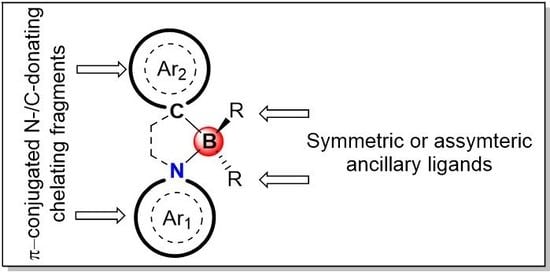
Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials
Abstract
:1. Introduction
2. Boron-Bridged π-Conjugated Materials: Properties and Features
3. π-Conjugated N^C-Chelate Organoboron Materials
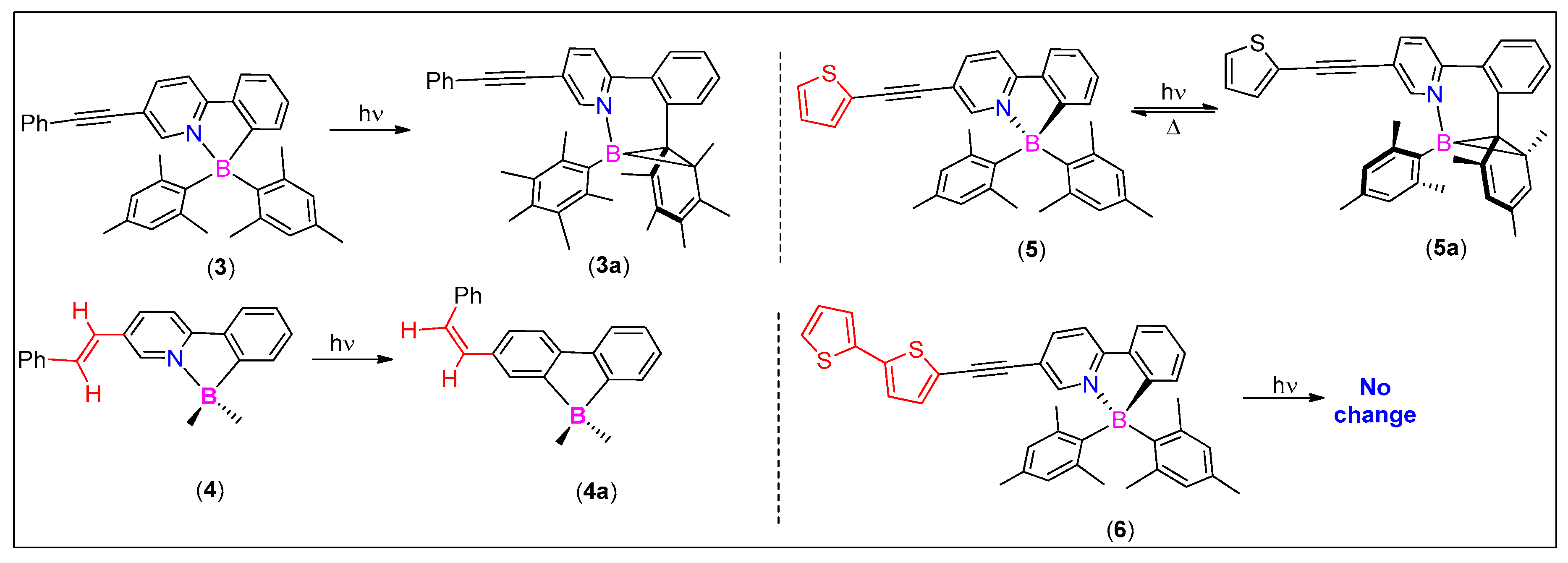
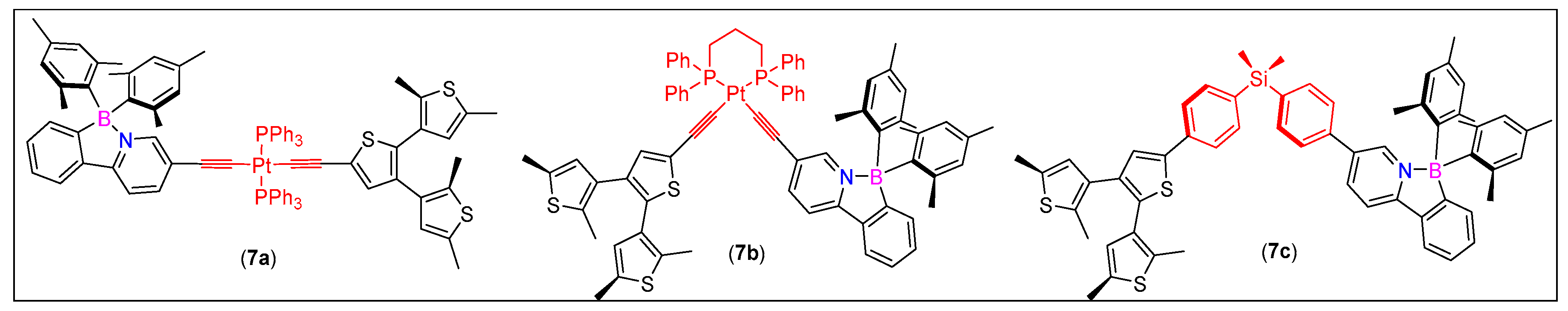
3.1. 2-Arylpyridine-Derived N^C-Chelates
3.2. 2-Arylthiazole and Aminobenzothiadiazole Derived N^C-Chelates
3.3. 2-Arylquinolines-Derived N^C-Chelates
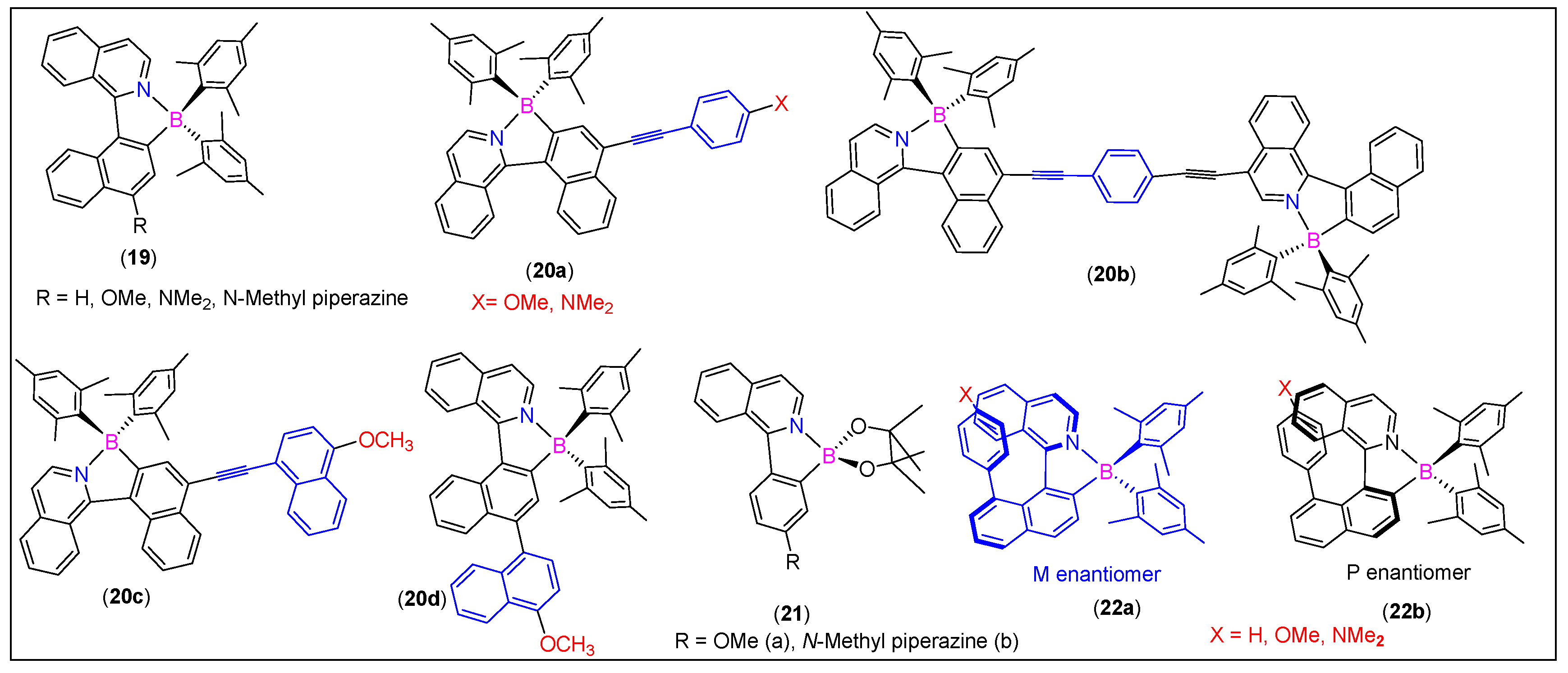
3.4. 1-Arylisoquinolines-Derived N^C-Chelates
4. Applications
4.1. O-E Applications
4.1.1. Organic Light Emitting Diodes (OLEDs)
4.1.2. Organic Field-Effect Transistors (OFETs)
4.1.3. Photovoltaics
4.2. Sensing
4.3. Bioimaging
4.4. Others
5. Opportunities and Challenges
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tour, J.M. Molecular electronics. Synthesis and testing of components. Acc. Chem. Res. 2000, 33, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhang, K.; Zhong, C.; Huang, F.; Cao, Y. Recent advances in water/alcohol-soluble π-conjugated materials: New materials and growing applications in solar cells. Chem. Soc. Rev. 2013, 42, 9071–9104. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Al-Balushi, R.A.; Al-Busaidi, I.J.; Khan, M.S.; Raithby, P.R. Rise of Conjugated Poly-ynes and Poly(Metalla-ynes): From Design Through Synthesis to Structure-Property Relationships and Applications. Chem. Rev. 2018, 118, 8474–8597. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Wang, Y. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs). Chem. Soc. Rev. 2013, 42, 8416–8433. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Mas-Montoya, M.; Xiao, M.; Duan, C.; Wang, Z.; Liu, X.; Janssen, R.A.J.; Yu, G.; Huang, F.; Cao, Y. Adjusting Aggregation Modes and Photophysical and Photovoltaic Properties of Diketopyrrolopyrrole-Based Small Molecules by Introducing B ← N Bonds. Chem. Eur. J. 2019, 25, 564–572. [Google Scholar] [CrossRef]
- Hertz, V.M.; Bolte, M.; Lerner, H.W.; Wagner, M. Boron-Containing Polycyclic Aromatic Hydrocarbons: Facile Synthesis of Stable, Redox-Active Luminophores. Angew. Chem. Int. Ed. Engl. 2015, 54, 8800–8804. [Google Scholar] [CrossRef]
- Glogowski, M.; Williams, J. Boron Photochemistry: XV. Determination of the Hammett Substituent Constant for the p-Dimesitylboryl Group. J. Organomet. Chem. 1981, 218, 137–146. [Google Scholar] [CrossRef]
- Santos, F.M.; Rosa, J.N.; Candeias, N.R.; Carvalho, C.P.; Matos, A.I.; Ventura, A.E.; Florindo, H.F.; Silva, L.C.; Pischel, U.; Gois, P.M. A Three-Component Assembly Promoted by Boronic Acids Delivers a Modular Fluorophore Platform (BASHY Dyes). Chem. Eur. J. 2016, 22, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Wang, F.; Wei, J.; Song, J.; Yan, L.; Luo, J.; Fang, Z.; Wang, Z.; Zhang, W.; He, G. Highly emissive B← N unit containing four-coordinate C, N-Chelated organoboron compound for the detection of fluoride ions. Dyes Pigm. 2019, 166, 410–415. [Google Scholar] [CrossRef]
