Compatibility of Phase Change Materials and Metals: Experimental Evaluation Based on the Corrosion Rate
Abstract
:1. Introduction
1.1. Literature Review on Compatibility Tests
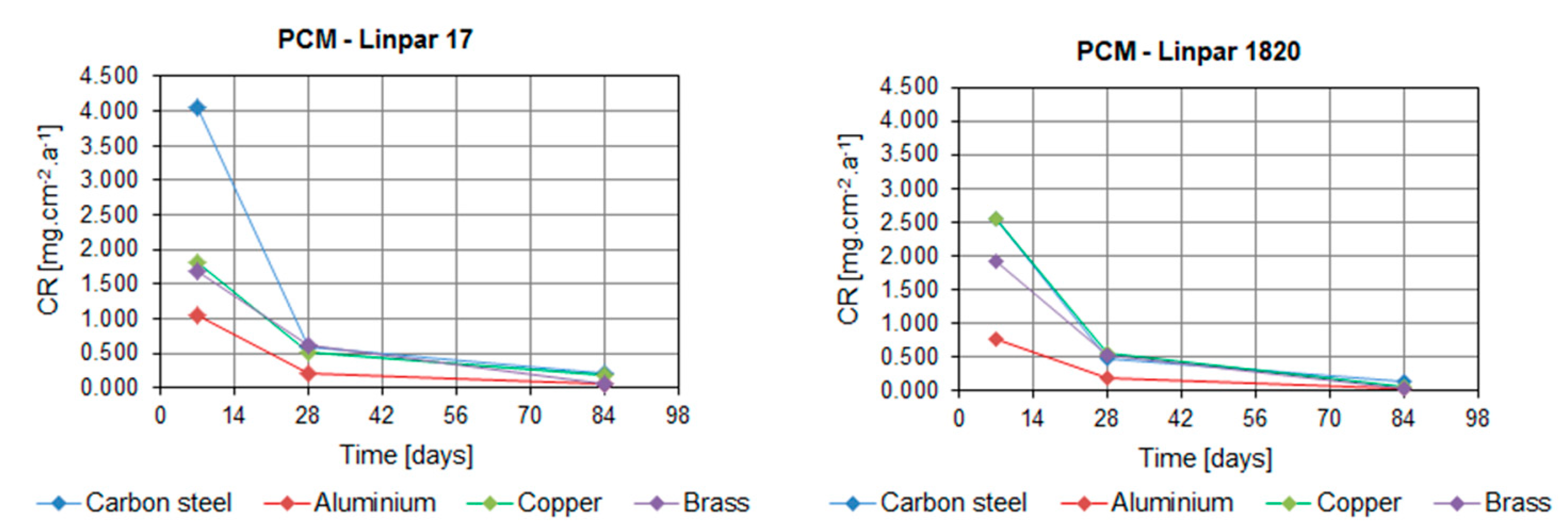
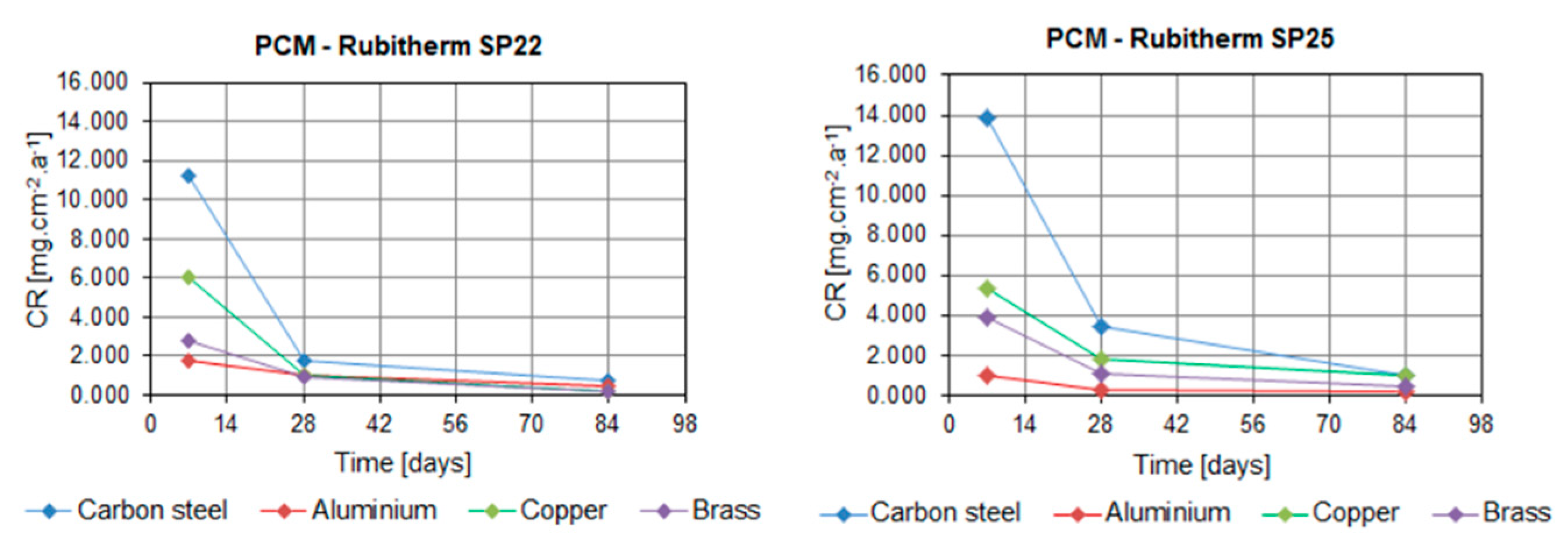
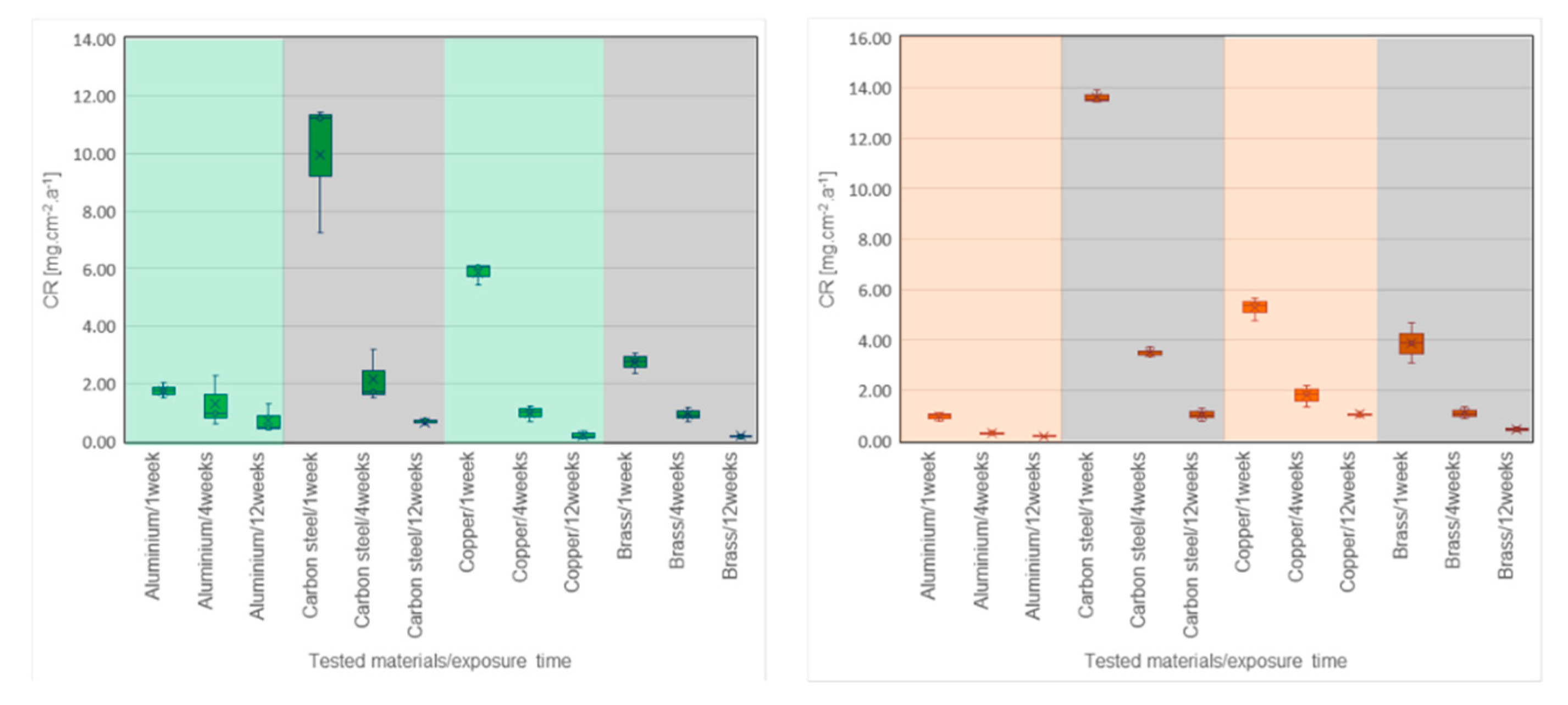
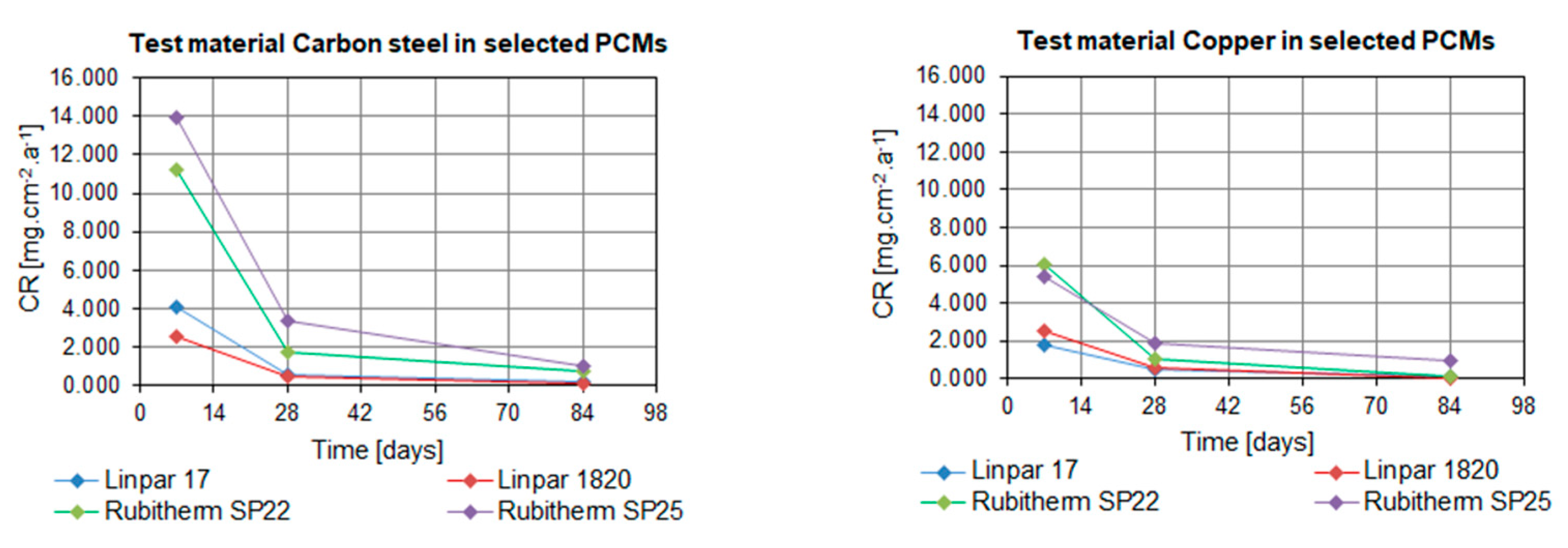
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
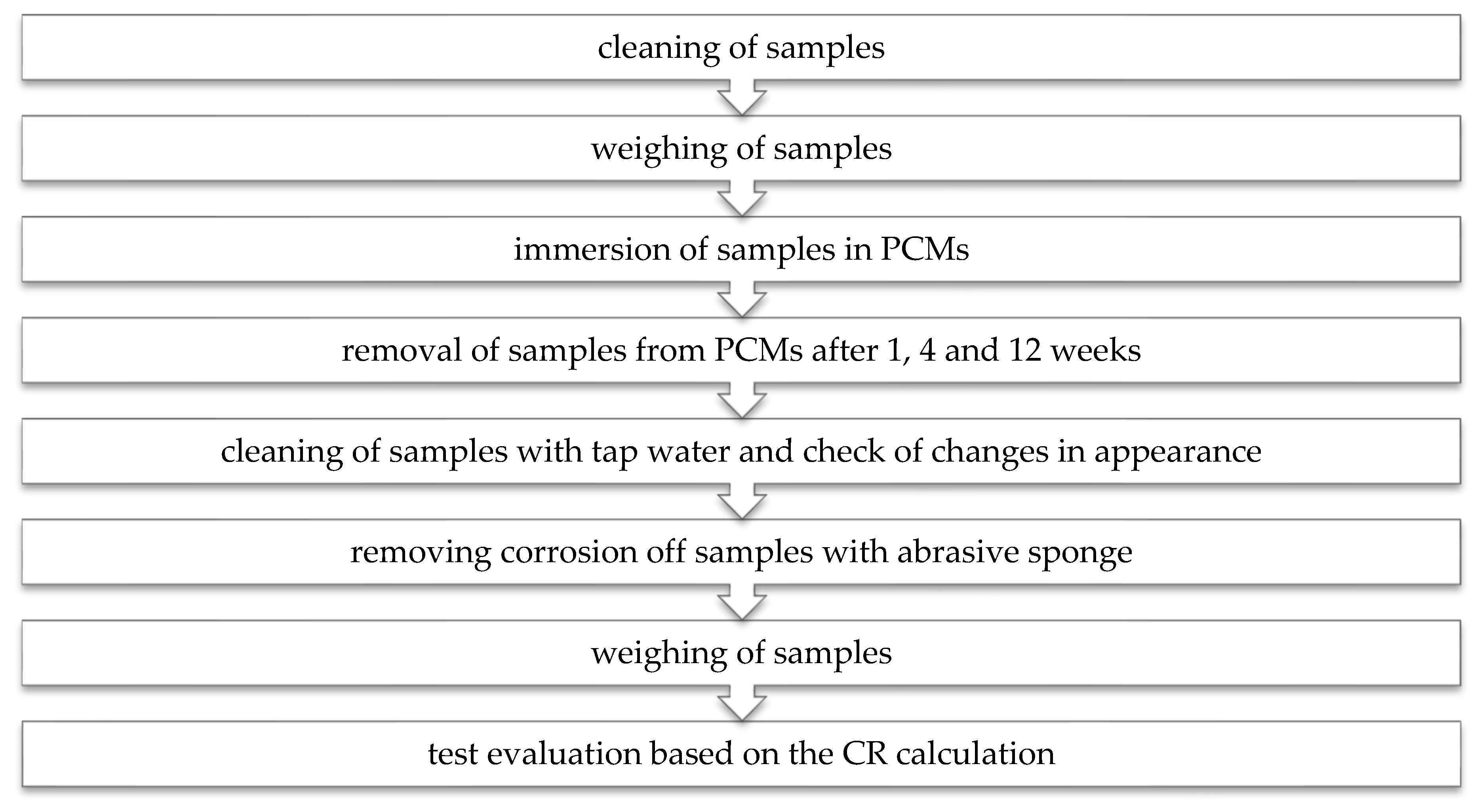
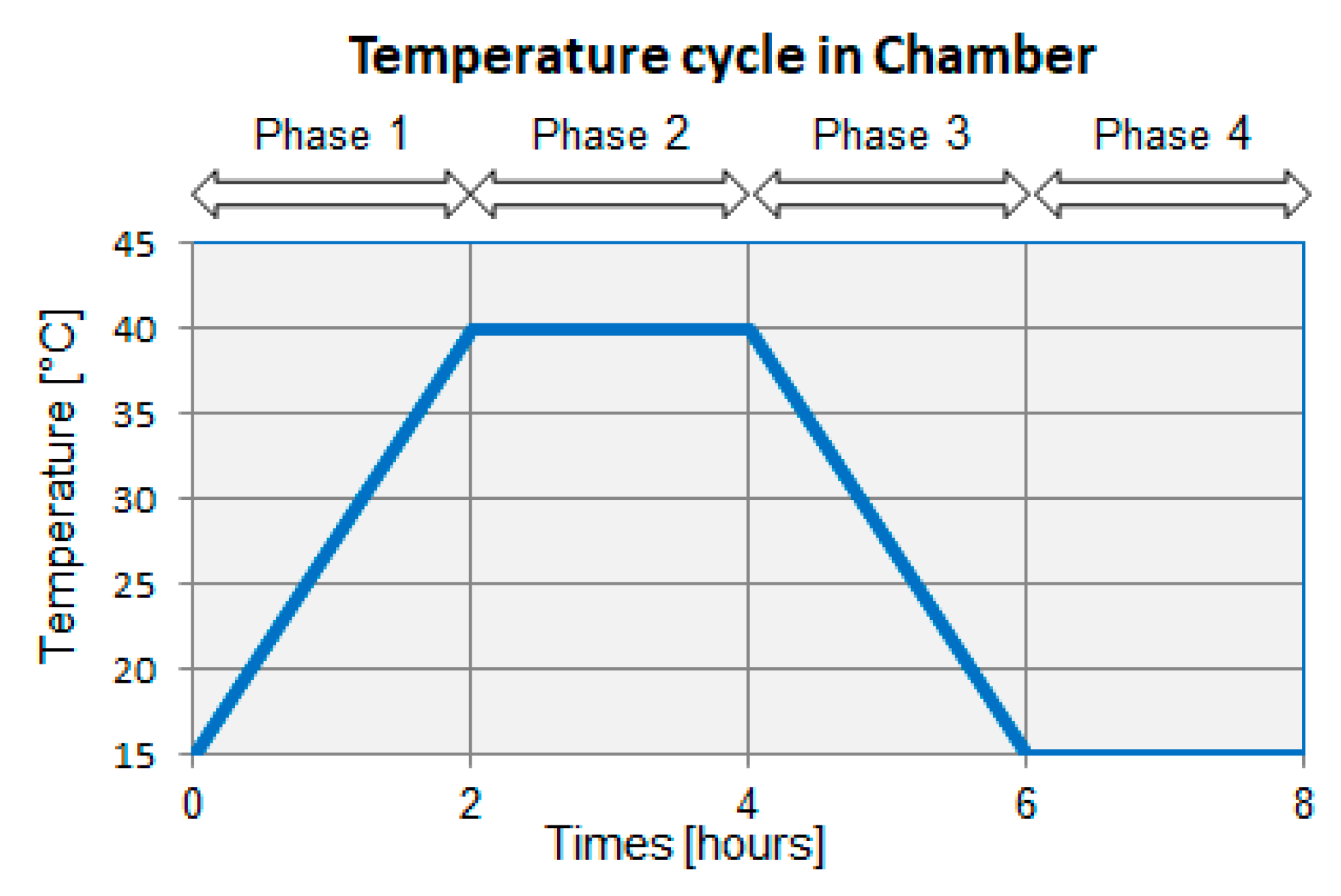
4.2. Experimental
