Role of Phase Change Materials Containing Carbonized Rice husks on the Roof-Surface and Indoor Temperatures for Cool Roof System Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CRHs
2.3. Preparation of PCM/CRH Packs and Set Up of Cool Roof Miniature Model
2.4. Characterization
3. Results and Discussion
3.1. XRD and Thermal Conductivity of PCM/CRHs
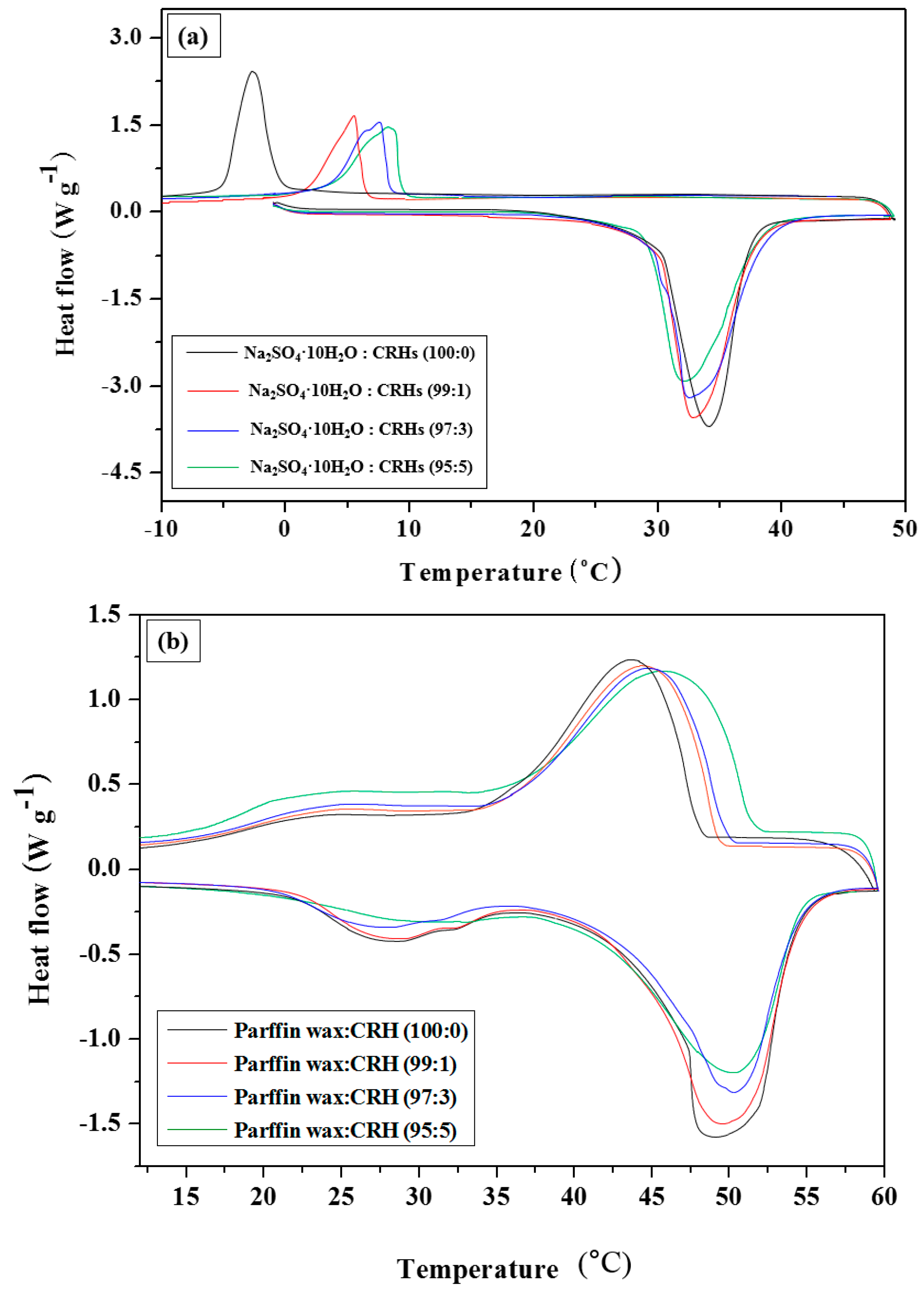
3.2. Latent heat of PCM/CRHs
3.3. Influence of Na2SO4·10H2O/CRH in the WPC on the Roof-Surface and Indoor Temperature of the Cool Roof Miniature Model
3.4. Influence of Parffin Wax/CRH in the WPC on the Roof-Surface and Indoor Temperature of the Cool Roof Miniature Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jayasinghe, M.T.R.; Attalage, R.A.; Jayawardena, A.I. Roof orientation, roofing materials and roof surface color: their influence on indoor thermal comfort in warm humid climates. Energy Sustain. Dev. 2003, 7, 16–27. [Google Scholar] [CrossRef]
- Ozel, M. Effect of indoor design temperature on the heating and cooling transmission loads. J. Build. Eng. 2016, 7, 46–52. [Google Scholar] [CrossRef]
- Arumugam, R.S.; Garg, V.; Ram, V.V.; Bhatia, A. Optimizing roof insulation for roofs with high albedo coating and radiant barriers in India. J. Build. Eng. 2015, 2, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Karam, M.A.; Mazran, I.; Abdul, M.A.R. A review of the potential of attic ventilation by passive and active turbine ventilators in tropical Malaysia. Sustain. Cities Soc. 2014, 10, 232–240. [Google Scholar]
- Anna, L.P. Thermal-energy analysis of roof cool clay tiles for application in historic buildings and cities. Sustain. Cities Soc. 2015, 19, 271–280. [Google Scholar]
- Latha, P.K.; Darshana, Y.; Venugopal, V. Role of building material in thermal comfort in tropical climates – a review. J. Build. Eng. 2015, 3, 104–113. [Google Scholar] [CrossRef]
- Fahmy, M.; Sharples, S. On the development of an urban passive thermal comfort system in Cario. Build. Environ. 2009, 44, 1907–1916. [Google Scholar]
- Giridharan, R.; Ganesan, S.; Lau, S. Daytime urban heat island effect in high-rise and high-density residential developments in Hong Kong. Energy Build. 2004, 36, 525–534. [Google Scholar] [CrossRef]
