A Triazole Hybrid of Neolignans as a Potential Antileishmanial Agent by Triggering Mitochondrial Dysfunction
Abstract
:1. Introduction
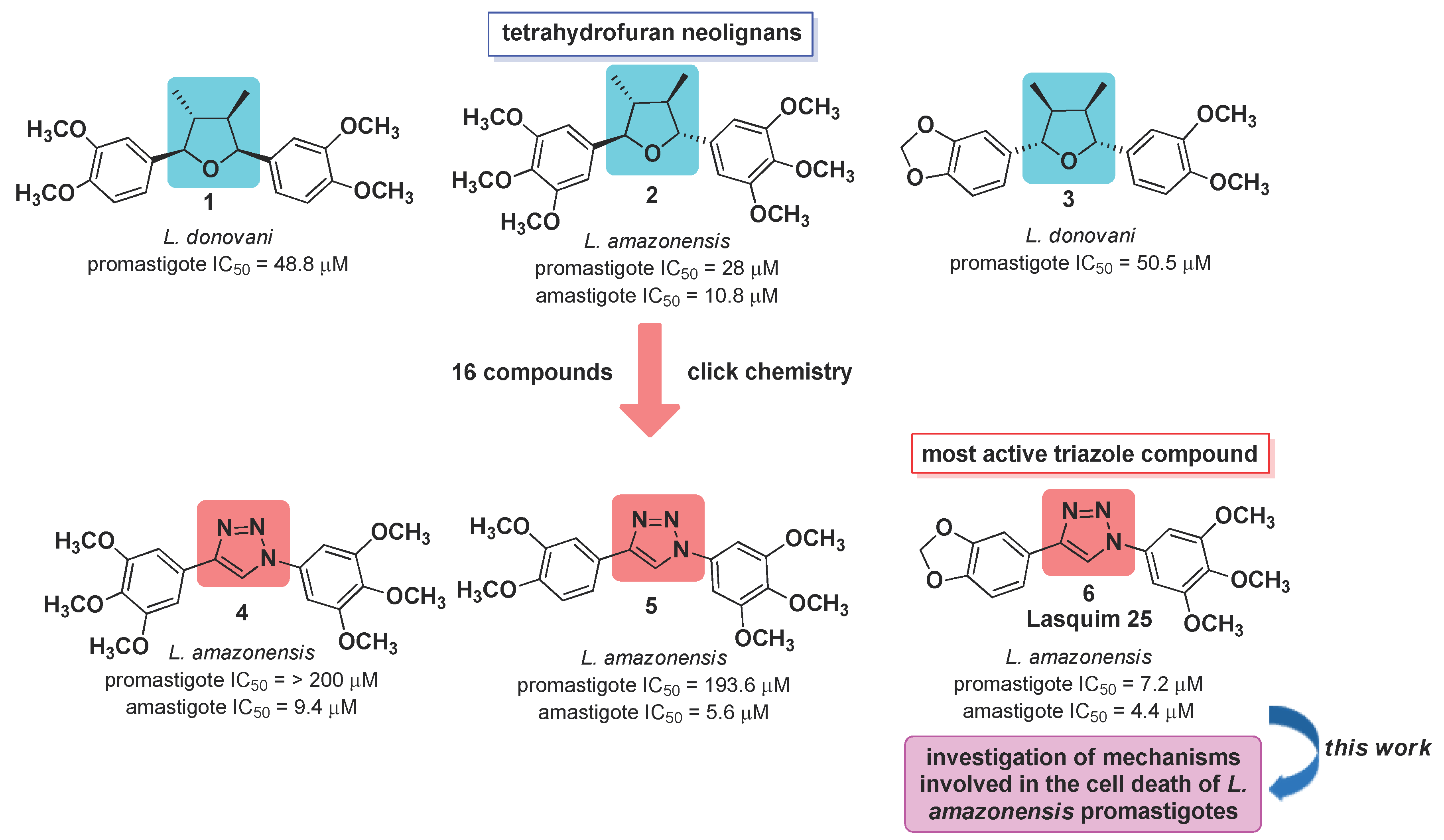