- Shi, Y.G.; Wang, J.W.; Li, H.; Hu, G.F.; Li, X.; Mellerup, S.K.; Wang, N.; Peng, T.; Wang, S. A simple multi-responsive system based on aldehyde functionalized amino-boranes. Chem. Sci. 2018, 9, 1902–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Xu, W.; Ding, N.; Chang, X.; Shang, C.; Peng, H.; Liu, T.; Fang, Y. A film-based fluorescent device for vapor phase detection of acetone and related peroxide explosives. Mater. Chem. Front. 2019, 3, 1218–1224. [Google Scholar] [CrossRef]
- Li, X.; Tang, P.; Yu, T.; Su, W.; Li, Y.; Wang, Y.; Zhao, Y.; Zhang, H. Two N, N-chelated difluoroboron complexes containing phenanthroimidazole moiety: Synthesis and luminescence properties. Dyes Pigm. 2019, 163, 9–16. [Google Scholar] [CrossRef]
- Dhanunjayarao, K.; Sa, S.; Aradhyula, B.P.R.; Venkatasubbaiah, K. Synthesis of phenanthroimidazole-based four coordinate organoboron compounds. Tetrahedron 2018, 74, 5819–5825. [Google Scholar] [CrossRef]
- Nagura, K.; Saito, S.; Fröhlich, R.; Glorius, F.; Yamaguchi, S. N-Heterocyclic Carbene Boranes as Electron-Donating and Electron-Accepting Components of π-Conjugated Systems. Angew. Chem. Int. Ed. Engl. 2012, 51, 7762–7766. [Google Scholar] [CrossRef]
- Oda, S.; Shimizu, T.; Katayama, T.; Yoshikawa, H.; Hatakeyama, T. Tetracoordinate Boron-Fused Double [5] Helicenes as Cathode Active Materials for Lithium Batteries. Org. Lett. 2019, 21, 1770–1773. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, dithienothiophenes, and thienoacenes: Syntheses, oligomers, polymers, and properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Wakamiya, A.; Yamaguchi, S. Designs of functional π-electron materials based on the characteristic features of boron. Bull. Chem. Soc. Jpn. 2015, 88, 1357–1377. [Google Scholar] [CrossRef] [Green Version]
- Mellerup, S.K.; Wang, S. Boron-doped molecules for optoelectronics. Trends Chem. 2019, 1, 77–89. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Amarne, H.; Wang, S. Photochromic four-coordinate N, C-chelate boron compounds. Coord. Chem. Rev. 2012, 256, 759–770. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Wang, S. Isomerization and rearrangement of boriranes: From chemical rarities to functional materials. Sci. China Mat. 2018, 61, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat’ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its role in energy related research and applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 2–19. [Google Scholar]
- Entwistle, C.D.; Marder, T.B. Boron chemistry lights the way: Optical properties of molecular and polymeric systems. Angew. Chem. Int. Ed. Engl. 2002, 41, 2927–2931. [Google Scholar] [CrossRef]
- Matsuo, K.; Yasuda, T. Enhancing thermally activated delayed fluorescence characteristics by intramolecular B–N coordination in a phenylpyridine-containing donor-acceptor π-system. Chem. Commun. 2017, 53, 8723–8726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, B.; Meng, H.; Xiang, Y.; Li, Y.; Huang, J. Synthesis of aromatic substituted B ← N embedded units with good stability and strong electron-affinity. Tetrahedron Lett. 2019, 60, 151286. [Google Scholar] [CrossRef]
- Patil, N.T.; Shaikh, A.C. N, C-Chelate Four-Coordinate Organoborons with Full Colourtunability. U.S. Patent 10,301,330, 28 May 2019. [Google Scholar]
- Wang, S.; Amarne, H.Y.; Rao, Y. Boron Compounds and Uses Thereof. U.S. Patent 8,697,872 B2, 15 April 2014. [Google Scholar]
