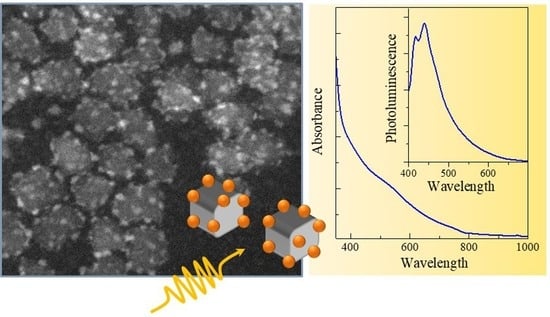
PbS Quantum Dots Decorating TiO2 Nanocrystals: Synthesis, Topology, and Optical Properties of the Colloidal Hybrid Architecture
Abstract
:1. Introduction
2. Results and Discussion
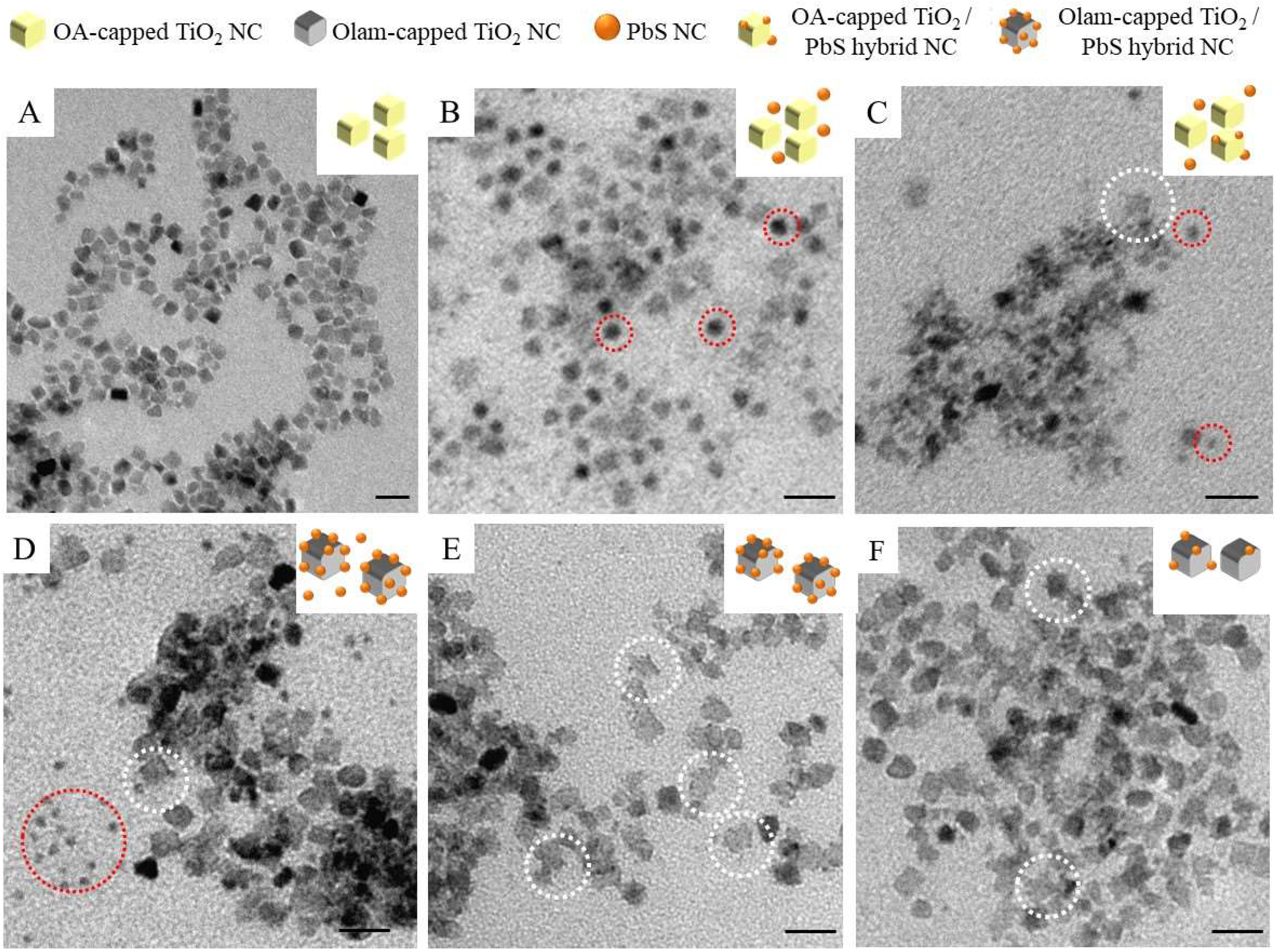
2.1. Fabrication of TiO2/PbS Hybrid NCs
2.2. Structure and Chemical Composition of TiO2/PbS Hybrid NCs
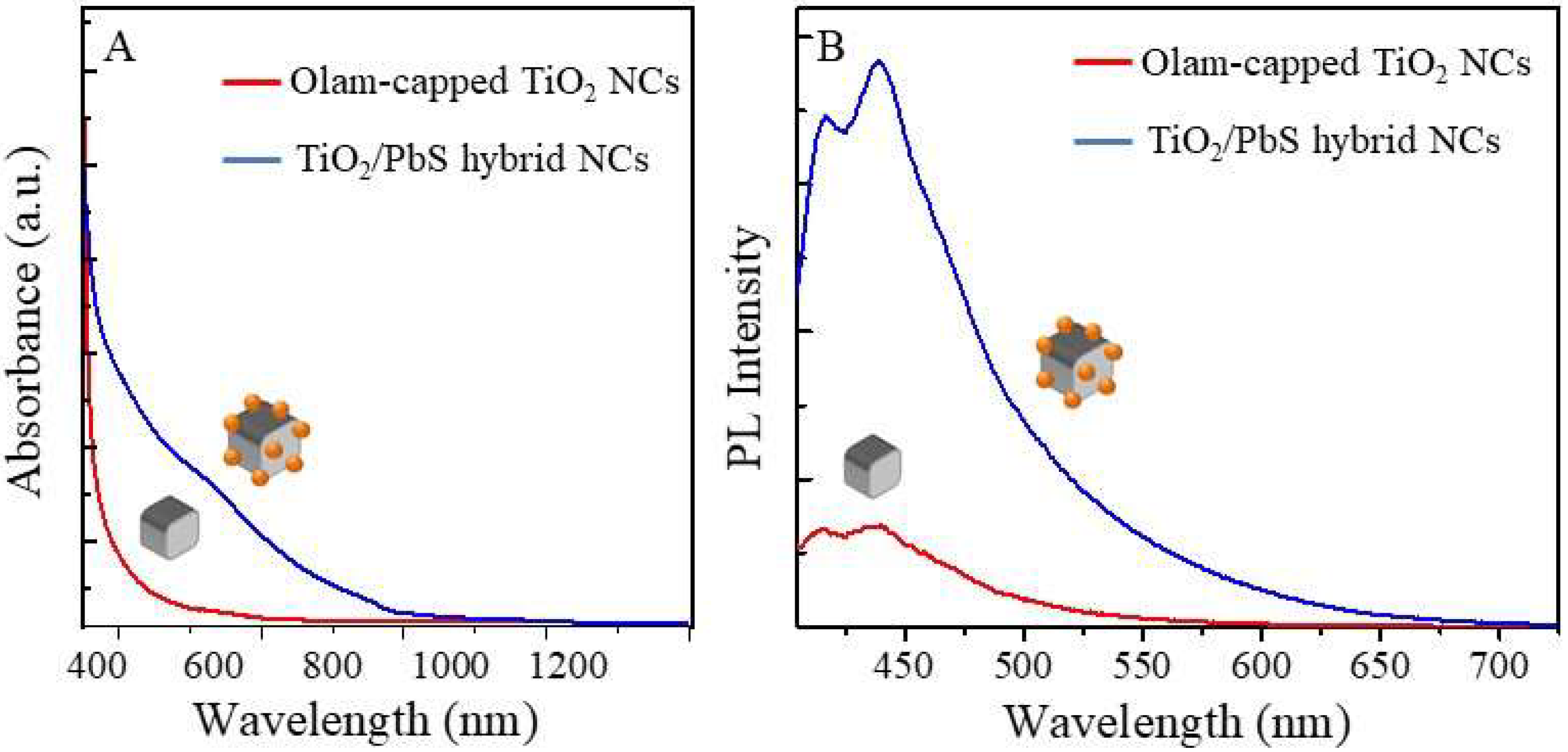
2.3. Spectroscopic Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Organic-Capped TiO2 NCs
3.3. Synthesis of TiO2/PbS Hybrid NPs
3.4. Sample Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The heat-up synthesis of colloidal nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-dimensional metal nanostructures: From colloidal syntheses to applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef] [PubMed]
- Nasilowski, M.; Mahler, B.; Lhuillier, E.; Ithurria, S.; Dubertret, B. Two-dimensional colloidal nanocrystals. Chem. Rev. 2016, 116, 10934–10982. [Google Scholar] [CrossRef] [PubMed]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot solar cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, J.-H.; You, M.; Li, Q.; Fan, C.; Nam, J.-M. Multicomponent plasmonic nanoparticles: From heterostructured nanoparticles to colloidal composite nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef] [PubMed]
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal strategies for preparing oxide-based hybrid nanocrystals. Eur. J. Inorg. Chem. 2008, 2008, 837–854. [Google Scholar] [CrossRef]
- Nag, A.; Kundu, J.; Hazarika, A. Seeded-growth, nanocrystal-fusion, ion-exchange and inorganic-ligand mediated formation of semiconductor-based colloidal heterostructured nanocrystals. Cryst. Eng. Comm. 2014, 16, 9391–9407. [Google Scholar] [CrossRef]
- Ma, D. Chapter 1—Hybrid nanoparticles: An introduction. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–6. [Google Scholar]
- Nguyen, K.T.; Zhao, Y. Engineered hybrid nanoparticles for on-demand diagnostics and therapeutics. Acc. Chem. Res. 2015, 48, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, L.; McGuire, S.C.; Hurley, N.; Wong, S.S. Metal chalcogenide quantum dot-sensitized 1D-based semiconducting heterostructures for optical-related applications. Energy Environ. Sci. 2019, 12, 1454–1494. [Google Scholar] [CrossRef]