2. Results and Discussion
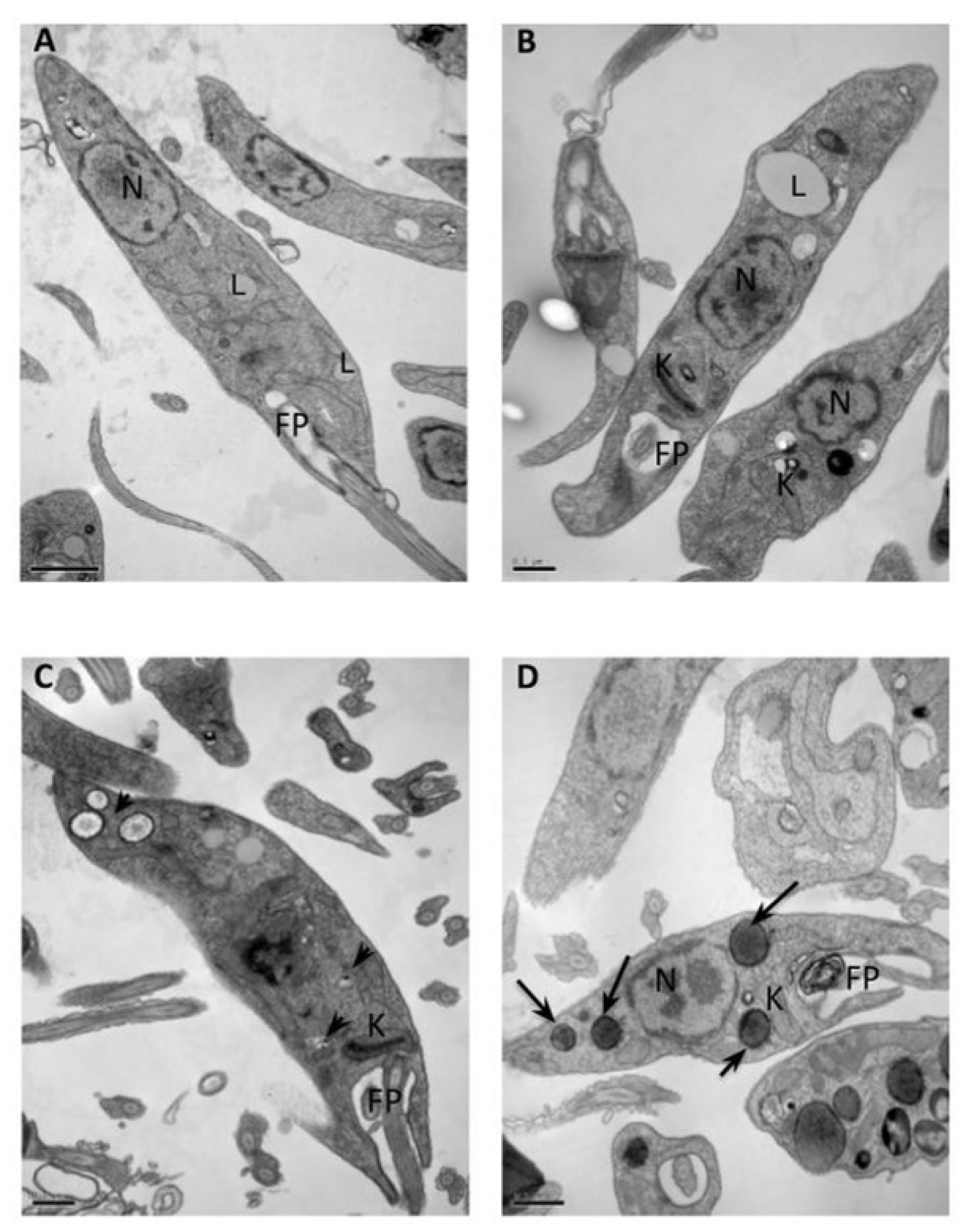
2.1. Transmission Electron Microscopy (TEM)
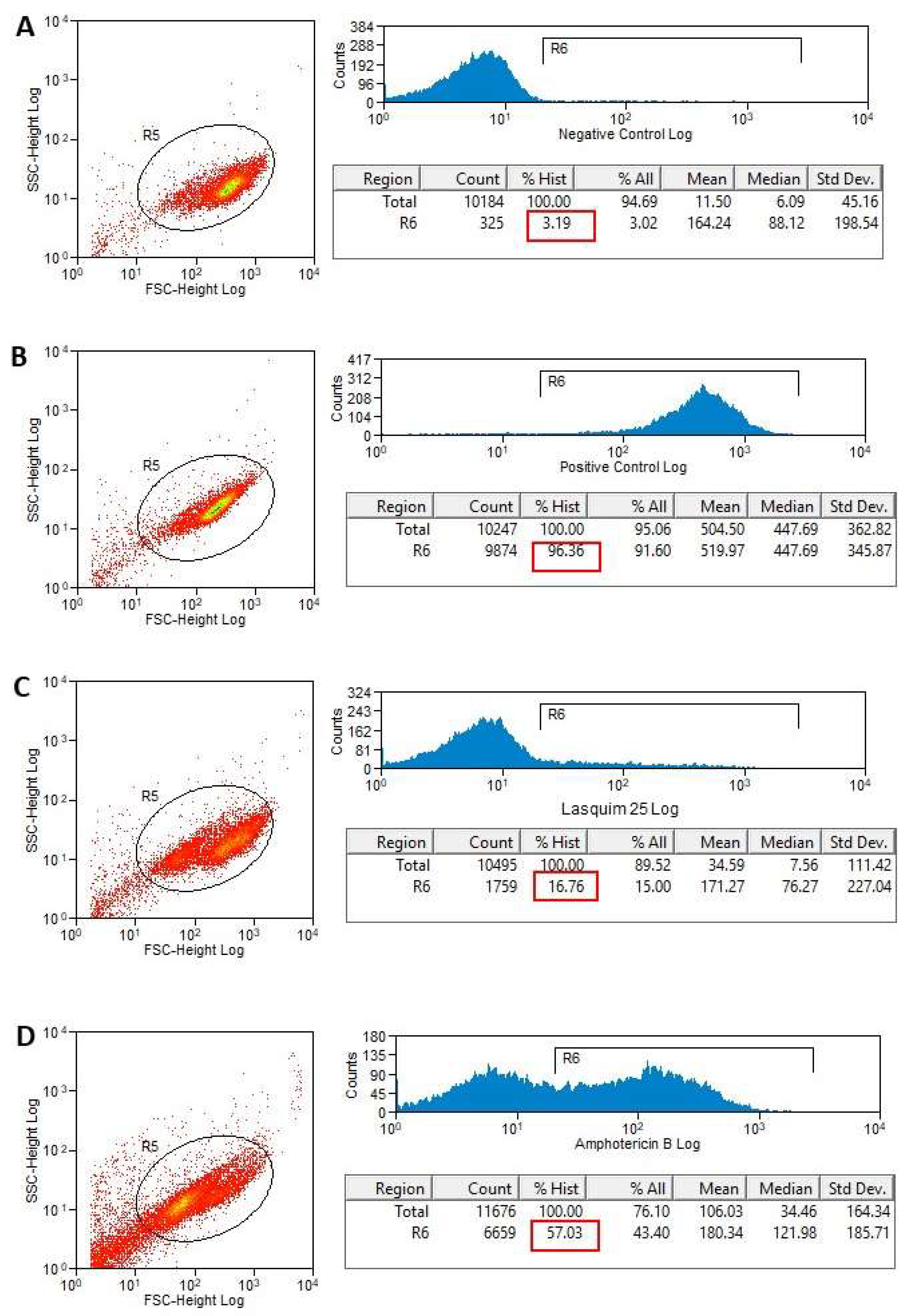
