Synthesis, Spectroscopy, Electrochemistry and DFT of Electron-Rich Ferrocenylsubphthalocyanines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. 1H NMR
2.3. UV/vis
2.4. Cyclic Voltammetry
2.4.1. Effect of SubPc on Ferrocenyl Moiety Oxidation
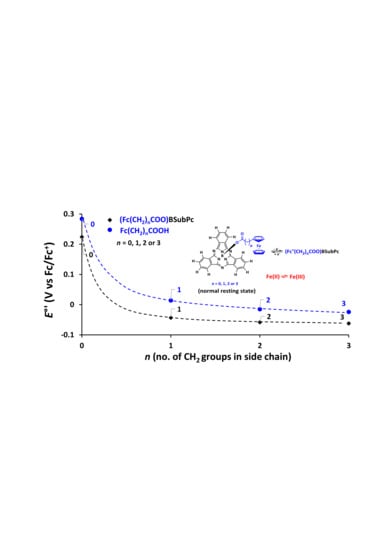
2.4.2. Effect of Chain Length on Ferrocenyl Moiety Oxidation
2.4.3. Effect of Carboxyl and Carbonyl Group on Ferrocenyl Moiety Oxidation
2.4.4. Ring Based Reductions
2.4.5. Ring Based Oxidation
2.5. Computational Analysis
3. Materials and Methods
3.1. Spectroscopy Measurements
3.2. Cyclic Voltammetry
3.3. DFT Calculations
3.4. Preparationof SubPcs 7, 8, 10 and 11
3.4.1. Preparation of FcCO2SubPc(H)12, 7
3.4.2. Preparation of FcCH2CO2SubPc(H)12, 8
3.4.3. Preparation of Fc(CH2)3CO2SubPc(H)12, 10
3.4.4. Preparation of FcCO(CH2)2CO2SubPc(H)12, 11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dagani, R. Fifty Years of Ferrocene Chemistry. Chem. Eng. News 2001, 79, 37–38. [Google Scholar] [CrossRef]
- Bublitz, D.E.; Rinehart, K.L. The Synthesis of Substituted Ferrocenes and other π-Cyclopentadienyl-Transition Metal Compounds. In Organic Reactions; Wiley online library, John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–154. [Google Scholar]
- Bruce, M.I. Comprehensive Organometallic Chemistry; Wilkinson, G., Stone, F.G.A., Abel, E.W., Eds.; Pergamon: Oxford, UK, 1982; Volume 4, p. 843. [Google Scholar]
- Bunton, C.A.; Carrasco, N.; Watts, W.E. Reactions of ferrocenyl-stabilised carbocations with water: Substituent, medium, salt, and solvent isotope effects on rates and equilibria. J. Chem. Soc. Perkin Trans. 2 1979, 8, 1267. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Kochetkova, N.S. Applications of Ferrocene and Its Derivatives. Russ. Chem. Rev. 1974, 5, 710–715. [Google Scholar] [CrossRef]
- Conradie, J.; Lamprecht, G.J.; Roodt, A.; Swarts, J.C. Kinetic study of the oxidative addition reaction between methyl iodide and [Rh(FcCOCHCOCF3)(CO)(PPh3)]: Structure of [Rh(FcCOCHCOCF3)(CO)(PPh3)(CH3)(I)]. Polyhedron 2007, 26, 5075–5087. [Google Scholar] [CrossRef]
- Shen, Q.; Shekhar, S.; Stambuli, J.P.; Hartwig, J.F. Highly reactive, general, and long-lived catalysts for coupling heteroaryl and aryl chlorides with primary nitrogen nucleophiles. Angew. Chem. Int. Ed. 2005, 44, 1371–1375. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Rahimpour, K.; Ghadari, R.; Ahmadi-Kandjani, S. Ferrocene based nonlinear optical chromophores: Synthesis, characterization and study of optical properties. J. Mol. Liq. 2017, 244, 322–329. [Google Scholar] [CrossRef]
- Nonjola, P.T.N.; Siegert, U.; Swarts, J.C. Synthesis, Electrochemistry and Cytotoxicity of Ferrocene-Containing Amides, Amines and Amino-Hydrochlorides. J. Inorg. Organomet. Polym. Mater. 2015, 25, 376–385. [Google Scholar] [CrossRef]
- Shago, R.F.; Swarts, J.C.; Kreft, E.; Van Rensburg, C.E.J. Antineoplastic activity of a series of ferrocene-containing alcohols. Anticancer Res. 2007, 27, 3431–3433. [Google Scholar]
- Peter, S.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.L.; Shago, R.F.; Langner, E.H.G.; Swarts, J.C. Synthesis and electrochemical properties of a series of ferrocene-containing alcohols. Polyhedron 2005, 24, 1611–1616. [Google Scholar] [CrossRef]