- Rao, Y.L.; Amarne, H.; Zhao, S.B.; McCormick, T.M.; Martic, S.; Sun, Y.; Wang, R.Y.; Wang, S. Reversible intramolecular C-C bond formation/breaking and color switching mediated by a N,C-chelate in (2-ph-py)BMes2 and (5-BMes2-2-ph-py)BMes2. J. Am. Chem. Soc. 2008, 130, 12898–12900. [Google Scholar] [CrossRef]
- Baik, C.; Murphy, S.K.; Wang, S. Switching of a single boryl center in pi-conjugated photochromic polyboryl compounds and its impact on fluorescence quenching. Angew. Chem. Int. Ed. Engl. 2010, 49, 8224–8227. [Google Scholar] [CrossRef]
- Rao, Y.L.; Amarne, H.; Chen, L.D.; Brown, M.L.; Mosey, N.J.; Wang, S. Photo- and thermal-induced multistructural transformation of 2-phenylazolyl chelate boron compounds. J. Am. Chem. Soc. 2013, 135, 3407–3410. [Google Scholar] [CrossRef]
- Baik, C.; Hudson, Z.M.; Amarne, H.; Wang, S. Enhancing the photochemical stability of N,C-chelate boryl compounds: C-C bond formation versus C=C bond cis,trans-isomerization. J. Am. Chem. Soc. 2009, 131, 14549–14559. [Google Scholar] [CrossRef]
- Novoseltseva, P.; Li, H.; Wang, X.; Sauriol, F.; Wang, S. Structural dynamics and stereoselectivity of chiral benzylidene-amine N, C-chelate borane photo-thermal isomerization. Chem. Eur. J. 2020, 26, 2276–2284. [Google Scholar] [CrossRef]
- Zeng, C.; Yuan, K.; Wang, N.; Peng, T.; Wu, G.; Wang, S. The opposite and amplifying effect of B←N coordination on photophysical properties of regioisomers with an unsymmetrical backbone. Chem. Sci. 2019, 10, 1724–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Ko, S.B.; Lu, J.S.; Chen, L.D.; Wang, S. Tuning the photoisomerization of a N^C-chelate organoboron compound with a metal-acetylide unit. Chem. Eur. J. 2013, 19, 5314–5323. [Google Scholar] [CrossRef] [PubMed]
- Baik, C.; Wang, S. Inhibiting olefin cis, trans-photoisomerization and enhancing electron-accepting ability of a diboryl compound by metal chelation. Chem. Commun. 2011, 47, 9432–9434. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Amarne, H.; Lu, J.S.; Wang, S. Impact of a dithienyl unit on photostability of N,C-chelating boron compounds. Dalton Trans. 2013, 42, 638–644. [Google Scholar] [CrossRef]
- Wong, H.-L.; Wong, W.-T.; Yam, V.W.-W. Photochromic thienylpyridine–bis (alkynyl) borane complexes: Toward readily tunable fluorescence dyes and photoswitchable materials. Org. Lett. 2012, 14, 1862–1865. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Wang, N.; Peng, T.; Wang, S. Photoisomerization of Pt(II) Complexes Containing Two Different Photochromic Chromophores: Boron Chromophore versus Dithienylethene Chromophore. Chem. Eur. J. 2019, 25, 5757–5767. [Google Scholar] [CrossRef]
- Amarne, H.; Baik, C.; Murphy, S.K.; Wang, S. Steric and electronic influence on photochromic switching of N,C-chelate four-coordinate organoboron compounds. Chem. Eur. J. 2010, 16, 4750–4761. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Wang, S. Impact of cyclometalation and π-conjugation on photoisomerization of an N, C-chelate organoboron compound. Organometallics 2011, 30, 4453–4458. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Yousefalizadeh, G.; Wang, S.; Stamplecoskie, K.G. Experimental Evidence for a Triplet Biradical Excited-State Mechanism in the Photoreactivity of N,C-Chelate Organoboron Compounds. J. Phys. Chem. A 2018, 122, 9267–9274. