Effect of a =X-NH-Fragment, (X = C, N), on Z/E Isomerization and ON/OFF Functionality of Isatin Arylhydrazones, ((Arylamino)Methylene)Indolin-2-Ones and Their Anions
Abstract
:1. Introduction
2. Results and Discussions
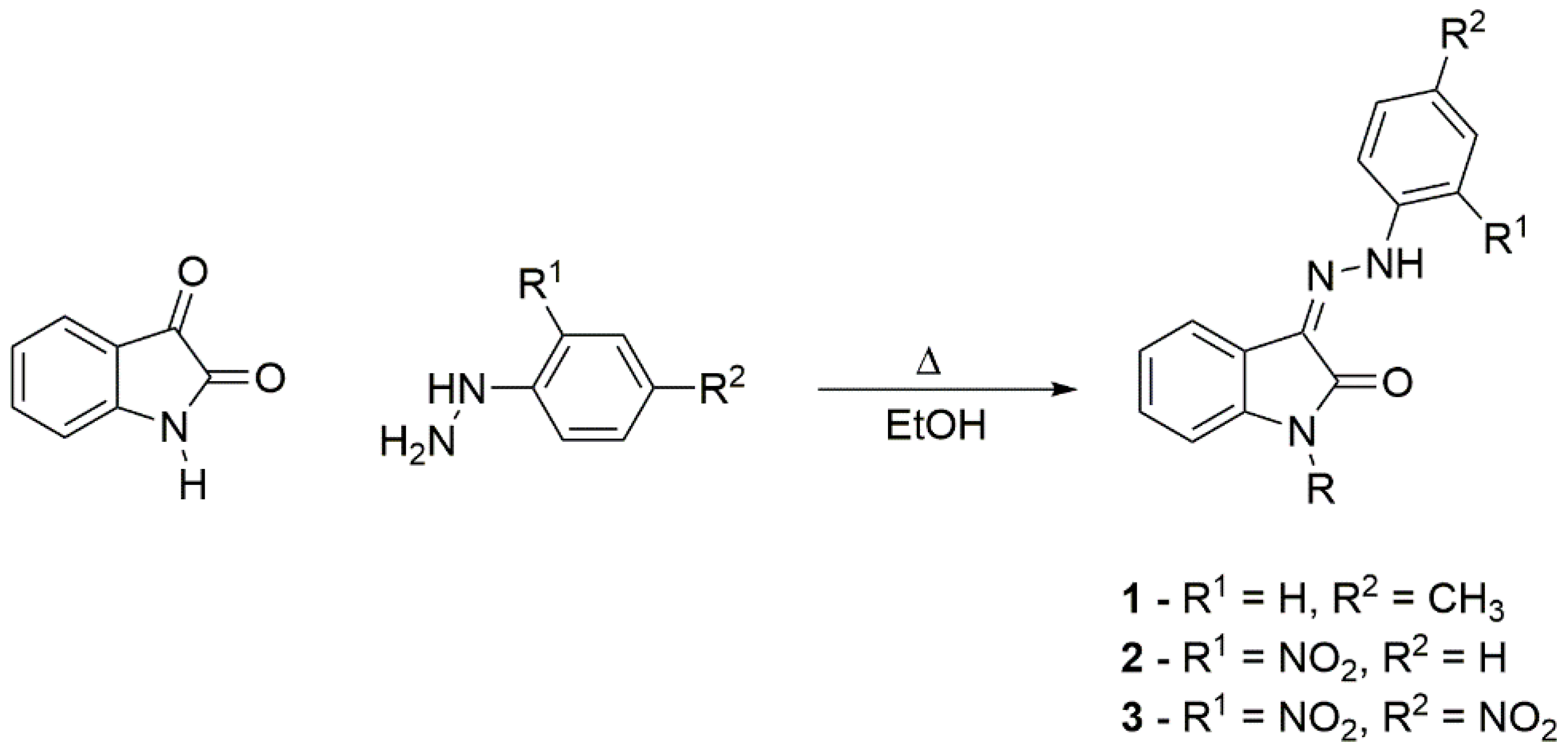