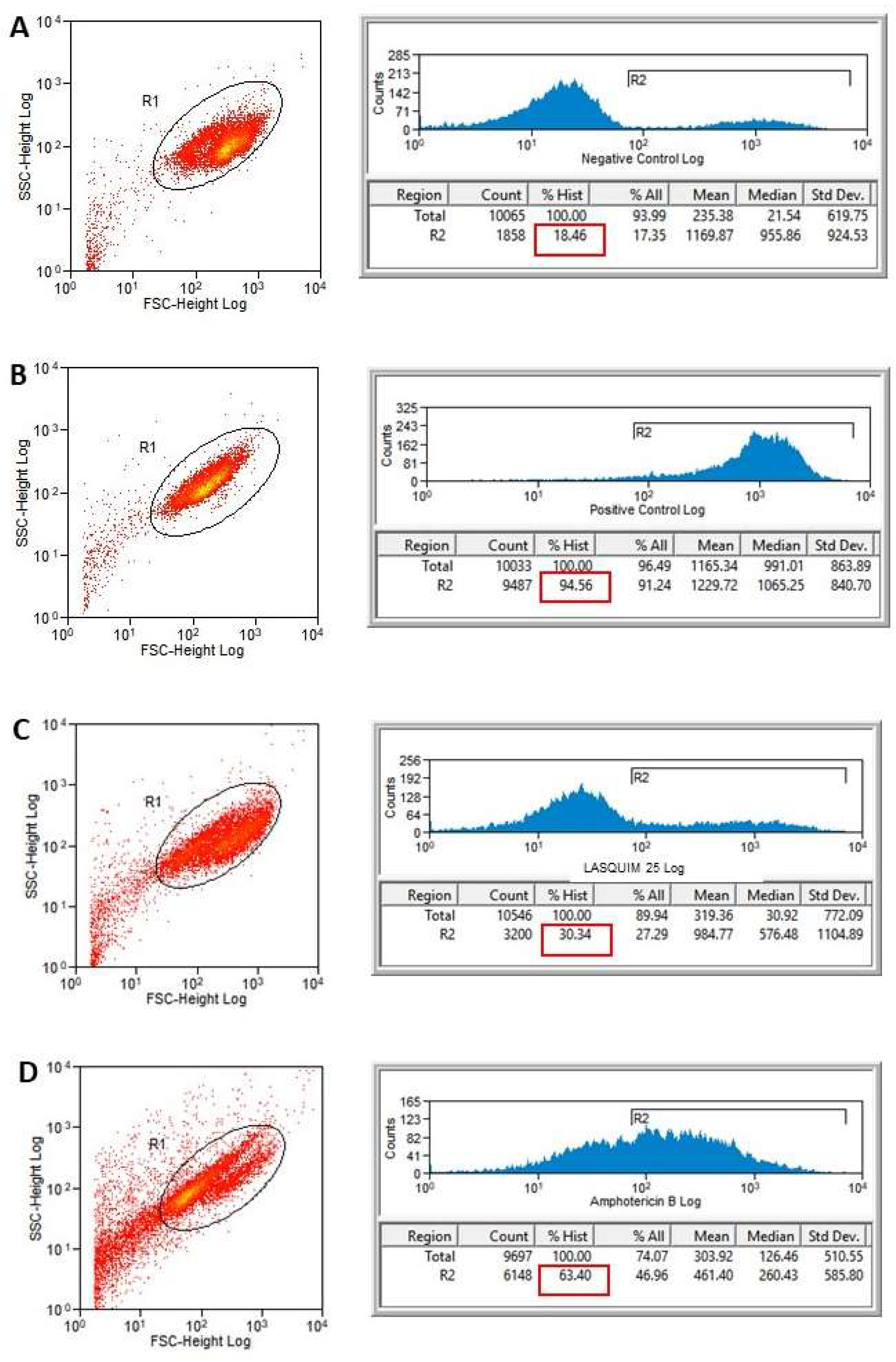
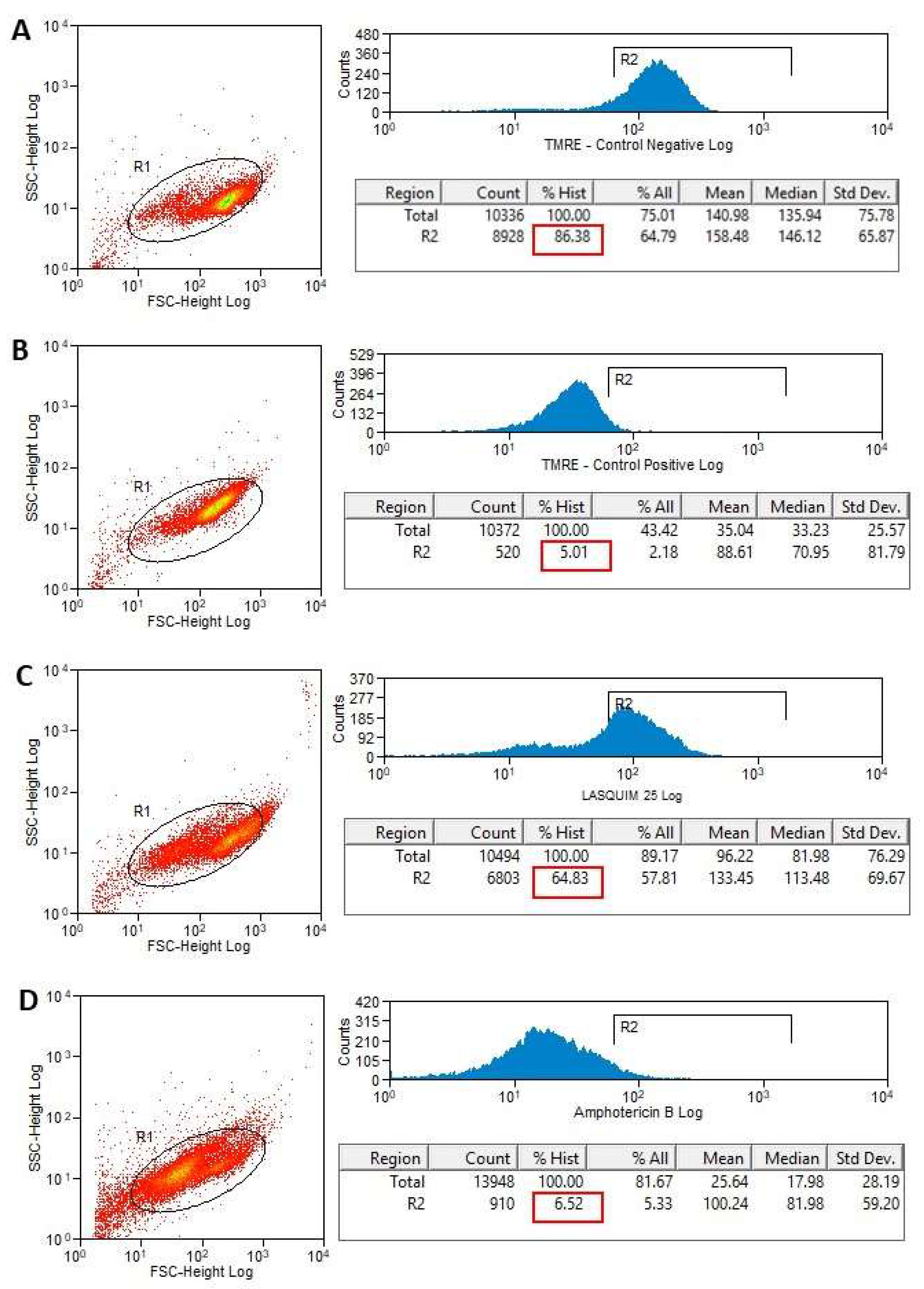
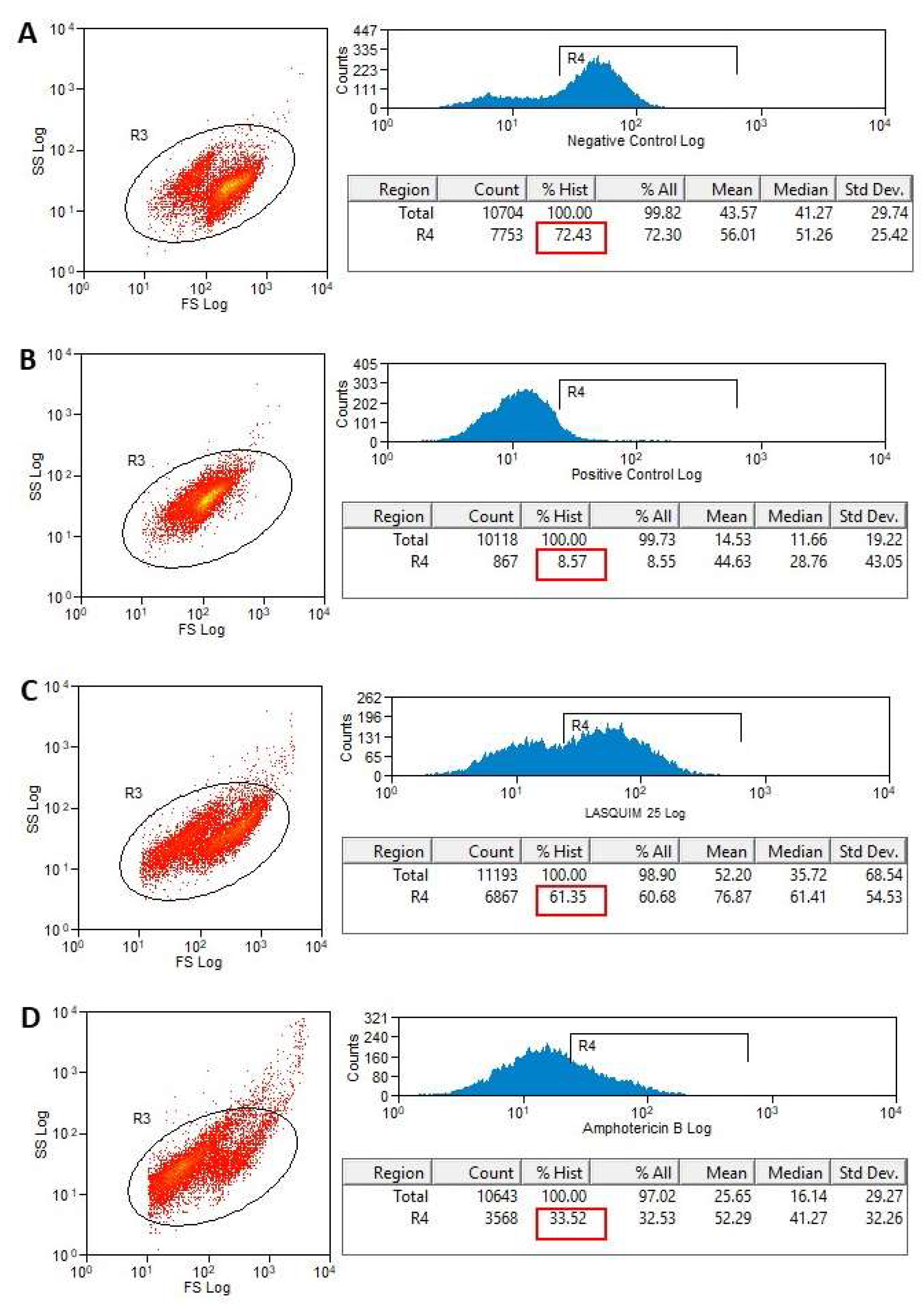
2.2. Flow Cytometric Analysis (FACS)
2.2.1. PI Staining
2.2.2. TMRE Staining
3. Materials and Methods
3.1. Chemistry
3.2. Parasites
3.3. In Vitro Tests
3.3.1. Transmission Electron Microscopy
3.3.2. Flow Cytometric Analysis for Detection of Cellular Membrane Alterations
3.3.3. Flow Cytometric Analysis for Detection of Mitochondrial Membrane Potential (ΔΨm) Alterations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Leishmaniasis—Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 22 February 2019).