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Ko, S.B.; Yamaguchi, S.; Wang, S. Modulating the photoisomerization of N,C-chelate organoboranes with triplet acceptors. Org. Lett. 2012, 14, 5610–5613. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhao, D.; Mellerup, S.K.; Peng, T.; Wang, H.; Wang, S. Lanthanide Complexes with Photochromic Organoboron Ligand: Synthesis and Luminescence Study. Inorg. Chem. 2018, 57, 10040–10049. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Liu, K.; Guo, F.; Lalancette, R.A.; Jäkle, F. Luminescent organoboron ladder compounds via directed electrophilic aromatic C–H borylation. Dalton Trans. 2016, 45, 4580–4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.-P.; Zhu, H.-Y.; Li, Q.-S.; Li, Z.-S. Theoretical study on the regioselective photoisomerization of asymmetric N, C-chelate organoboron compounds. Phys. Chem. Chem. Phys. 2019, 21, 8376–8383. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-S.; Zhu, H.-Y. Insights into the Photo-induced Isomerization Mechanisms of a N, C-Chelate Organoboron Compound: A Theoretical Study. ChemPhysChem 2020, 21, 510–517. [Google Scholar]
- Li, C.; Mellerup, S.K.; Wang, X.; Wang, S. Accessing Two-Stage Regioselective Photoisomerization in Unsymmetrical N, C-Chelate Organoboron Compounds: Reactivity of B (ppz)(Mes) Ar. Organometallics 2018, 37, 3360–3367. [Google Scholar] [CrossRef]
- Wakamiya, A.; Taniguchi, T.; Yamaguchi, S. Intramolecular B–N Coordination as a Scaffold for Electron-Transporting Materials: Synthesis and Properties of Boryl-Substituted Thienylthiazoles. Angew. Chem. Int. Ed. Engl. 2006, 45, 3170–3173. [Google Scholar] [CrossRef] [PubMed]
- Job, A.; Wakamiya, A.; Kehr, G.; Erker, G.; Yamaguchi, S. Electronic tuning of thiazolyl-capped pi-conjugated compounds via a coordination/cyclization protocol with B(C6F5)3. Org. Lett. 2010, 12, 5470–5473. [Google Scholar] [CrossRef]
- Shimogawa, H.; Endo, M.; Taniguchi, T.; Nakaike, Y.; Kawaraya, M.; Segawa, H.; Murata, Y.; Wakamiya, A. D–π–A dyes with an intramolecular B-N coordination bond as a key scaffold for electronic structural tuning and their application in dye-sensitized solar cells. Bull. Chem. Soc. Jpn. 2017, 90, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Dou, C.; Ding, Z.; Zhang, Z.; Xie, Z.; Liu, J.; Wang, L. Developing Conjugated Polymers with High Electron Affinity by Replacing a C—C Unit with a B← N Unit. Angew. Chem. Int. Ed. Engl. 2015, 54, 3648–3652. [Google Scholar] [CrossRef]
- Khan, M.S.; Al-Suti, M.K.; Maharaja, J.; Haque, A.; Al-Balushi, R.; Raithby, P.R. Conjugated Poly-ynes and Poly (metalla-ynes) Incorporating Thiophene-based Spacers for Solar Cell (SC) Applications. J. Organomet. Chem. 2015, 812, 13–33. [Google Scholar] [CrossRef] [Green Version]
- Crossley, D.L.; Goh, R.; Cid, J.; Vitorica-Yrezabal, I.; Turner, M.L.; Ingleson, M.J. Borylated arylamine–benzothiadiazole donor–acceptor materials as low-LUMO, low-band-gap chromophores. Organometallics 2017, 36, 2597–2604. [Google Scholar] [CrossRef]