- Giridharan, R.; Lau, S.; Ganesan, S.; Givoni, B. Urban design factors influencing heat island intensity in high-rise high-density environments of Hong Kong. Build. Environ. 2007, 42, 3669–3684. [Google Scholar] [CrossRef]
- Giridharan, R.; Lau, S.; Ganesan, S.; Givoni, B. Lowering the outdoor temperature in high-rise high-density residential developments of coastal Hong Kong: The vegetation influence. Build. Environ. 2008, 43, 1583–1595. [Google Scholar] [CrossRef]
- Ibáñez, M.; Lazaro, A.; Zalba, B.; Cabeza, L.F. An approach to the simulation of PCMs in building applications using TRNSYS. Appl. Therm. Eng. 2005, 25, 1796–1807. [Google Scholar] [CrossRef]
- Cabeza, L.F.; de Gracia, A. Thermal energy storage (TES) systems for cooling in residential buildings. In Advances in Thermal Energy Storage Systems; Cabeza, L.F., Ed.; Elsevier: Cambridge, UK, 2015; pp. 549–572. [Google Scholar]
- Tabares-Velasco, P.C.; Christensen, C.; Bianchi, M. Verification and validation of EnergyPlus phase change material model for opaque wall assemblies. Build. Environ. 2012, 54, 186–196. [Google Scholar] [CrossRef]
- Kuznik, F.; Virgone, J.; Johannes, K. Development and validation of a new TRNSYS type for the simulation of external building walls containing PCM. Energy Build. 2010, 42, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Kheradmand, M.; Azenha, M.; De Aguiar, J.L.B.; Krakowiak, K. Thermal behavior of cement based plastering mortar containing hybrid microencapsulated phase change materials. Energy Build. 2014, 84, 526–536. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, M.; Kim, H.Y.; Park, S.-J. Thermal property and latent heat energy storage behavior of sodium acetate trihydrate composites containing expanded graphite and carboxymethyl cellulose for phase change materials. Appl. Therm. Eng. 2015, 75, 978–983. [Google Scholar] [CrossRef]