- Selinsky, R.S.; Ding, Q.; Faber, M.S.; Wright, J.C.; Jin, S. Quantum dot nanoscale heterostructures for solar energy conversion. Chem. Soc. Rev. 2013, 42, 2963–2985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, M.; Guo, Z.; Miao, L.; Han, S.-T.; Wang, Z.; Zhang, X.; Zhang, H.; Peng, Z. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horizons 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-based nanoheterostructures for promoting gas sensitivity performance: Designs, developments, and prospects. Sensors 2017, 17, 1971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Vomiero, A.; Rosei, F. Ultrasensitive, biocompatible, self-calibrating, multiparametric temperature sensors. Small 2015, 11, 5741–5746. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Niu, J.; Wang, H.; Shen, H.; Li, L.S. Size, shape-dependent growth of semiconductor heterostructures mediated by Ag2Se nanocrystals as seeds. ACS Appl. Mater. Interfaces 2013, 5, 7537–7543. [Google Scholar] [CrossRef]
- Milleville, C.C.; Chen, E.Y.; Lennon, K.R.; Cleveland, J.M.; Kumar, A.; Zhang, J.; Bork, J.A.; Tessier, A.; LeBeau, J.M.; Chase, D.B.; et al. Engineering efficient photon upconversion in semiconductor heterostructures. ACS Nano 2019, 13, 489–497. [Google Scholar] [CrossRef]
- Acharya, K.P.; Hewa-Kasakarage, N.N.; Alabi, T.R.; Nemitz, I.; Khon, E.; Ullrich, B.; Anzenbacher, P.; Zamkov, M. Synthesis of PbS/TiO2 colloidal heterostructures for photovoltaic applications. J. Phys. Chem. C 2010, 114, 12496–12504. [Google Scholar] [CrossRef] [Green Version]
- Acharya, K.P.; Alabi, T.R.; Schmall, N.; Hewa-Kasakarage, N.N.; Kirsanova, M.; Nemchinov, A.; Khon, E.; Zamkov, M. Linker-free modification of TiO2 nanorods with PbSe nanocrystals. J. Phys. Chem. C 2009, 113, 19531–19535. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Y.; Chuang, C.-H.; Kobayashi, Y.; Coombs, N.; Gorantla, S.; Botton, G.A.; Winnik, M.A.; Burda, C.; Scholes, G.D. Synthesis and optical properties of linker-free TiO2/CdSe nanorods. J. Phys. Chem. C 2014, 118, 3347–3358. [Google Scholar] [CrossRef]
- Kwon, K.-W.; Lee, B.H.; Shim, M. Structural evolution in metal oxide/semiconductor colloidal nanocrystal heterostructures. Chem. Mater. 2006, 18, 6357–6363. [Google Scholar] [CrossRef]
- Fenton, J.L.; Hodges, J.M.; Schaak, R.E. Synthetic deconvolution of interfaces and materials components in hybrid nanoparticles. Chem. Mater. 2017, 29, 6168–6177. [Google Scholar] [CrossRef]