- Cassamale, T.B.; Costa, E.C.; Carvalho, D.B.; Cassemiro, N.S.; Tomazela, C.C.; Marques, M.C.S.; Ojeda, M.; Matos, M.F.C.; Albuquerque, S.; Arruda, C.C.P.; et al. Synthesis and antitrypanosomatid activity of 1,4-Diaryl-1,2,3-triazole analogues of neolignans veraguensin, grandisin and machilin G. J. Braz. Chem. Soc. 2016, 27, 1217–1228. [Google Scholar]
- Costa, E.C.; Cassamale, T.B.; Carvalho, D.B.; Bosquiroli, L.S.S.; Ojeda, M.; Ximenes, T.V.; Matos, M.F.C.; Kadri, M.C.T.; Baroni, A.C.M.; Arruda, C.C.P. Antileishmanial activity and structure-activity relationship of triazolic compounds derived from the neolignans grandisin, veraguensin, and machilin G. Molecules 2016, 21, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Filho, A.A.; Costa, E.S.; Cunha, W.R.; Silva, M.L.; Nanayakkara, N.P.; Bastos, J.K. In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae). Phytother. Res. 2008, 22, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Trefzger, O.F.; Neves, A.R.; Barbosa, N.V.; Carvalho, D.B.; Pereira, I.C.; Perdomo, R.T.; Matos, M.F.C.; Yoshida, N.C.; Kato, M.J.; Albuquerque, S.; et al. Design, synthesis and antitrypanosomatid activities of 3,5-diaryl-isoxazole analogues based on neolignans veraguensin, grandisin and machilin G. Chem. Biol. Drug Des. 2019, 93, 313–324. [Google Scholar] [CrossRef]
- Neves, A.R.; Trefzger, O.F.; Barbosa, N.V.; Honorato, A.M.; Carvalho, D.B.; Moslaves, I.S.; Kadri, M.C.T.; Yoshida, N.C.; Kato, M.J.; Arruda, C.C.P.; et al. Effect of isoxazole derivatives of tetrahydrofuran neolignans on intracellular amastigotes of Leishmania (Leishmania) amazonensis: A structure–activity relationship comparative study with triazole-neolignan-based compounds. Chem. Biol. Drug Des. 2019. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–967. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.N.J.; Docampo, R. The role of acidocalcisomes in parasitic protists. J. Eukar. Microbiol. 2009, 56, 208–213. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Ueda-Nakamura, T.; Garcia Cortez, D.A.; Dias-Filho, B.P.; Morgado-Díaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Souza, F.; Taniwaki, N.N.; Amaral, A.C.F.; Souza, C.S.F.; Calabrese, K.S.; Abreu-Silva, A.L. Ultrastructural changes and death of Leishmania infantum promastigotes induced by Morinda citrifolia Linn. fruit (Noni) juice treatment. Evid. Based Complement. Alternat. Med. 2016, 2016, 5063540. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Ruiz, A.; Alzate, J.F.; Macleod, E.T.; Lüder, C.G.K.; Fasel, N.; Hurd, H. Apoptotic markers in protozoan parasites. Parasite Vector 2010, 3, 104. [Google Scholar] [CrossRef] [Green Version]
- Aureliano, D.P.; Lindoso, J.A.L.; de Castro Soares, S.R.; Takakura, C.F.H.; Pereira, T.M.; Ribeiro, M.S. Cell death mechanisms in Leishmania amazonensis triggered by methylene blue-mediated antiparasitic photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Proto, W.R.; Coombs, G.H.; Mottram, J.C. Cell death in parasitic protozoa: Regulated or incidental? Nat. Rev. Microbiol. 2013, 11, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Temel, H.E.; Sever, B.; Çiftçi, G.A.; Kaplancıklı, Z.A. Synthesis and evaluation of new benzodioxole-based thiosemicarbazone derivatives as potential antitumor agents. Molecules 2016, 21, 1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.-J.; Moon, K.-W.; Upreti, V.V.; Eddington, N.D.; Lee, I.J. Mechanisms of MDMA (Ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr. Pharm. Biotechnol. 2010, 11, 434–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, L.M. Safrole and the versatility of a natural biophore. Rev. Virtual Quim. 2015, 7, 495–538. [Google Scholar] [CrossRef]
- Hara, Y.; Nakajima, M.; Miyamoto, K.I.; Yokoi, T. Inhibitory effects of psychotropic drugs on mexiletine metabolism in human liver microsomes: Prediction of in vivo drug interactions. Xenobiotica 2005, 35, 549–560. [Google Scholar] [CrossRef]
- Walsh, C.M.; Edinger, A.L. The complex interplay between autophagy, apoptosis, and necrotic signals promotes t-cell homeostasis. Immunol. Rev. 2010, 23, 95–109. [Google Scholar] [CrossRef]
