Molecules 25, no. 1: 223. https://doi.org/10.3390/molecules25010223
APA StyleSchmier, S., Hosoda, N., & Speck, T. (2020). Hierarchical Structure of the Cocos nucifera (Coconut) Endocarp: Functional Morphology and its Influence on Fracture Toughness. Molecules, 25(1), 223. https://doi.org/10.3390/molecules25010223