- Shaviv, E.; Schubert, O.; Alves-Santos, M.; Goldoni, G.; Di Felice, R.; Vallée, F.; Del Fatti, N.; Banin, U.; Sönnichsen, C. Absorption properties of metal–semiconductor hybrid nanoparticles. ACS Nano 2011, 5, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Comparelli, R.; Fanizza, E.; Curri, M.L.; Agostiano, A.; Laub, D. Photocatalytic synthesis of silver nanoparticles stabilized by TiO2 nanorods: A semiconductor/metal nanocomposite in homogeneous nonpolar solution. J. Am. Chem. Soc. 2004, 126, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Fanizza, E.; Curri, M.L.; Laub, D.; Agostiano, A. Low-dimensional chainlike assemblies of TiO2 nanorod-stabilized Au nanoparticles. Chem. Commun. 2005, 942–944. [Google Scholar] [CrossRef]
- He, S.; Zhang, H.; Delikanli, S.; Qin, Y.; Swihart, M.T.; Zeng, H. Bifunctional magneto-optical FePt−CdS hybrid nanoparticles. J. Phys. Chem. C 2009, 113, 87–90. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Curri, M.L.; Innocenti, C.; Sangregorio, C.; Achterhold, K.; Parak, F.G.; Agostiano, A.; et al. Seeded growth of asymmetric binary nanocrystals made of a semiconductor TiO2 rodlike section and a magnetic γ-Fe2O3 spherical domain. J. Am. Chem. Soc. 2006, 128, 16953–16970. [Google Scholar] [CrossRef]
- Santana Vega, M.; Guerrero Martínez, A.; Cucinotta, F. Facile strategy for the synthesis of gold@ silica hybrid nanoparticles with controlled porosity and janus morphology. Nanomaterials 2019, 9, 348. [Google Scholar] [CrossRef] [Green Version]
- Sashchiuk, A.; Yanover, D.; Rubin-Brusilovski, A.; Maikov, G.I.; Čapek, R.K.; Vaxenburg, R.; Tilchin, J.; Zaiats, G.; Lifshitz, E. Tuning of electronic properties in IV–VI colloidal nanostructures by alloy composition and architecture. Nanoscale 2013, 5, 7724–7745. [Google Scholar] [CrossRef]
- Kundu, P.; Anumol, E.A.; Nethravathi, C.; Ravishankar, N. Existing and emerging strategies for the synthesis of nanoscale heterostructures. PCCP 2011, 13, 19256–19269. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-R.; Liu, D.-Y.; Wu, Y.-F.; Duan, S.; Xie, Z.-X.; Jiang, Z.-Y.; Tian, Z.-Q. Epitaxial growth of heterogeneous metal nanocrystals: from gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951. [Google Scholar] [CrossRef] [PubMed]
- Rivest, J.B.; Jain, P.K. Cation exchange on the nanoscale: An emerging technique for new material synthesis, device fabrication, and chemical sensing. Chem. Soc. Rev. 2013, 42, 89–96. [Google Scholar] [CrossRef] [PubMed]
- De Trizio, L.; Manna, L. Forging colloidal nanostructures via cation exchange reactions. Chem. Rev. 2016, 116, 10852–10887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriegel, I.; Wisnet, A.; Srimath Kandada, A.R.; Scotognella, F.; Tassone, F.; Scheu, C.; Zhang, H.; Govorov, A.O.; Rodríguez-Fernández, J.; Feldmann, J. Cation exchange synthesis and optoelectronic properties of type II CdTe–Cu2−xTe nano-heterostructures. J. Mater. Chem. C 2014, 2, 3189–3198. [Google Scholar] [CrossRef] [Green Version]