- Shin, H.K.; Rhee, K.-Y.; Park, S.-J. Effects of exfoliated graphite on the thermal properties of erythritol-based composites used as phase-change materials. Compos. Part B Eng. 2016, 96, 350–353. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.J.; Lee, S.O.; Lee, U.S.; Shin, H.K. Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material. Nanomaterials 2019, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Haillot, D.; Bauer, T.; Tamme, R. Thermal analysis of phase change materials in the temperature range 120–150 °C. Themochim. Acta 2011, 513, 49–59. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Waste heat transportation system, using phase change material (PCM) from steelworks to chemical plant. Resour. Conserv. Recycl. 2010, 54, 1000–1006. [Google Scholar] [CrossRef]
- Yang, Y.K.; Kang, I.S.; Chung, M.H.; Kim, S.; Park, J.C. Effect of PCM cool roof system on the reduction in urban heat island phenomenon. Build. Environ. 2017, 122, 411–421. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y.; Liu, C.; Wu, G. Numerical analysis on thermal performance of roof contained PCM of a single residential building. Energy Convers. Manag. 2015, 100, 147–156. [Google Scholar] [CrossRef]
- Jayalath, A.; Aye, L.; Mendis, P.; Ngo, T. Effects of phase change material roof layers on thermal performance of a residential building in Melbourne and Sydney. Energy Build. 2016, 121, 152–158. [Google Scholar] [CrossRef]
- Saffari, M.; de Gracia, A.; Ushak, S.; Cabeza, L.F. Passive cooling of buildings with phase change materials using whole building energy simulation tools: A review. Renew. Sust. Energ. Rev. 2017, 80, 1239–1255. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, V.; Evola, G.; Marletta, L.; Nocera, F. The effectiveness of phase change materials in relation to summer thermal comfort in air-conditioned office buildings. Build. Simul. 2018, 11, 1145–1161. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Wang, L.; Metcalf, S.; Critoph, R.E.; Thorpe, R.; Tamainot-Telto, Z. Thermal conductivity and permeability of consolidated expanded natural graphite treated with sulphuric acid. Carbon 2011, 49, 4812–4819. [Google Scholar] [CrossRef]
- Marín, J.M.; Zalba, B.; Cabeza, L.F.; Mehling, H. Improvement of a thermal energy storage using plates with paraffin–graphite composite. Int. J. Heat Mass Transf. 2005, 48, 2561–2570. [Google Scholar] [CrossRef]