- Blom, N.F.; Neuse, E.W.; Thomas, H.G. Electrochemical characterization of some ferrocenylcarboxylic acids. Transit. Met. Chem. 1987, 12, 301–306. [Google Scholar] [CrossRef]
- Swarts, P.J.; Conradie, J. Solvent and substituent effect on electrochemistry of ferrocenylcarboxylic acids. J. Electroanal. Chem. 2020, 866, 114164. [Google Scholar] [CrossRef]
- Meller, A.; Ossko, A. Phthalocyaninartige Bor-Komplexe. Mon. Chem. 1972, 155, 150–151. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, S.; Hu, S.; Yu, J. Highly efficient tandem organic light-emitting diodes based on SubPc:C60 bulk heterojunction as charge generation layer. J. Lumin. 2016, 169, 29–34. [Google Scholar] [CrossRef]
- Ince, M.; Medina, A.; Yum, J.H.; Yella, A.; Claessens, C.G.; Martínez-Díaz, M.V.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Peripherally and axially carboxylic acid substituted subphthalocyanines for dye-sensitized solar cells. Chem. Eur. J. 2014, 20, 2016–2021. [Google Scholar] [CrossRef]
- Claessens, C.G.; González-Rodríguez, D.; Rodríguez-Morgade, M.S.; Medina, A.; Torres, T. Subphthalocyanines, Subporphyrazines, and Subporphyrins: Singular Nonplanar Aromatic Systems. Chem. Rev. 2014, 114, 2192–2277. [Google Scholar] [CrossRef]
- van de Winckel, E.; Mascaraque, M.; Zamarrón, A.; Juarranz de la Fuente, Á.; Torres, T.; de la Escosura, A. Dual Role of Subphthalocyanine Dyes for Optical Imaging and Therapy of Cancer. Adv. Funct. Mater. 2018, 28, 1705938. [Google Scholar] [CrossRef]
- Swarts, P.J.; Conradie, J. Electrochemical behaviour of chloro- and hydroxy- subphthalocyanines. Electrochim. Acta 2020, 329, 135165. [Google Scholar] [CrossRef]
- Solntsev, P.V.; Spurgin, K.L.; Sabin, J.R.; Heikal, A.A.; Nemykin, V.N. Photoinduced Charge Transfer in Short-Distance Ferrocenylsubphthalocyanine Dyads. Inorg. Chem. 2012, 51, 6537–6547. [Google Scholar] [CrossRef]
- Maligaspe, E.; Hauwiller, M.R.; Zatsikha, Y.V.; Hinke, J.A.; Solntsev, P.V.; Blank, D.A.; Nemykin, V.N. Redox and photoinduced electron-transfer properties in short distance organoboryl ferrocene-subphthalocyanine dyads. Inorg. Chem. 2014, 53, 9336–9347. [Google Scholar] [CrossRef]
- Guilleme, J.; González-Rodríguez, D.; Torres, T. Triflate-subphthalocyanines: Versatile, reactive intermediates for axial functionalization at the boron atom. Angew. Chem. Int. Ed. 2011, 50, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Swarts, P.J.; Conradie, J. Redox and photophysical properties of four subphthalocyanines containing ferrocenylcarboxylic acid as axial ligands. Inorg. Chem. 2020, 59, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Lewtak, J.P.; Landman, M.; Fernández, I.; Swarts, J.C. A DFT-Elucidated Comparison of the Solution-Phase and SAM Electrochemical Properties of Short-Chain Mercaptoalkylferrocenes: Synthetic and Spectroscopic Aspects, and the Structure of Fc–CH2CH2–S–S–CH2CH2–Fc. Inorg. Chem. 2016, 55, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, W.C.; Erasmus, J.J.C.; Lamprecht, G.J.; Conradie, J.; Cameron, T.S.; Aquino, M.A.S.; Swarts, J.C. Cyclic voltammetry of ferrocene-containing β-diketones as a tool to obtain group electronegativities. The structure of 3-ferrocenoyl-1,1,1- trifluoro-2-hydroxyprop-2-ene. Can. J. Chem. 1999, 77, 378–386. [Google Scholar] [CrossRef]