- Dibenedetto, C.N.; Fanizza, E.; Brescia, R.; Kolodny, Y.; Remennik, S.; Panniello, A.; Depalo, N.; Yochelis, S.; Comparelli, R.; Agostiano, A.; et al. Coupling effects in QD dimers at sub-nanometer interparticle distance. Nano Res. 2020, 13, 1071–1080. [Google Scholar] [CrossRef]
- Hyun, B.-R.; Zhong, Y.-W.; Bartnik, A.C.; Sun, L.; Abruña, H.D.; Wise, F.W.; Goodreau, J.D.; Matthews, J.R.; Leslie, T.M.; Borrelli, N.F. Electron injection from colloidal PbS quantum dots into titanium dioxide nanoparticles. ACS Nano 2008, 2, 2206–2212. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, L.; Liu, W.; Su, G.; Gao, R.; Zhao, Y. The key role of nanoparticle seeds during site-selective growth of silver to fabricate core-shell or asymmetric dumbbell heterostructures. Dalton Trans. 2014, 43, 4822–4829. [Google Scholar] [CrossRef]
- Enright, M.J.; Sarsito, H.; Cossairt, B.M. Kinetically controlled assembly of cadmium chalcogenide nanorods and nanorod heterostructures. Mater. Chem. Front. 2018, 2, 1296–1305. [Google Scholar] [CrossRef]
- Corrias, A.; Conca, E.; Cibin, G.; Mountjoy, G.; Gianolio, D.; De Donato, F.; Manna, L.; Casula, M.F. Insights into the structure of dot@rod and dot@octapod cdse@cds heterostructures. J. Phys. Chem. C 2015, 119, 16338–16348. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, X.; Hou, J.G. Colloidal hybrid nanocrystals: Synthesis, properties, and perspectives. In Nanocrystal; Masuda, Y., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Liu, X.Y. A new kinetic model for three-dimensional heterogeneous nucleation. J. Chem. Phys. 1999, 111, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.G.; Yang, F.; Xi, J.F.; Jia, Y.F.; Cheng, C.H.; Zhao, Y. Ultrasound effect: Preparation of PbS/TiO2 heterostructure nanotube arrays through successive ionic layer adsorption and the reaction method. Mater. Lett. 2013, 107, 39–41. [Google Scholar] [CrossRef]
- Hajjaji, A.; Jemai, S.; Rebhi, A.; Trabelsi, K.; Gaidi, M.; Alhazaa, A.N.; Al-Gawati, M.A.; El Khakani, M.A.; Bessais, B. Enhancement of photocatalytic and photoelectrochemical properties of TiO2 nanotubes sensitized by SILAR-Deposited PbS nanoparticles. J. Mater. 2020, 6, 62–69. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, T.G.; Pacio, M.; Agustín-Serrano, R.; Juárez-Santiesteban, H.; Muñiz, J. Understanding structure of small TiO2 nanoparticles and adsorption mechanisms of PbS quantum dots for solid-state applications: A combined theoretical and experimental study. Theor. Chem. Acc. 2019, 138, 92. [Google Scholar] [CrossRef]