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Directive (EU) 2018/844 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2010/31/EU on the Energy Performance of Buildings and Directive 2012/27/EU on Energy Efficiency. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_2018.156.01.0075.01.ENG (accessed on 10 January 2020).
- Shahid, U.B.; Bicer, Y.; Ahzi, S.; Abdala, A. Thermodynamic assessment of an integrated renewable energy multigeneration system including ammonia as hydrogen carrier and phase change material energy storage. Energy Convers. Manag. 2019, 198, 111809. [Google Scholar] [CrossRef]
- Dréau, J.L.; Heiselberg, P. Energy flexibility of residential buildings using short term heat storage in the thermal mass. Energy 2016, 111, 991–1002. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Arunachalam, S. Latent Heat Storage: Container Geometry, Enhancement Techniques, and Applications—A Review. J. Sol. Energy Eng. 2019, 141, 050801-1–050801-14. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grádea, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G.D. Heat storage materials, geometry and applications: A review. J. Energy Inst. 2017, 90, 1–11. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM. An up to Date Introduction into Basics and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 97–100. [Google Scholar]
- Lane, G.A. Solar Heat Storage: Latent Heat Materials. Volume II. Technology; CRC Press, Inc.: Boca Raton, FL, USA, 1986; pp. 95–98. [Google Scholar]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Krishna, D.J.; Shinde, A. Step by Step Methodology for the Assessment of Metal Corrosion Rate with PCMs Suitable for Low Temperature Heat Storage Applications. Mater. Today Proc. 2017, 4, 10039–10042. [Google Scholar] [CrossRef]
- ASTM G1–03 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. Available online: http://materialstandard.com/astm-g/ (accessed on 10 July 2019).