- Sampson, K.L.; Josey, D.S.; Li, Y.; Virdo, J.D.; Lu, Z.-H.; Bender, T.P. Ability To Fine-Tune the Electronic Properties and Open-Circuit Voltage of Phenoxy-Boron Subphthalocyanines through Meta-Fluorination of the Axial Substituent. J. Phys. Chem. C 2018, 122, 1091–1102. [Google Scholar] [CrossRef]
- Conradie, J.; Swarts, J.C. Relationship between electrochemical potentials and substitution reaction rates of ferrocene-containing β-diketonato rhodium(i) complexes; Cytotoxicity of [Rh(FcCOCHCOPh)(cod)]. Dalt. Trans. 2011, 40, 5844. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Fabelo, O.; Castellano, M.; Cano, J.; Fordham, S.; Zhou, H.C. Multielectron oxidation in a ferromagnetically coupled dinickel(II) triple mesocate. Chem. Commun. 2015, 51, 13381–13384. [Google Scholar] [CrossRef]
- Buitendach, B.E.; Conradie, J.; Malan, F.P.; Niemantsverdriet, J.W.; Swarts, J.C. Synthesis, Spectroscopy and Electrochemistry in Relation to DFT Computed Energies of Ferrocene- and Ruthenocene-Containing -Diketonato Iridium(III) Heteroleptic Complexes. Structure of [(2-Pyridylphenyl)2Ir(RcCOCHCOCH3]. Molecules 2019, 24, 3923. [Google Scholar] [CrossRef] [Green Version]
- Malan, F.P.; Singleton, E.; Conradie, J.; Landman, M. Electrochemistry of a series of symmetric and asymmetric CpNiBr(NHC) complexes: Probing the electrochemical environment due to push-pull effects. J. Electroanal. Chem. 2018, 814, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Gouterman, M. Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Elsevier: Amsterdam, The Netherlands, 1978; pp. 1–165. ISBN 9780122201035. [Google Scholar]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702–706. [Google Scholar] [CrossRef]
- Gericke, H.J.; Barnard, N.I.; Erasmus, E.; Swarts, J.C.; Cook, M.J.; Aquino, M.A.S. Solvent and electrolyte effects in enhancing the identification of intramolecular electronic communication in a multi redox-active diruthenium tetraferrocenoate complex, a triple-sandwiched dicadmium phthalocyanine and a ruthenocene-containing β-diketone. Inorg. Chim. Acta 2010, 363, 2222–2232. [Google Scholar] [CrossRef]
- Birke, R.L.; Kim, M.-H.; Strassfeld, M. Diagnosis of reversible, quasi-reversible, and irreversible electrode processes with differential pulse polarography. Anal. Chem. 1981, 53, 852–856. [Google Scholar] [CrossRef]
- Mirkin, M.V.; Bard, A.J. Simple Analysis of Quasi-Reversible Steady-State Voltammograms. Anal. Chem. 1992, 64, 2293–2302. [Google Scholar] [CrossRef]
- Myland, J.C.; Oldham, K.B. Quasireversible cyclic voltammetry of a surface confined redox system: A mathematical treatment. Electrochem. Commun. 2005, 7, 282–287. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple (ERRATA). Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Erratum: Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Skyner, R.E.; Mcdonagh, J.L.; Groom, C.R.; Mourik, T. Van A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solntsev, P.V.; Dudkin, S.V.; Sabin, J.R.; Nemykin, V.N. Electronic Communications in (Z)-Bis(ferrocenyl)ethylenes with Electron-Withdrawing Substituents. Organometallics 2011, 30, 3037–3046. [Google Scholar] [CrossRef]