- Wu, H.; Lu, C.; Zhang, W.; Zhang, X. Preparation of low-density polyethylene/low-temperature expandable graphite composites with high thermal conductivity by an in situ expansion melt blending process. Mater. Des. 2013, 52, 621–629. [Google Scholar] [CrossRef]
- Chen, B.; Han, M.; Zhang, B.; Ouyang, G.; Shafei, B.; Wang, X.; Hu, S. Efficient Solar-to-Thermal Energy Conversion and Storage with High-Thermal-Conductivity and Form-Stabilized Phase Change Composite Based on Wood-Derived Scaffolds. Energies 2019, 12, 1283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Tian, Y.; Jin, X.; Lo, Y.T.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 2205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wen, R.; Huang, Z.; Tang, C.; Huang, Y.; Min, X.; Fang, M.; Wu, X.; Min, X.; Xu, Y. Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build. 2017, 149, 463–470. [Google Scholar] [CrossRef]
- Agrawal, R.; Hanna, J.; Gunduz, I.E.; Luhrs, C.C. Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers. Molecules 2019, 24, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Wang, S.; Tian, Y.; Zhou, D. Comprehensive evaluation of Paraffin-HDPE shape stabilized PCM with hybrid carbon nano-additives. Appl. Therm. Eng. 2019, 163, 114404. [Google Scholar] [CrossRef]
- Vivekananthan, M.; Amirtham, V.A.; Mayilvelnathan, V.; Arasu, A.V. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells 2019, 192, 72–80. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


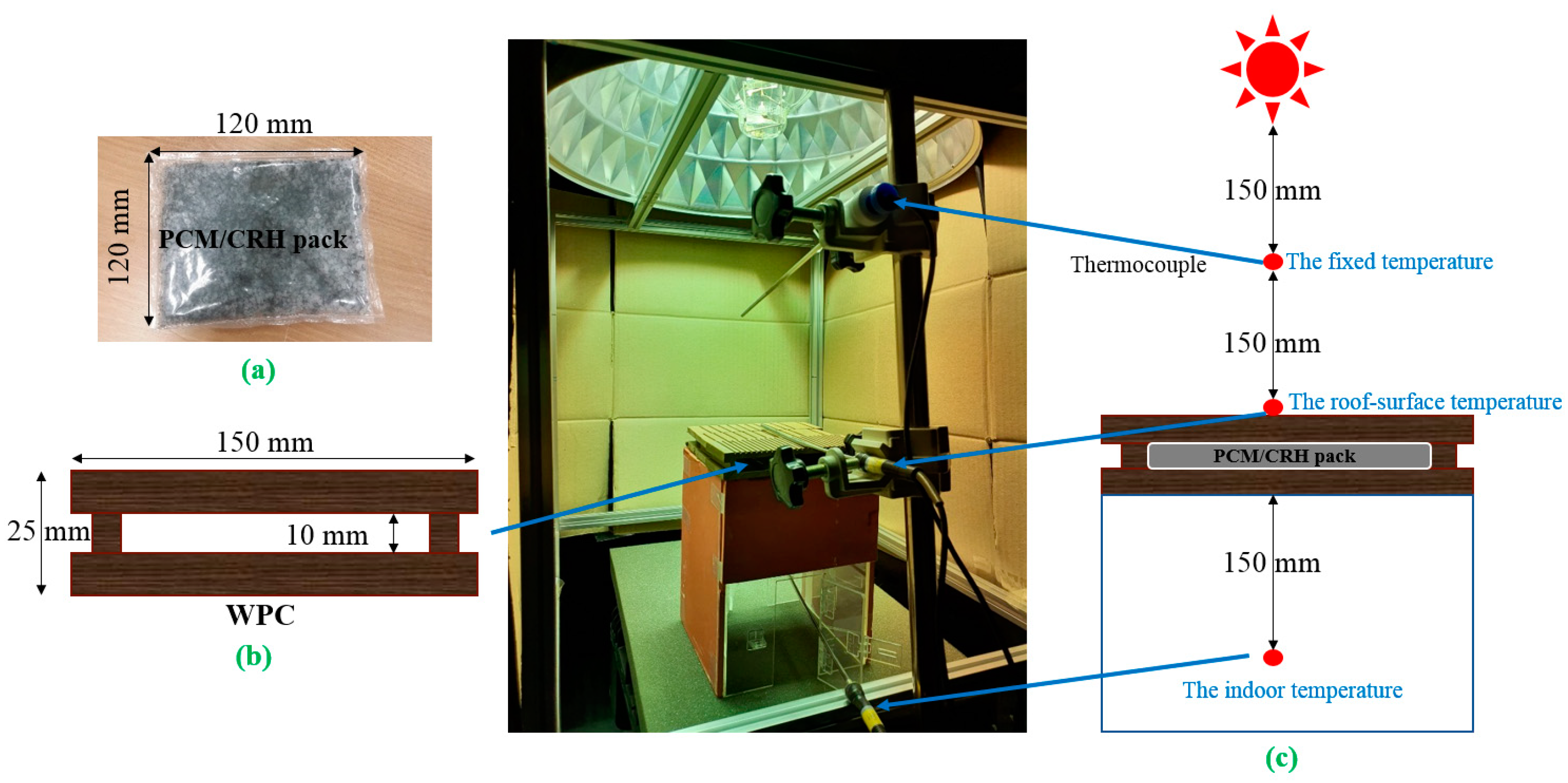

 depicts the fixed temperature): (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C.
depicts the fixed temperature): (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C.
 depicts the fixed temperature): (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C.
depicts the fixed temperature): (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C.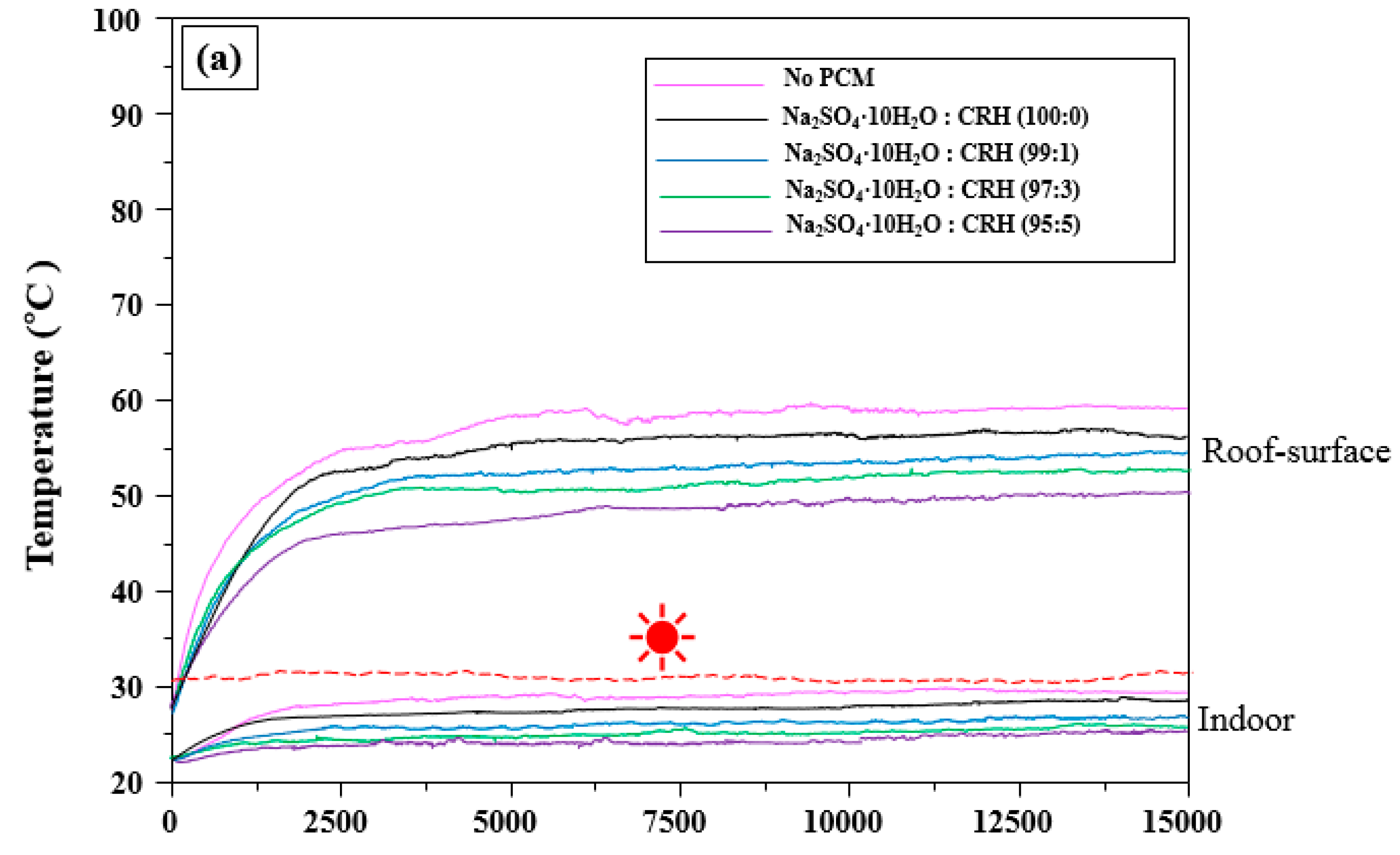

 depicts the fixed temperature: (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C).