- Zangger, H.; Mottram, J.C.; Fasel, N. Cell death in Leishmania induced by stress and differentiation: Programmed cell death or necrosis? Cell Death Differ. 2002, 9, 1126–1139. [Google Scholar] [CrossRef] [Green Version]
- Schurigt, U.; Glowa, C.; Schirmeister, T. Aziridine-2,3-dicarboxylate-based cysteine cathepsin inhibitors induce cell death in Leishmania major associated with accumulation of debris in autophagy-related lysosome-like vacuoles. Antimicrob. Agents Chemother. 2010, 54, 5028–5041. [Google Scholar] [CrossRef] [Green Version]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Decaudin, D.; Macho, A.; Hirsch, T.; Susin, S.A.; Petit, P.X.; Mignotte, B.; Kroemer, G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995, 182, 367–377. [Google Scholar] [CrossRef]
- Overbeeke, M.; Yildirim, C.P.M.; Reutelingsperger, C.; Haanen, I.; Vermes, R. Sequential occurrence of mitochondrial and plasma membrane alterations, fluctuations in cellular Ca2+ and pH during initial and later phases of cell death. Apoptosis 1999, 4, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Basmaciyan, L.; Azas, N.; Casanova, M. Different apoptosis pathways in Leishmania parasites. Cell Death Discov. 2018, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.; Gonzalez, J.; Sprissler, C.; Zalila, H.; Dacher, M.; Basmaciyan, L.; Späth, G.L.; Azas, N.; Fasel, N. Implication of different domains of the Leishmania major metacaspase in cell death and autophagy. Cell Death Dis. 2015, 6, e1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, I.A.; Azevedo, M.M.B.; Chaves, F.C.M.; Alviano, C.S.; Alviano, D.S.; Vermelho, A.B. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. Biomed. Res. Int. 2014, 2014, 985171. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.V.; Desoti, V.C.; Caleare Ade, O.; Ueda-Nakamura, T.; Silva, S.O.; Nakamura, C.V. Mitochondria superoxide anion production contributes to geranylgeraniol-induced death in Leishmania amazonensis. Evid. Based Complement. Altern. Med. 2012, 2012, 298320. [Google Scholar] [CrossRef] [Green Version]
- Inacio, J.D.; Canto-Cavalheiro, M.M.; Menna-Barreto, R.F.; Almeida-Amaral, E.E. Mitochondrial damage contribute to epigallocatechin-3-gallate induced death in Leishmania amazonensis. Exp. Parasitol. 2012, 13, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lui, X.; Ma, S. The roles of mitochondria in autophagic cell death. Cancer Biother. Radiopharm. 2016, 31, 269–276. [Google Scholar] [CrossRef]
- Rottini, M.M.; Amaral, A.C.F.; Ferreira, J.L.P.; Andrade Silva, J.R.; Taniwaki, N.N.; Souza, C.S.F.; D’Escoffier, L.N.; Almeida-Souza, F.; Hardoim, D.J.; Gonçalves da Costa, S.C.; et al. In vitro evaluation of (−)α-bisabolol as a promising agent against Leishmania amazonensis. Exp. Parasitol. 2015, 148, 66–72. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4–6 are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardozo Pinto de Arruda, C.; de Jesus Hardoim, D.; Silva Rizk, Y.; da Silva Freitas de Souza, C.; Zaverucha do Valle, T.; Bento Carvalho, D.; Nosomi Taniwaki, N.; de Morais Baroni, A.C.; da Silva Calabrese, K. A Triazole Hybrid of Neolignans as a Potential Antileishmanial Agent by Triggering Mitochondrial Dysfunction. Molecules 2020, 25, 37. https://doi.org/10.3390/molecules25010037
Cardozo Pinto de Arruda C, de Jesus Hardoim D, Silva Rizk Y, da Silva Freitas de Souza C, Zaverucha do Valle T, Bento Carvalho D, Nosomi Taniwaki N, de Morais Baroni AC, da Silva Calabrese K. A Triazole Hybrid of Neolignans as a Potential Antileishmanial Agent by Triggering Mitochondrial Dysfunction. Molecules. 2020; 25(1):37. https://doi.org/10.3390/molecules25010037
Chicago/Turabian StyleCardozo Pinto de Arruda, Carla, Daiana de Jesus Hardoim, Yasmin Silva Rizk, Celeste da Silva Freitas de Souza, Tânia Zaverucha do Valle, Diego Bento Carvalho, Noemi Nosomi Taniwaki, Adriano Cesar de Morais Baroni, and Kátia da Silva Calabrese. 2020. "A Triazole Hybrid of Neolignans as a Potential Antileishmanial Agent by Triggering Mitochondrial Dysfunction" Molecules 25, no. 1: 37. https://doi.org/10.3390/molecules25010037