- Ostry, M.; Beckovsky, D. Influence of panels with phase change materials on the thermal stability of attic spaces. In Proceedings of the 8th IIR Conference on Phase Change Materials and Slurries for Refrigeration and Air Conditioning, Karlsruhe, Germany, 3–5 June 2009; Kauffeld, M., Ed.; Int. Inst. Refrigeration: 177 Blvd. Malesherbes: Paris, France, 2009. [Google Scholar]
- Charvat, P.; Mauder, T.; Ostry, M. Simulation of latent-heat thermal storage integrated with room structures. Mater. Tehnol. 2012, 46, 239–242. [Google Scholar]
- Calabrese, L.; Brancato, V.; Palomba, V.; Proverbio, E. An experimental study on the corrosion sensitivity of metal alloys for usage in PCM thermal energy storages. Renew. Energy 2019, 138, 1018–1027. [Google Scholar] [CrossRef]
- Farrell, A.J.; Norton, B.; Kennedy, D.M. Corrosive effect of salt hydrate phase change materials used with aluminium and copper. J. Mater. Process. Technol. 2006, 176, 198–205. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, I.J.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 32 to 36 °C temperature range. Mater. Corros. 2001, 52, 140–146. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, J.; Nogués, M.; Mehling, H.; Hiebler, S. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 48 to 58 °C temperature range. Mater. Corros. 2002, 53, 902–907. [Google Scholar] [CrossRef]
- Fernández, A.I.; Solé, A.; Giró-Paloma, J.; Martínez, M.; Hadjieva, M.; Boudenne, A.; Constantinescu, M.; Anghel, E.M.; Malikova, M.; Krupa, I.; et al. Unconventional experimental technologies used for phase change materials (PCM) characterization: Part 2–morphological and structural characterization, physico-chemical stability and mechanical properties. Renew. Sustain. Energy Rev. 2015, 43, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Ushak, S.; Marín, P.; Galazutdinova, Y.; Cabeza, L.F.; Farid, M.M.; Grágeda. Compatibility of materials for macroencapsulation of inorganic phase change materials: Experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Moreno, P.; Miró, L.; Solé, A.; Barreneche, C.; Solé, C.; Martonell, I.; Cabeza, L.F. Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 2014, 125, 238–245. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Barreneche, C.; Martonell, I.; Farid, M.M.; Cabeza, L.F. Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 2013, 109, 449–453. [Google Scholar]
- Danielik, V.; Šoška, P.; Felgerová, K.; Zemanová, M. The corrosion of carbon steel in nitrate hydrates used as phase change materials. Mater. Corros. 2017, 68, 416–422. [Google Scholar] [CrossRef]
- Danielik, V.; Šoška, P.; Felgerová, K. Corrosive effects of nitrate-containing phase change materials used with copper. Acta Chim. Slovaca 2016, 9, 75–83. [Google Scholar]
- Zhao, T.; Munis, A.; Rehman, A.U.; Zheng, M. Corrosion behavior of aluminum in molten hydrated salt phase change materials for thermal energy storage. Mater. Res. Express 2020, 7, 015529. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Durham, R.N.; Galetz, M.C. Corrosion behavior of stainless and low-chromium steels and IN625 in molten nitrate salts at 600 °C. Sol. Energy Mater. Sol. Cells 2016, 144, 109–116. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty acids and related phase change materials for reliable thermal energy storage at moderate temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar] [CrossRef]
- Ferrer, G.; Solé, A.; Barreneche, C.; Martonell, I.; Cabeza, L.F. Corrosion of metal containers for use in PCM energy storage. Renew. Energy 2015, 76, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Sari, A.; Kaygusuz, K. Some fatty acids used for latent heat storage: Thermal stability and corrosion of metals with respect to thermal cycling. Renew. Energy 2003, 28, 939–948. [Google Scholar] [CrossRef]
- Browne, M.; Boyd, E.; McCormack, S.J. Investigation of the corrosive properties of phase change materials in contact with metals and plastic. Renew. Energy 2017, 108, 555–568. [Google Scholar] [CrossRef]
- Dheep, G.R.; Sreekumar, A. Thermal reliability and corrosion characteristics of an organic phase change materials for solar space heating applications. J. Energy Storage 2019, 23, 98–105. [Google Scholar] [CrossRef]
- Dheep, G.R.; Sreekumar, A. Investigation on thermal reliability and corrosion characteristics of glutaric acid as an organic phase change material for solar thermal energy storage applications. Appl. Therm. Eng. 2018, 129, 1189–1196. [Google Scholar] [CrossRef]