- Crossley, D.L.; Cid, J.; Curless, L.D.; Turner, M.L.; Ingleson, M.J. Facile Arylation of Four-Coordinate Boron Halides by Borenium Cation Mediated Boro-desilylation and-destannylation. Organometallics 2015, 34, 5767–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crossley, D.L.; Vitorica-Yrezabal, I.; Humphries, M.J.; Turner, M.L.; Ingleson, M.J. Highly Emissive Far Red/Near-IR Fluorophores Based on Borylated Fluorene–Benzothiadiazole Donor–Acceptor Materials. Chem. Eur. J. 2016, 22, 12439–12448. [Google Scholar] [CrossRef] [PubMed]
- Shimogawa, H.; Yoshikawa, O.; Aramaki, Y.; Murata, M.; Wakamiya, A.; Murata, Y. 4,7-Bis[3-(dimesitylboryl)thien-2-yl]benzothiadiazole: Solvato-, Thermo-, and Mechanochromism Based on the Reversible Formation of an Intramolecular B-N Bond. Chemistry 2017, 23, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.C.; Ranade, D.S.; Thorat, S.; Maity, A.; Kulkarni, P.P.; Gonnade, R.G.; Munshi, P.; Patil, N.T. Highly emissive organic solids with remarkably broad color tunability based on N,C-chelate, four-coordinate organoborons. Chem. Commun. (Camb.) 2015, 51, 16115–16118. [Google Scholar] [CrossRef] [PubMed]
- Bachollet, S.P.; Volz, D.; Fiser, B.; Munch, S.; Ronicke, F.; Carrillo, J.; Adams, H.; Schepers, U.; Gomez-Bengoa, E.; Brase, S.; et al. A Modular Class of Fluorescent Difluoroboranes: Synthesis, Structure, Optical Properties, Theoretical Calculations and Applications for Biological Imaging. Chem. Eur. J. 2016, 22, 12430–12438. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Chang, Z.; He, B.; Chen, B.; Deng, C.; Lu, P.; Qiu, H.; Tang, B.Z. Aggregation-Induced Emission and Efficient Solid-State Fluorescence from Tetraphenylethene-Based N, C-Chelate Four-Coordinate Organoborons. Chem. Eur. J. 2013, 19, 11512–11517. [Google Scholar] [CrossRef]
- Boscá Mayans, F.; Cuquerella Alabort, M.C.; Fernandes Pais, V.C.; Ros Lao, A.; Pischel, U. Excited-State Pathways of Four-Coordinate N, C-Chelate Organoboron Dyes. ChemPhotoChem 2018, 2, 34–41. [Google Scholar] [CrossRef]
- Domínguez, Z.; Pais, V.N.F.; Collado, D.; Vázquez-Domínguez, P.; Albendín, F.N.; Pérez-Inestrosa, E.; Ros, A.; Pischel, U. π-Extended Four-Coordinate Organoboron N, C-Chelates as Two-Photon Absorbing Chromophores. J. Org. Chem. 2019, 84, 13384–13393. [Google Scholar] [CrossRef]
- Pais, V.F.; Lassaletta, J.M.; Fernandez, R.; El-Sheshtawy, H.S.; Ros, A.; Pischel, U. Organic fluorescent thermometers based on borylated arylisoquinoline dyes. Chemistry 2014, 20, 7638–7645. [Google Scholar] [CrossRef]
- Domínguez, Z.; López-Rodríguez, R.; Álvarez, E.; Abbate, S.; Longhi, G.; Pischel, U.; Ros, A. Azabora [5] helicene Charge-Transfer Dyes Show Efficient and Spectrally Variable Circularly Polarized Luminescence. Chem. Eur. J. 2018, 24, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Z.; Wang, S.; Guo, Y.; Liu, Y. Insight into high-performance conjugated polymers for organic field-effect transistors. Chem 2018, 4, 2748–2785. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Chang, Z.; Jiang, Y.; Chen, B.; Lu, P.; Kwok, H.S.; Qin, A.; Zhao, Z.; Qiu, H. Impacts of intramolecular B–N coordination on photoluminescence, electronic structure and electroluminescence of tetraphenylethene-based luminogens. Dyes Pigm. 2014, 101, 247–253. [Google Scholar] [CrossRef]