depicts the fixed temperature: (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C).
 depicts the fixed temperature: (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C).
depicts the fixed temperature: (a) 30 to 32 °C, (b) 35 to 37 °C, and (c) 40 to 42 °C).

| PCM | PCM:CRH wt.% | |||
|---|---|---|---|---|
| Na2SO4·10H2O:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Density (kg m−3) | 858.88 ± 15.6 | 867.55 ± 3.3 | 883.00 ± 7.0 | 894.80 ± 4.2 |
| Paraffin wax:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Density (kg m−3) | 450.12 ± 8.4 | 477.07 ± 6.2 | 501.56 ± 6.9 | 521.25 ± 9.3 |
| PCM | PCM:CRH wt.% | |||
|---|---|---|---|---|
| Na2SO4·10H2O:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Thermal conductivity (W mK−1) | 0.718 ± 0.007 | 0.826 ± 0.008 | 1.01 ± 0.007 | 1.43 ± 0.004 |
| Paraffin wax:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Thermal conductivity (W mK−1) | 0.14 ± 0.013 | 0.22 ± 0.012 | 0.34 ± 0.009 | 0.40 ± 0.009 |
| PCM | PCM:CRH wt.% | |||
|---|---|---|---|---|
| Na2SO4·10H2O:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Latent heat (kJ kg−1) | 216.13 ± 2.70 | 201.62 ± 1.03 | 192.45 ± 1.78 | 185.28 ± 2.01 |
| Paraffin wax:CRHs | 100:0 | 99:1 | 97:3 | 95:5 |
| Latent heat (kJ kg−1) | 220.45 ± 1.62 | 208.23 ± 2.01 | 194.55 ± 2.45 | 188.09 ± 1.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Park, M.; Shin, H.K. Role of Phase Change Materials Containing Carbonized Rice husks on the Roof-Surface and Indoor Temperatures for Cool Roof System Application. Molecules 2020, 25, 3280. https://doi.org/10.3390/molecules25143280
Kim HG, Kim Y-S, Kwac LK, Park M, Shin HK. Role of Phase Change Materials Containing Carbonized Rice husks on the Roof-Surface and Indoor Temperatures for Cool Roof System Application. Molecules. 2020; 25(14):3280. https://doi.org/10.3390/molecules25143280
Chicago/Turabian StyleKim, Hong Gun, Yong-Sun Kim, Lee Ku Kwac, Mira Park, and Hye Kyoung Shin. 2020. "Role of Phase Change Materials Containing Carbonized Rice husks on the Roof-Surface and Indoor Temperatures for Cool Roof System Application" Molecules 25, no. 14: 3280. https://doi.org/10.3390/molecules25143280