- Ghadiri, E.; Liu, B.; Moser, J.-E.; Grätzel, M.; Etgar, L. Investigation of interfacial charge separation at PbS QDs/(001) TiO2 nanosheets heterojunction solar cell. Part. Part. Syst. Character 2015, 32, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-heterojunction colloidal quantum dot solar cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Leventis, H.C.; Moon, S.-J.; Chen, P.; Ito, S.; Haque, S.A.; Torres, T.; Nüesch, F.; Geiger, T.; Zakeeruddin, S.M.; et al. PbS and CdS quantum dot-sensitized solid-state solar cells: “Old concepts, new results”. Adv. Funct. Mater. 2009, 19, 2735–2742. [Google Scholar] [CrossRef]
- Fu, H.; Tsang, S.-W. Infrared colloidal lead chalcogenide nanocrystals: Synthesis, properties, and photovoltaic applications. Nanoscale 2012, 4, 2187–2201. [Google Scholar] [CrossRef]
- Trejo, O.; Roelofs, K.E.; Xu, S.; Logar, M.; Sarangi, R.; Nordlund, D.; Dadlani, A.L.; Kravec, R.; Dasgupta, N.P.; Bent, S.F.; et al. Quantifying geometric strain at the PbS QD-TiO2 anode interface and its effect on electronic structures. Nano Lett. 2015, 15, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Spooner, N.A.; Qiao, S.Z.; Dai, S. Mechanistic insight into the nucleation and growth of oleic acid capped lead sulphide quantum dots. PCCP 2016, 18, 14055–14062. [Google Scholar] [CrossRef]
- Abel, K.A.; Shan, J.; Boyer, J.-C.; Harris, F.; van Veggel, F.C.J.M. Highly photoluminescent PbS nanocrystals: The beneficial effect of trioctylphosphine. Chem. Mater. 2008, 20, 3794–3796. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv. Mater. (Weinh. Ger.) 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Corricelli, M.; Enrichi, F.; Altamura, D.; De Caro, L.; Giannini, C.; Falqui, A.; Agostiano, A.; Curri, M.L.; Striccoli, M. Near infrared emission from monomodal and bimodal PbS nanocrystal superlattices. J. Phys. Chem. C 2012, 116, 6143–6152. [Google Scholar] [CrossRef]
- Depalo, N.; Corricelli, M.; De Paola, I.; Valente, G.; Iacobazzi, R.M.; Altamura, E.; Debellis, D.; Comegna, D.; Fanizza, E.; Denora, N.; et al. NIR emitting nanoprobes based on cyclic RGD motif conjugated PbS quantum dots for integrin-targeted optical bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 43113–43126. [Google Scholar] [CrossRef] [PubMed]