- Crossley, D.; Cade, I.; Clark, E.R.; Escande, A.; Humphries, M.; King, S.; Vitorica-Yrezabal, I.; Ingleson, M.; Turner, M. Enhancing electron affinity and tuning band gap in donor–acceptor organic semiconductors by benzothiadiazole directed C-H borylation. Chem. Sci. 2015, 6, 5144–5151. [Google Scholar] [CrossRef] [Green Version]
- Shiu, Y.-J.; Chen, Y.-T.; Lee, W.-K.; Wu, C.-C.; Lin, T.-C.; Liu, S.-H.; Chou, P.-T.; Lu, C.-W.; Cheng, I.-C.; Lien, Y.-J. Efficient thermally activated delayed fluorescence of functional phenylpyridinato boron complexes and high performance organic light-emitting diodes. J. Mat. Chem. C 2017, 5, 1452–1462. [Google Scholar] [CrossRef]
- Wong, B.Y.; Wong, H.L.; Wong, Y.C.; Chan, M.Y.; Yam, V.W. Air-Stable Spirofluorene-Containing Ladder-Type Bis(alkynyl)borane Compounds with Readily Tunable Full Color Emission Properties. Chem. Eur. J. 2016, 22, 15095–15106. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Yuan, K.; Nguyen, C.; Lu, Z.H.; Wang, S. Donor-Appended N,C-Chelate Organoboron Compounds: Influence of Donor Strength on Photochromic Behaviour. Chem. Eur. J. 2016, 22, 12464–12472. [Google Scholar] [CrossRef]
- Mamada, M.; Tian, G.; Nakanotani, H.; Su, J.; Adachi, C. The Importance of Excited-State Energy Alignment for Efficient Exciplex Systems Based on a Study of Phenylpyridinato Boron Derivatives. Angew. Chem. Int. Ed. Engl. 2018, 57, 12380–12384. [Google Scholar] [CrossRef]
- Zhao, R.; Min, Y.; Dou, C.; Lin, B.; Ma, W.; Liu, J.; Wang, L. A Conjugated Polymer Containing a B ← N Unit for Unipolar n-Type Organic Field-Effect Transistors. ACS Appl. Polym. Mater. 2020, 2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Hecht, R.; Kade, J.; Schmidt, D.; Nowak-Krol, A. n-Channel Organic Semiconductors Derived from Air-Stable Four-Coordinate Boron Complexes of Substituted Thienylthiazoles. Chem. Eur. J. 2017, 23, 11620–11628. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, T.; Xiao, Y.; Tang, Z.; Pang, B.; Li, Y.; Xiang, Y.; Zhang, G.; Lu, X.; et al. 8.78% Efficient All-Polymer Solar Cells Enabled by Polymer Acceptors Based on a B ← N Embedded Electron-Deficient Unit. Adv. Mater. 2019, 31, e1904585. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Tang, Z.; Li, Y.; Meng, H.; Xiang, Y.; Li, Y.; Huang, J. Synthesis of Conjugated Polymers Containing B ← N Bonds with Strong Electron Affinity and Extended Absorption. Polymers 2019, 11, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Wang, J.; Jiao, J.; Huang, L.; Tang, J. Recent advances of polymer acceptors for high-performance organic solar cells. J. Mat. Chem. C 2020, 8, 28–43. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Pang, B.; Li, Y.; Xiang, Y.; Guo, L.; Li, X.; Zhan, C.; Huang, J. Effects of Halogenation in B ← N Embedded Polymer Acceptors on Performance of All-polymer Solar Cells. ACS Appl. Mater. Inter. 2020, 12, 2733–2742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Dou, C.; Xie, Z.; Liu, J.; Wang, L. Polymer Acceptor Based on B ← N Units with Enhanced Electron Mobility for Efficient All-Polymer Solar Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 5313–5317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, Z.; Dou, C.; Liu, J.; Wang, L. Development of a donor polymer using a B ← N unit for suitable LUMO/HOMO energy levels and improved photovoltaic performance. Poly. Chem. 2015, 6, 8029–8035. [Google Scholar] [CrossRef]