- Goetsch, W.R.; Solntsev, P.V.; Van Stappen, C.; Purchel, A.A.; Dudkin, S.V.; Nemykin, V.N. Electron-Transfer Processes in 3,4-Diferrocenylpyrroles: Insight into a Missing Piece of the Polyferrocenyl-Containing Pyrroles Family. Organometallics 2014, 33, 145–157. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Soret Band | Q Band | |||
|---|---|---|---|---|
| Max | 1st Shoulder | 2nd Shoulder | Max | |
| ClBSubPc(H)12, 6, [20] | 308 | 525 | 532 | 564 |
| FcCO2BSubPc(H)12, 7 | 299 | 515 | 530 | 563 |
| FcCH2CO2BSubPc(H)12, 8 | 300 | 518 | 534 | 563 |
| Fc(CH2)2CO2BSubPc(H)12, 9, [24] | 302 | 505 | 544 | 563 |
| Fc(CH2)3CO2BSubPc(H)12, 10 | 328 | 521 | 539 | 563 |
| FcCO(CH2)2CO2BSubPc(H)12, 11 | 327 | 523 | 542 | 563 |
| Description | Epa | E°’ (V), ΔEp (V) | ip (μA) b, Current Ratio c | |
|---|---|---|---|---|
| FcCOOHd 1 | Fc | 0.321 | 0.284, 0.074 | 3.60, 0.99 |
| FcCH2COOH d 2 | Fc | 0.047 | 0.014, 0.066 | 3.79, 0.99 |
| Fc(CH2)2COOH d 3 | Fc | 0.020 | −0.015, 0.070 | 3.87, 0.99 |
| Fc(CH2)3COOH d 4 | Fc | 0.011 | −0.024, 0.070 | 3.98, 0.99 |
| FcCO(CH2)2COOH d 5 | Fc | 0.330 | 0.295, 0.070 | 3.66, 0.99 |
| SubPc 6 | DmFc | −0.647 | −0.610, 0.076 | 3.89, 0.99 |
| ClBSubPc(H)12 e | Wave I | 0.674 | 0.628, 0.086 | 3.08, 0.99 |
| Wave II | −1.519 | -, - | 3.63, - | |
| Wave III | −2.050 | -, - | -, - | |
| SubPc 7 | DmFc | −0.647 | −0.610, 0.074 | 3.91, 0.99 |
| FcCO2SubPc(H)12 | Fc | 0.262 | 0.224, 0.076 | 3.61, 0.99 |
| (n = 0) | Wave I | 0.711 | 0.670, 0.082 | 3.34, 0.99 |
| Wave II | −1.643 | −1.601, 0.084 | 3.47, 0.99 | |
| Wave III | −2.124 | -, - | -, - | |
| SubPc 8 | DmFc | −0.647 | −0.610, 0.075 | 3.84, 0.99 |
| FcCH2CO2SubPc(H)12 | Fc | −0.005 | −0.043, 0.076 | 3.61, 0.99 |
| (n = 1) | Wave I | 0.710 | 0.670, 0.080 | 3.38, 0.99 |
| Wave II | −1.715 | −1.674, 0.082 | 3.49,0.99 | |
| Wave III | −2.154 | -, - | -, - | |
| SubPc 9 | DmFc | −0.647 | −0.610,0.074 | 3.89, 0.99 |
| Fc(CH2)2CO2SubPc(H)12f | Fc | −0.021 | −0.058, 0.074 | 3.66, 0.99 |
| (n = 2) | Wave I | 0.712 | 0.670, 0.084 | 3.31, 0.99 |
| Wave II | −1.783 | −1.741, 0.084 | 3.42, 0.99 | |
| Wave III | −2.264 | -, - | -, - | |
| SubPc 10 | DmFc | −0.647 | −0.610, 0.075 | 3.97, 0.99 |
| Fc(CH2)3CO2SubPc(H)12 | Fc | −0.024 | −0.062, 0.076 | 3.58, 0.99 |
| (n = 3) | Wave I | 0.711 | 0.670, 0.082 | 3.27, 0.99 |
| Wave II | −1.871 | −1.829, 0.084 | 3.39, 0.99 | |
| Wave III | −2.344 | -, - | -, - | |
| SubPc 11 | DmFc | −0.647 | −0.610, 0.074 | 3.84, 0.99 |
| FcCO(CH2)2CO2SubPc(H)12 | Fc | 0.300 | 0.262, 0.076 | 3.57, 0.99 |
| Wave I | 0.711 | 0.670, 0.082 | 3.35, 0.99 | |
| Wave II | −1.525 | −1.482, 0.086 | 3.41, 0.99 | |
| Wave III | −2.004 | -, - | -, - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swarts, P.J.; Conradie, J. Synthesis, Spectroscopy, Electrochemistry and DFT of Electron-Rich Ferrocenylsubphthalocyanines. Molecules 2020, 25, 2575. https://doi.org/10.3390/molecules25112575
Swarts PJ, Conradie J. Synthesis, Spectroscopy, Electrochemistry and DFT of Electron-Rich Ferrocenylsubphthalocyanines. Molecules. 2020; 25(11):2575. https://doi.org/10.3390/molecules25112575
Chicago/Turabian StyleSwarts, Pieter J., and Jeanet Conradie. 2020. "Synthesis, Spectroscopy, Electrochemistry and DFT of Electron-Rich Ferrocenylsubphthalocyanines" Molecules 25, no. 11: 2575. https://doi.org/10.3390/molecules25112575