- Ostrý, M.; Přikryl, R.; Charvát, P. Characterization of selected phase- change materials for proposed use in building applications. Mater. Tehnol. 2013, 47, 185–188. [Google Scholar]




| Authors | Subject of Investigation | Main Results |
|---|---|---|
| Browne et al. [31] | Compatibility tests of aluminum, brass, copper, stainless steel and mild steel in contact with capric acid, capric-palmitic acid, capric-lauric acid, salt hydrate SP22, and Micronal®. | Stainless steel is suitable container material for all tested PCMs. |
| Cabeza et al. [18] | Tests of aluminum, brass, copper, steel and stainless steel in contact with Zn(NO3)2 × 6H2O, Na2HPO4 × 12H2O and CaCl2 × 6H2O | Aluminum and steel should be avoided in PCM containers due to their high corrosion rates; slower corrosion was observed for brass and copper. Stainless steel is corrosion resistant. |
| Cabeza et al. [19] | Tests of aluminum, brass, copper, steel, and stainless steel in contact with NaOAc × 3H2O and Na2S2O3 × 5H2O (in pure form and enhanced with graphite). | Aluminum, steel, and stainless steel are suitable container materials for the tested PCMs, although authors recommended monitoring of steel in contact with graphite. |
| Calabrese et al. [16] | Compatibility between magnesium nitrate PlusIce S83 and aluminum alloy, copper alloy, and carbon steel | Aluminum alloy is suitable container material for Plus Ice S83 PCM. Carbon steel and copper alloy suffer too low electrochemical stability. |
| Danielik et al. [24,25] | Corrosion resistance of carbon steel and copper in contact with Mg(NO3)2 × 6H2O, Mg(NO3)2 × 6H2O + 0.5 wt% Mg(OH)2, Mg(NO3)2 × 6H2O + 0.5 wt% Sr(OH)2, Mg(NO3)2 × 6H2O + Ca(NO3)2 × 4H2O (1:1) and Ca(NO3)2 × 4H2O. | Corrosion rate of carbon steel is much higher than corrosion rate of copper when exposed to tested PCMs. |
| Dheep and Sreekumar [32] | Corrosion analysis of phenyl acetic acid in contact with copper, aluminum, and stainless steel. | Phenyl acetic acid is less corrosive and compatible with stainless steel. The higher corrosion rate was calculated for copper. |
| Dheep and Sreekumar [33] | Compatibility tests for glutaric acid and copper, aluminum, and stainless steel | The lowest corrosion rate and the lowest mass loss were calculated for stainless steel |
| Dorcheh et al. [27] | Tests for two low chromium alloys, two high chromium stainless steels, and Ni-alloy immersed in molten eutectic composition. | The best material is Ni-alloy, alloyed steels are recommended with additional protective coatings. |
| Farrell et al. [17] | Corrosion resistance of aluminum alloy and copper in contact with PCMs PlusICE E17 and ClimSel C18. | Aluminum combined with copper in a heat exchanger is prone to galvanic corrosion due to exposure to tested PCMs. |
| Fernández et al. [20] | Compatibility tests of aluminum, brass, copper, steel, stainless steel and carbon steel in contact with 13 salt hydrates. | Carbon steel is not recommended or cautiously recommended as a container material for the tested PCMs. Stainless steel is recommended as container material for inorganic PCMs |
| Ferrer et al. [29] | Compatibility tests of stainless-steel SS-316 and SS-304, copper, aluminum, and carbon steel in contact with inorganic SP21E, bio-based PureTemp 23, and two fatty acid eutectics. | All tested metals are recommended as container materials for bio-based PCM. Stainless steel and copper also proved resistant to inorganic PCM. |
| Kahvaji et al. [28] | Study focused on the compatibility potential of 16 samples of container materials or supplementary gasket materials with five fatty acids and one alcohol. | Stainless steel alloys and aluminum alloys have shown good compatibility with all PCMs. |
| Moreno et al. [22] | Corrosion potential of five inorganic PCMs for cooling applications and six inorganic PCMs for heating application on copper, aluminum, stainless steel | Confirmed corrosive resistance of stainless steel exposed to the tested inorganic PCMs. |
| Oró et al. [23] | Study on corrosion of copper, aluminum, stainless steel carbon steel exposed to three commercial PCMs and seven PCMs with special formulation based on the NH4Cl + H2O | Only stainless steel is recommended as PCM container material. Copper and carbon steel should be avoided, and aluminum is not recommended for long term PCM storage. |