- De Trizio, L.; Buonsanti, R.; Schimpf, A.M.; Llordes, A.; Gamelin, D.R.; Simonutti, R.; Milliron, D.J. Nb-doped colloidal TiO2 nanocrystals with tunable infrared absorption. Chem. Mater. 2013, 25, 3383–3390. [Google Scholar] [CrossRef]
- Kuo, M.-S.; Chang, S.-J.; Hsieh, P.-H.; Huang, Y.-C.; Li, C.-C. Efficient dispersants for TiO2 nanopowder in organic suspensions. J. Am. Ceram. Soc. 2016, 99, 445–451. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Yin, S.; Sato, T.; Wang, Y. Visible light-driven photocatalytic activity of oleic acid-coated TiO2 nanoparticles synthesized from absolute ethanol solution. Nanoscale Res. Lett. 2015, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Fanizza, E.; Cozzoli, P.D.; Curri, M.L.; Striccoli, M.; Sardella, E.; Agostiano, A. UV-light-driven immobilization of surface-functionalized oxide nanocrystals onto silicon. Adv. Funct. Mater. 2007, 17, 201–211. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Liu, S.; Li, D.; Han, M. Aminolysis route to monodisperse titania nanorods with tunable aspect ratio. Angew. Chem. Int. Ed. 2005, 44, 3466–3470. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.P.; Toolan, D.T.W.; Kilbride, R.C.; Penfold, N.J.W.; Washington, A.L.; King, S.M.; Xiao, J.; Zhang, Z.; Gray, V.; Dowland, S.; et al. Ligand shell structure in lead sulfide–Oleic acid colloidal quantum dots revealed by small-angle scattering. J. Phys. Chem. Lett. 2019, 10, 4713–4719. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: Spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Thompson, R.L.; Ohodnicki, P.; Baltrus, J.; Matranga, C. Size-dependent photocatalytic reduction of CO2 with PbS quantum dot sensitized TiO2 heterostructured photocatalysts. J. Mater. Chem. 2011, 21, 13452–13457. [Google Scholar] [CrossRef]
- Malgras, V.; Nattestad, A.; Yamauchi, Y.; Dou, S.X.; Kim, J.H. The effect of surface passivation on the structure of sulphur-rich PbS colloidal quantum dots for photovoltaic application. Nanoscale 2015, 7, 5706–5711. [Google Scholar] [CrossRef] [Green Version]
- Cant, D.J.H.; Syres, K.L.; Lunt, P.J.B.; Radtke, H.; Treacy, J.; Thomas, P.J.; Lewis, E.A.; Haigh, S.J.; O’Brien, P.; Schulte, K.; et al. Surface properties of nanocrystalline PbS films deposited at the water-oil interface: A study of atmospheric aging. Langmuir 2015, 31, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J.; Gaarenstroom, S.W. NIST X-ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps/Default.aspx (accessed on 16 April 2019).