- Shimogawa, H.; Endo, M.; Nakaike, Y.; Murata, Y.; Wakamiya, A. D-π-A Dyes with Diketopyrrolopyrrole and Boryl-substituted Thienylthiazole Units for Dye-sensitized Solar Cells with High J SC Values. Chem. Lett. 2017, 46, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ding, Z.; Zhao, R.; Jirui, F.; Ma, W.; Liu, J.; Wang, L. Effect of polymer donor aggregation on the active layer morphology of amorphous polymer acceptor-based all-polymer solar cells. J. Mat. Chem. C 2020, 8, 5613–5619. [Google Scholar] [CrossRef]
- Zhao, R.; Lin, B.; Feng, J.; Dou, C.; Ding, Z.; Ma, W.; Liu, J.; Wang, L. Amorphous Polymer Acceptor Containing B← N Units Matches Various Polymer Donors for All-Polymer Solar Cells. Macromolecules 2019, 52, 7081–7088. [Google Scholar] [CrossRef]
- Liu, K.; Lalancette, R.A.; Jäkle, F. B–N Lewis pair functionalization of anthracene: Structural dynamics, optoelectronic properties, and O2 sensitization. J. Am. Chem. Soc. 2017, 139, 18170–18173. [Google Scholar] [CrossRef]
- Liu, K.; Lalancette, R.A.; Jäkle, F. Tuning the structure and electronic properties of B-N fused dipyridylanthracene and implications on the self-sensitized reactivity with singlet oxygen. J. Am. Chem. Soc. 2019, 141, 7453–7462. [Google Scholar] [CrossRef] [PubMed]
- Schraff, S.; Sun, Y.; Pammer, F. Tuning of electronic properties via labile N → B-coordination in conjugated organoboranes. J. Mat. Chem. C 2017, 5, 1730–1741. [Google Scholar] [CrossRef]
- Koch, R.; Sun, Y.; Orthaber, A.; Pierik, A.J.; Pammer, F.D. Turn-on Fluorescence Sensors Based on Dynamic Intramolecular N → B-Coordination. Org. Chem. Front. 2020. [Google Scholar] [CrossRef]
- Haque, A.; Faizi, M.S.H.; Rather, J.A.; Khan, M.S. Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: A review. Bioorg. Med. Chem. 2017, 25, 2017–2034. [Google Scholar] [CrossRef]
- Haque, A.; Al-Balushi, R.A.; Khan, M.S. σ-Acetylide Complexes for Biomedical Applications: Features, Challenges and Future directions. J. Organomet. Chem. 2019, 897, 95–106. [Google Scholar] [CrossRef]
- DeRosa, C.A.; Fraser, C.L. Tetracoordinate Boron Materials for Biological Imaging; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fernandes Pais, V.C.; Alcaide, M.M.; Pischel, U. Strongly Emissive and Photostable Four-Coordinate Organoboron N, C Chelates and Their Use in Fluorescence Microscopy. Chem. Eur. J. 2015, 21, 15369–15376. [Google Scholar] [CrossRef]
- Neumann, P.R.; Crossley, D.L.; Turner, M.; Ingleson, M.; Green, M.; Rao, J.; Dailey, L.A. In Vivo Optical Performance of a New Class of Near-Infrared-Emitting Conjugated Polymers: Borylated PF8-BT. ACS Appl. Mater. Inter. 2019, 11, 46525–46535. [Google Scholar] [CrossRef]
- Chen, J.; Lalancette, R.A.; Jäkle, F. Stereoselective ortho borylation of pyridylferrocenes. Organometallics 2013, 32, 5843–5851. [Google Scholar] [CrossRef]
- Chen, J.; Lalancette, R.A.; Jakle, F. Chiral organoborane Lewis pairs derived from pyridylferrocene. Chem. Eur. J. 2014, 20, 9120–9129. [Google Scholar] [CrossRef]
- Chen, J.; Murillo Parra, D.A.; Lalancette, R.A.; Jäkle, F. Redox-Switchable Chiral Anions and Cations Based on Heteroatom-Fused Biferrocenes. Organometallics 2015, 34, 4323–4330. [Google Scholar] [CrossRef]