| Sari et Kaygusuz [30] | Study on compatibility of stearic acid, palmitic acid, myristic acid and lauric acid with carbon steel, stainless steel, aluminum, and copper. | The highest corrosion rates were calculated for carbon steel and copper in contact with myristic acid, the lowest corrosion rate was calculated for stainless steel. |
| Ushak et al. [21] | Corrosion effect of bischofite and commercial MgCl2 × 6H2O on copper, aluminum, and stainless steel. | Copper has the highest and stainless steel the lowest mass loss. |
| Zhao et al. [26] | Study on corrosion of aluminum Al-1060 immersed in molten hydrated salt PCM composite Na2HPO4 × 12H2O − Na2SO4 × 10H2O | Corrosion property of composite PCM is highly related with the pH. |
| Metal | Exposure Time | Corrosion Rate (mg·cm−2·year−1) | |||
|---|---|---|---|---|---|
| Linpar 17 | Linpar 1820 | RT SP22 | RT SP25 | ||
| Carbon steel | 1 week | 4.058 | 2.562 | 11.236 | 13.897 |
| 4 weeks | 0.589 | 0.492 | 1.723 | 3.413 | |
| 12 weeks | 0.214 | 0.135 | 0.712 | 1.029 | |
| Aluminum | 1 week | 1.049 | 0.762 | 1.765 | 1.024 |
| 4 weeks | 0.219 | 0.190 | 0.991 | 0.292 | |
| 12 weeks | 0.053 | 0.033 | 0.460 | 0.194 | |
| Copper | 1 week | 1.807 | 2.564 | 6.049 | 5.400 |
| 4 weeks | 0.525 | 0.572 | 1.013 | 1.863 | |
| 12 weeks | 0.174 | 0.072 | 0.152 | 1.004 | |
| Brass | 1 week | 1.685 | 1.926 | 2.795 | 3.882 |
| 4 weeks | 0.621 | 0.527 | 0.894 | 1.061 | |
| 12 weeks | 0.044 | 0.040 | 0.168 | 0.476 | |
| Material (Metals) | Criterion | PCMs (Organic Group Linpar and Inorganic Group RT) | |||
|---|---|---|---|---|---|
| Linpar 17 | Linpar 1820 | RT SP22 | RT SP25 | ||
| carbon steel | CR | 0.21 to 4.05 | 0.13 to 2.56 | 0.71 to 11.23 | 1.02 to 13.89 |
| corrosion | no corrosion surface w/o change | no corrosion surface w/o change | corrosion–red color | corrosion–red color | |
| recommendation | recommended | recommended | cautiously recommended | cautiously recommended | |
| aluminium | CR | 0.05 to 1.05 | 0.03 to 0.76 | 0.46 to 1.76 | 0.19 to 1.02 |
| corrosion | no corrosion, shiny surface | no corrosion, shiny surface | no corrosion, surface tarnish, occurrence of cracks | no corrosion, surface tarnish | |
| recommendation | recommended | recommended | recommended | recommended | |
| copper | CR | 0.17 to 1.80 | 0.07 to 2.56 | 0.15 to 2.79 | 1.00 to 5.40 |
| corrosion | no corrosion, shiny to matt surface with time | no corrosion, shiny to matt surface with time | corrosion–blue color, surface tarnish | corrosion–blue color, surface tarnish | |
| recommendation | recommended | recommended | recommended | recommended | |
| brass | CR | 0.04 to 1.68 | 0.04 to 1.92 | 0.16 to 6.04 | 0.47 to 3.88 |
| corrosion | no corrosion, shiny surface | no corrosion, shiny surface | no corrosion, surface tarnish | no corrosion, surface tarnish | |
| recommendation | recommended | recommended | recommended | recommended | |
| Type | Product Name | Manufacturer | Latent Heat [J∙g−1] | Onset Temperature [°C] | Peak Temperature [°C] |
|---|---|---|---|---|---|
| inorganic | SP22 | Rubitherm Technologies GmbH, Berlin, Germany | 145 | 14 | 25 |
| inorganic | SP25 | Rubitherm Technologies GmbH, Berlin, Germany | 122 | 18 | 28 |
| organic | Linpar 17 | Sasol Germany GmbH, Hamburg, Germany | 152 | 21 | 22 |
| organic | Linpar 1820 | Sasol Germany GmbH, Hamburg, Germany | 141 | 24 | 27 |
| CR [mg·cm−2·year−1] | Recommendation |
|---|---|
| >1000 | Completely destroyed within days |
| 100–999 | Not recommended for service greater than a month |
| 50–99 | Not recommended for service greater than one year |
| 10–49 | Caution recommended, based on the specific application |
| 0.3–9.9 | Recommended for long term service |
| <0.2 | Recommended for long term service; no corrosion, other than as a result of surface cleaning, was evidenced |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrý, M.; Bantová, S.; Struhala, K. Compatibility of Phase Change Materials and Metals: Experimental Evaluation Based on the Corrosion Rate. Molecules 2020, 25, 2823. https://doi.org/10.3390/molecules25122823
Ostrý M, Bantová S, Struhala K. Compatibility of Phase Change Materials and Metals: Experimental Evaluation Based on the Corrosion Rate. Molecules. 2020; 25(12):2823. https://doi.org/10.3390/molecules25122823
Chicago/Turabian StyleOstrý, Milan, Sylva Bantová, and Karel Struhala. 2020. "Compatibility of Phase Change Materials and Metals: Experimental Evaluation Based on the Corrosion Rate" Molecules 25, no. 12: 2823. https://doi.org/10.3390/molecules25122823