- Saini, C.P.; Barman, A.; Banerjee, D.; Grynko, O.; Prucnal, S.; Gupta, M.; Phase, D.M.; Sinha, A.K.; Kanjilal, D.; Skorupa, W.; et al. Impact of self-trapped excitons on blue photoluminescence in TiO2 nanorods on chemically etched Si pyramids. J. Phys. Chem. C 2017, 121, 11448–11454. [Google Scholar] [CrossRef]
- Saini, C.P.; Barman, A.; Satpati, B.; Bhattacharyya, S.R.; Kanjilal, D.; Kanjilal, A. Defect-engineered optical bandgap in self-assembled TiO2 nanorods on Si pyramids. Appl. Phys. Lett. 2016, 108, 011907. [Google Scholar] [CrossRef]
- Hou, B.; Cho, Y.; Kim, B.-S.; Ahn, D.; Lee, S.; Park, J.B.; Lee, Y.-W.; Hong, J.; Im, H.; Morris, S.M.; et al. Red green blue emissive lead sulfide quantum dots: Heterogeneous synthesis and applications. J. Mater. Chem. C 2017, 5, 3692–3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.M.; Kroupa, D.M.; Zhang, J.; Schulz, P.; Marshall, A.R.; Kahn, A.; Lany, S.; Luther, J.M.; Beard, M.C.; Perkins, C.L.; et al. Revisiting the valence and conduction band size dependence of PbS quantum dot thin films. ACS Nano 2016, 10, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence mechanisms in anatase and rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Materiomics 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal synthesis of rGO-TiO2 composites as high-performance UV photocatalysts for ethylparaben degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Ti% | O% | Pb% | S% | |
|---|---|---|---|---|
| TiO2 NCs | 33 | 66 | - | - |
| TiO2/PbS NCs | 20 | 73 | 8 | low |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibenedetto, C.N.; Sibillano, T.; Brescia, R.; Prato, M.; Triggiani, L.; Giannini, C.; Panniello, A.; Corricelli, M.; Comparelli, R.; Ingrosso, C.; et al. PbS Quantum Dots Decorating TiO2 Nanocrystals: Synthesis, Topology, and Optical Properties of the Colloidal Hybrid Architecture. Molecules 2020, 25, 2939. https://doi.org/10.3390/molecules25122939
Dibenedetto CN, Sibillano T, Brescia R, Prato M, Triggiani L, Giannini C, Panniello A, Corricelli M, Comparelli R, Ingrosso C, et al. PbS Quantum Dots Decorating TiO2 Nanocrystals: Synthesis, Topology, and Optical Properties of the Colloidal Hybrid Architecture. Molecules. 2020; 25(12):2939. https://doi.org/10.3390/molecules25122939
Chicago/Turabian StyleDibenedetto, Carlo Nazareno, Teresa Sibillano, Rosaria Brescia, Mirko Prato, Leonardo Triggiani, Cinzia Giannini, Annamaria Panniello, Michela Corricelli, Roberto Comparelli, Chiara Ingrosso, and et al. 2020. "PbS Quantum Dots Decorating TiO2 Nanocrystals: Synthesis, Topology, and Optical Properties of the Colloidal Hybrid Architecture" Molecules 25, no. 12: 2939. https://doi.org/10.3390/molecules25122939