- Jiménez, C.C.; Enríquez-Cabrera, A.; González-Antonio, O.; Ordóñez-Hernández, J.; Lacroix, P.G.; Labra-Vázquez, P.; Farfán, N.; Santillan, R. State of the Art of Boron and Tin Complexes in Second-and Third-Order Nonlinear Optics. Inorganics 2018, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Mukundam, V.; Sa, S.; Kumari, A.; Das, R.; Venkatasubbaiah, K. B–N coordinated triaryl pyrazole: Effect of dimerization, and optical and NLO properties. J. Mat. Chem. C 2019, 7, 12725–12737. [Google Scholar] [CrossRef]
- Wang, J.; Jin, B.; Wang, N.; Peng, T.; Li, X.; Luo, Y.; Wang, S. Organoboron-based photochromic copolymers for erasable writing and patterning. Macromolecules 2017, 50, 4629–4638. [Google Scholar] [CrossRef]








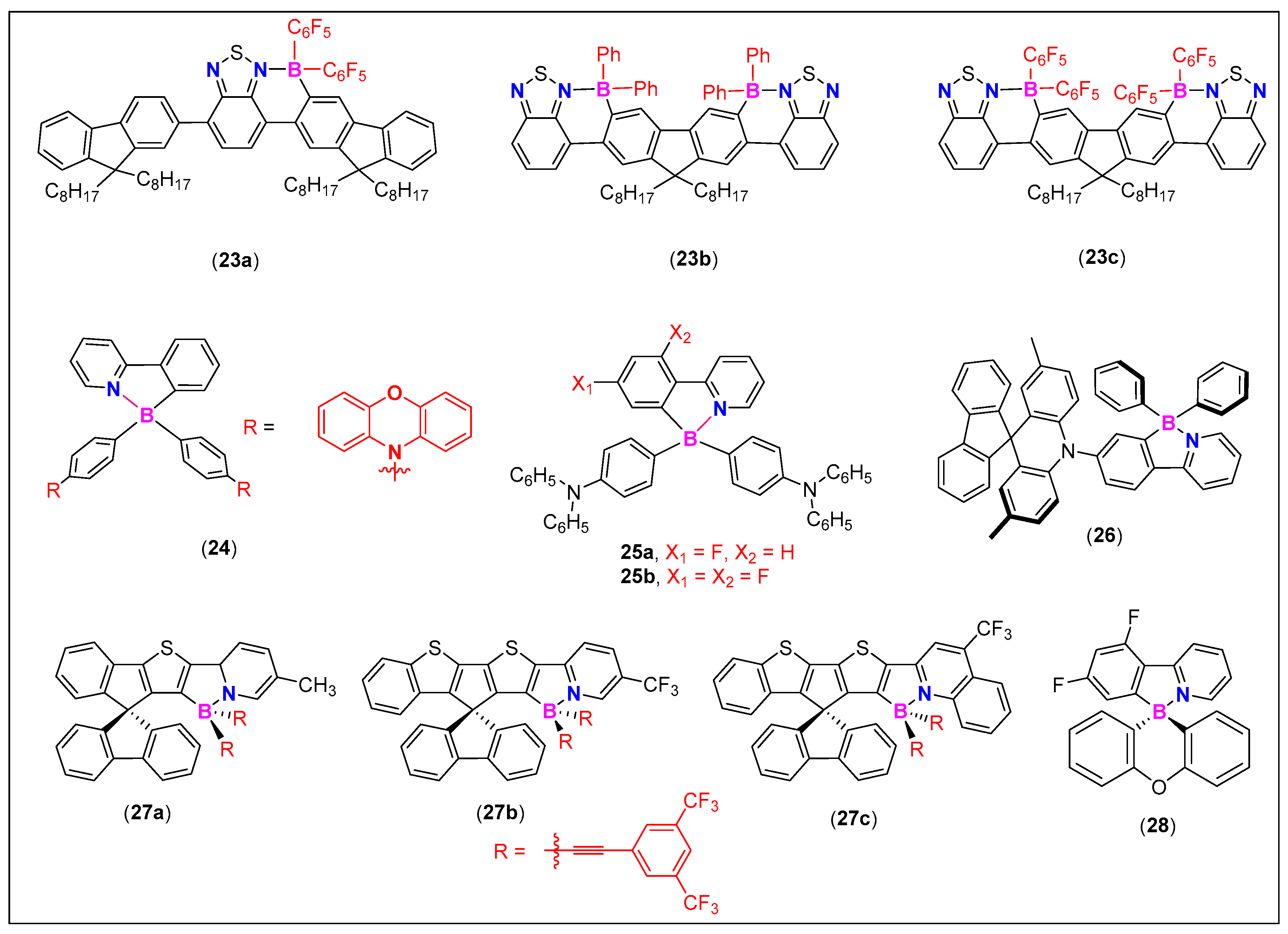
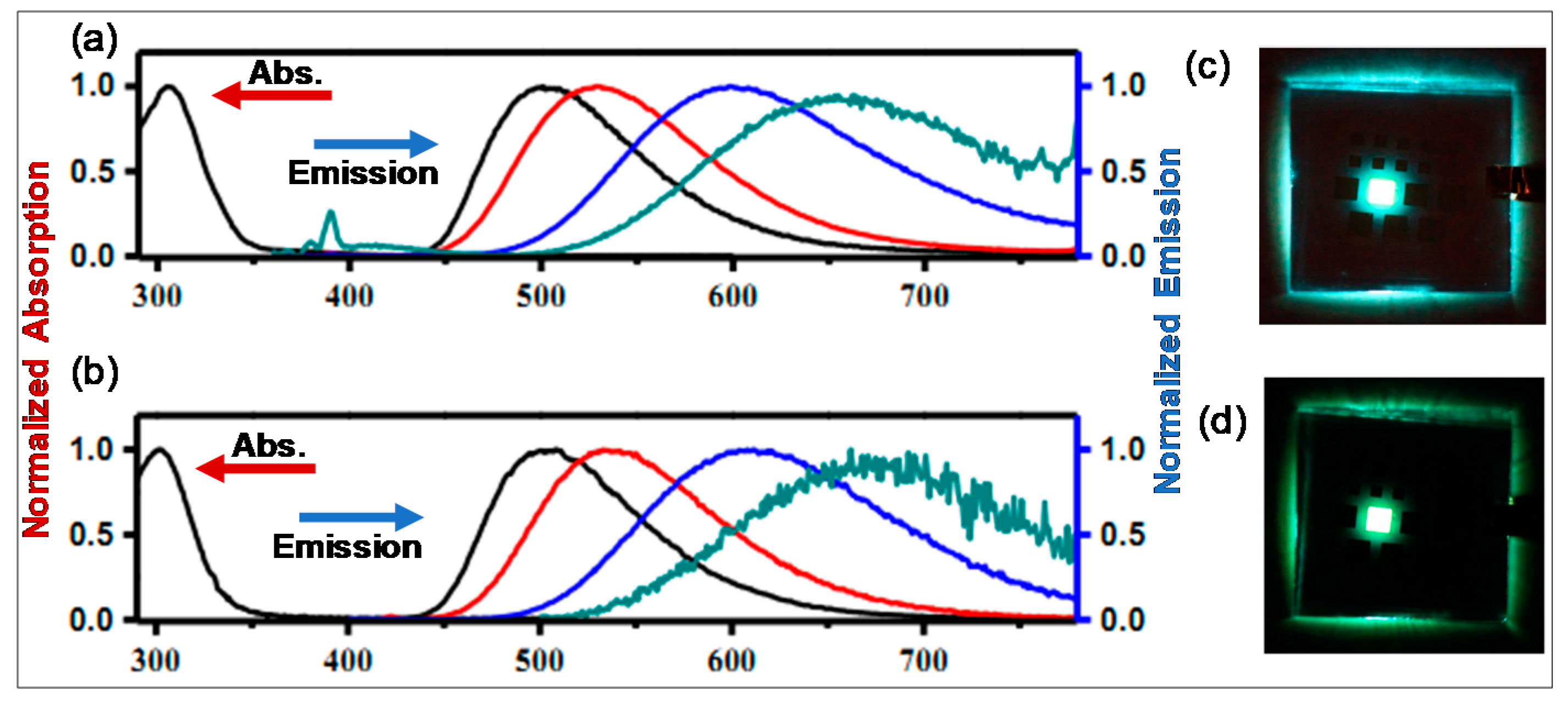




| Comp. # | Device Architecture | EQE (%) | Current Efficiency (Cd/A) | Power Efficiency (lm/W) | Ref. |
|---|---|---|---|---|---|
| 23a | ITO/Plexcore OC/PF8-TFB/PF8-BT/PF8-TFB/23a)/Ba | 0.46 | - | - | [66] |
| 23b | ITO/Plexcore OC/PF8-TFB/PF8-BT/PF8-TFB/23b)/Ba | 0.14 | - | - | [66] |
| 23c | ITO/Plexcore OC/PF8-TFB/PF8-BT/PF8-TFB/23c)/Ba | 0.13 | - | - | [66] |
| 25a | ITO/PEDOT:PSS/TAPC/mCP/mCPCN doped with 25a)/3TPYMB/LiF/Al | 20.2 | 63.9 a | 66.9 a | [67] |
| 25b | ITO/PEDOT:PSS/TAPC/mCP/mCPCN doped with 25b)/3TPYMB/LiF/Al | 26.6 | 88.2 b | 81.5 b | [67] |
| 26 | ITO/HAT-CN/α-NPD/CCP/EML/PPF/TPBi/Liq/Al | 22.7 | 56.4 | 44.3 | [24] |
| 27a | ITO/PEDOT:PSS/27a/MCP/3TPYMB/TmPyPB/LiF/Al | 1.1 | 1.6 | 1.0 | [68] |
| 27b | ITO/PEDOT:PSS/27b/MCP/3TPYMB/TmPyPB/LiF/Al | 1.3 | 4.8 | 3.0 | [68] |
| 27c | ITO/PEDOT:PSS/27b/MCP/3TPYMB/TmPyPB/LiF/Al | 0.9 | 1.4 | 0.9 | [68] |
| Comp. # | Device Architecture | Voc (V) | Jsc (mA/cm−2) | FF (%) | PCE a (%) | Ref. |
|---|---|---|---|---|---|---|
| 32a | ITO/PEDOT:PSS/PTB7-Th:32a/Ca/Al | 0.92 | 11.37 | 48 | 4.95 | [77] |
| ITO/PEDOT:PSS/PTB7:32a/Ca/Al | 0.93 | 9.05 | 45 | 3.71 | ||
| 32c | ITO/PEDOT:PSS/PTB7-Th:32c/Ca/Al | 1.08 | 0.51 | 22 | 0.10 | |
| ITO/PEDOT:PSS/PTB7:32c/Ca/Al | 1.00 | 2.48 | 30 | 0.63 | ||
| 33 | ITO/PEDOT:PSS/PBDB-T:33/PDINO/Al | 0.97 (X = H) 0.95 (X = F) 0.95 (X = Cl) | 4.15 8.74 9.19 | 38.12 43.66 46.55 | 1.54 3.65 4.10 | [76] |
| 34 | ITO/PEDOT:PSS/PBDB-T:34/PNDIT-F3N/Al | 0.92 (X = H) 0.92 (X = F) | 8.01 13.01 | 48.7 69.8 | 3.79 8.42 | [73] |
| 36 | ITO/PEDOT:PSS/36:PC71BM/LiF/Al | 0.82 | 9.89 | 46.1 | 3.62 | [78] |
| 37 | 37/TiO2 | 0.51–0.73 | 10.3–14.2 | 54–72 | 3.9–6.1 | [50] |
| 38 | 38TBP/TiO2 or 38/TiO2 or 38+DCA-TBP/TiO2 or 38+DCA/TiO2 | 0.44–0.68 | 7.8–19.8 | 49–68 | 3.2–6.1 | [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, A.; Al-Balushi, R.A.; Raithby, P.R.; Khan, M.S. Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials. Molecules 2020, 25, 2645. https://doi.org/10.3390/molecules25112645
Haque A, Al-Balushi RA, Raithby PR, Khan MS. Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials. Molecules. 2020; 25(11):2645. https://doi.org/10.3390/molecules25112645
Chicago/Turabian StyleHaque, Ashanul, Rayya A. Al-Balushi, Paul R. Raithby, and Muhammad S. Khan. 2020. "Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials" Molecules 25, no. 11: 2645. https://doi.org/10.3390/molecules25112645