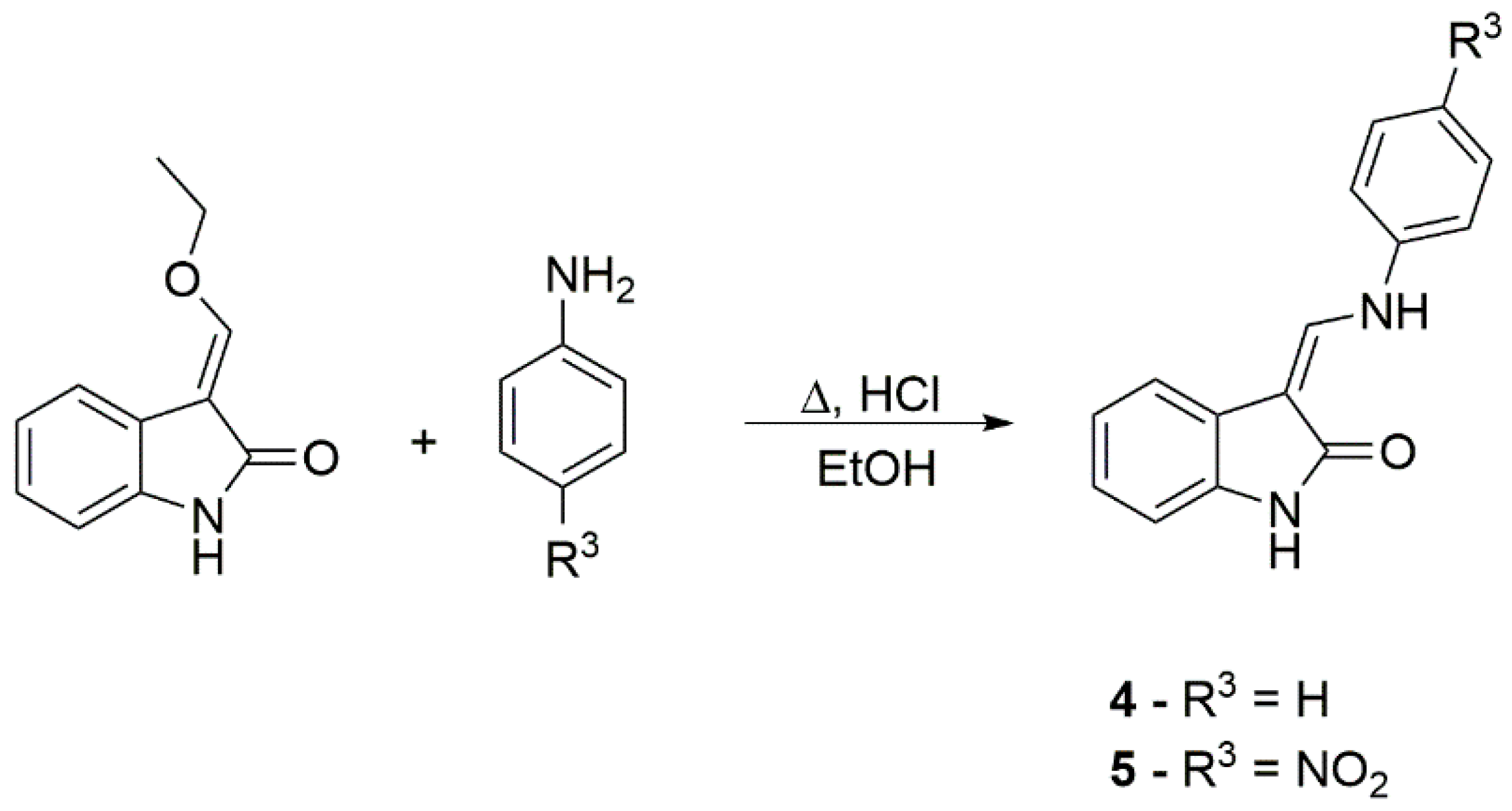
2.1. Synthesis of Studied Compounds
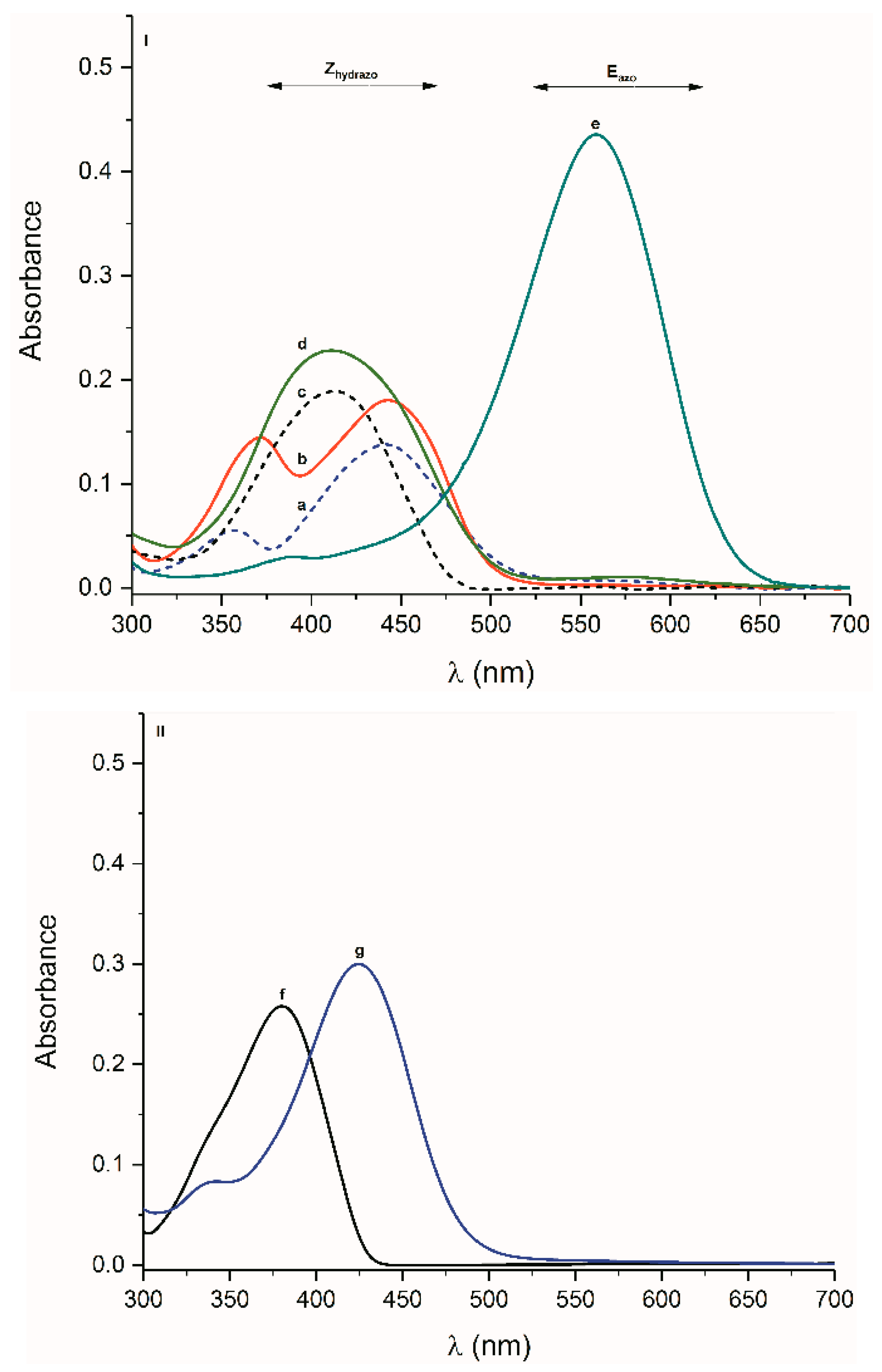
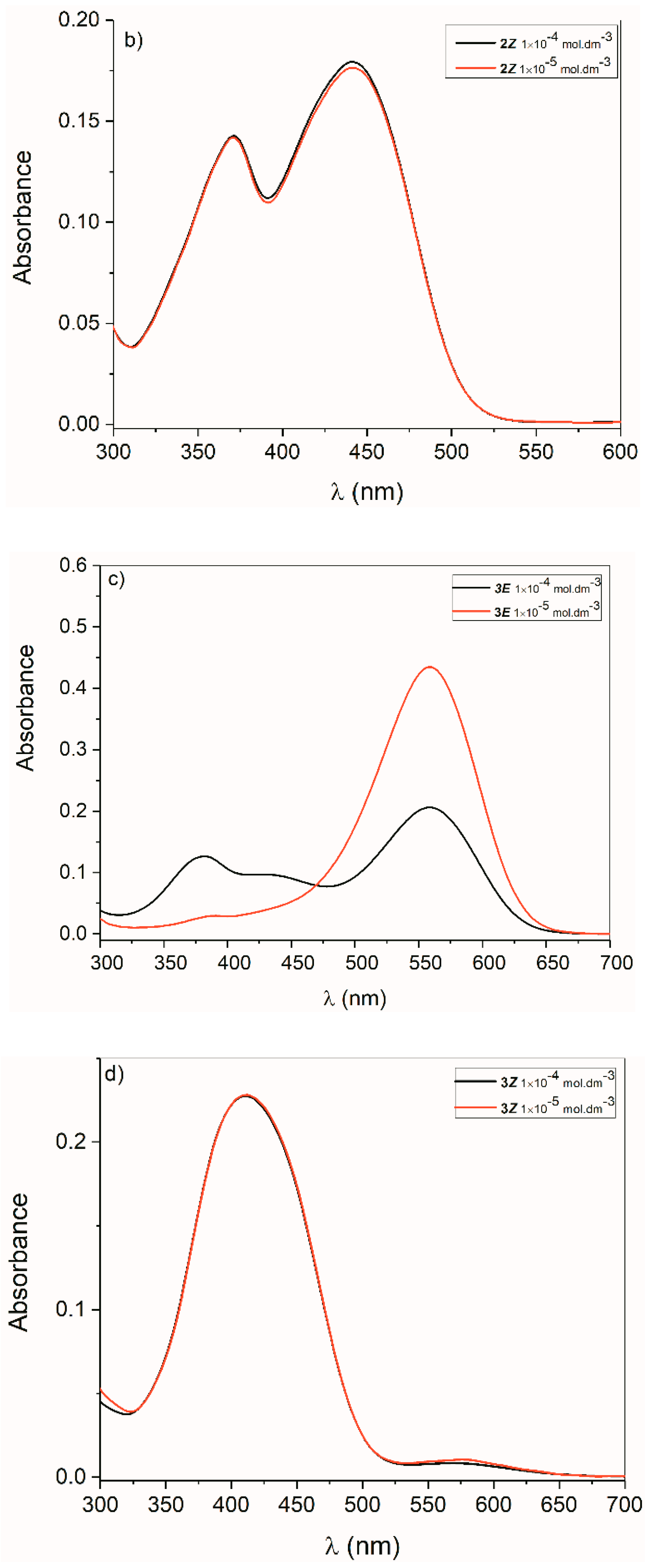
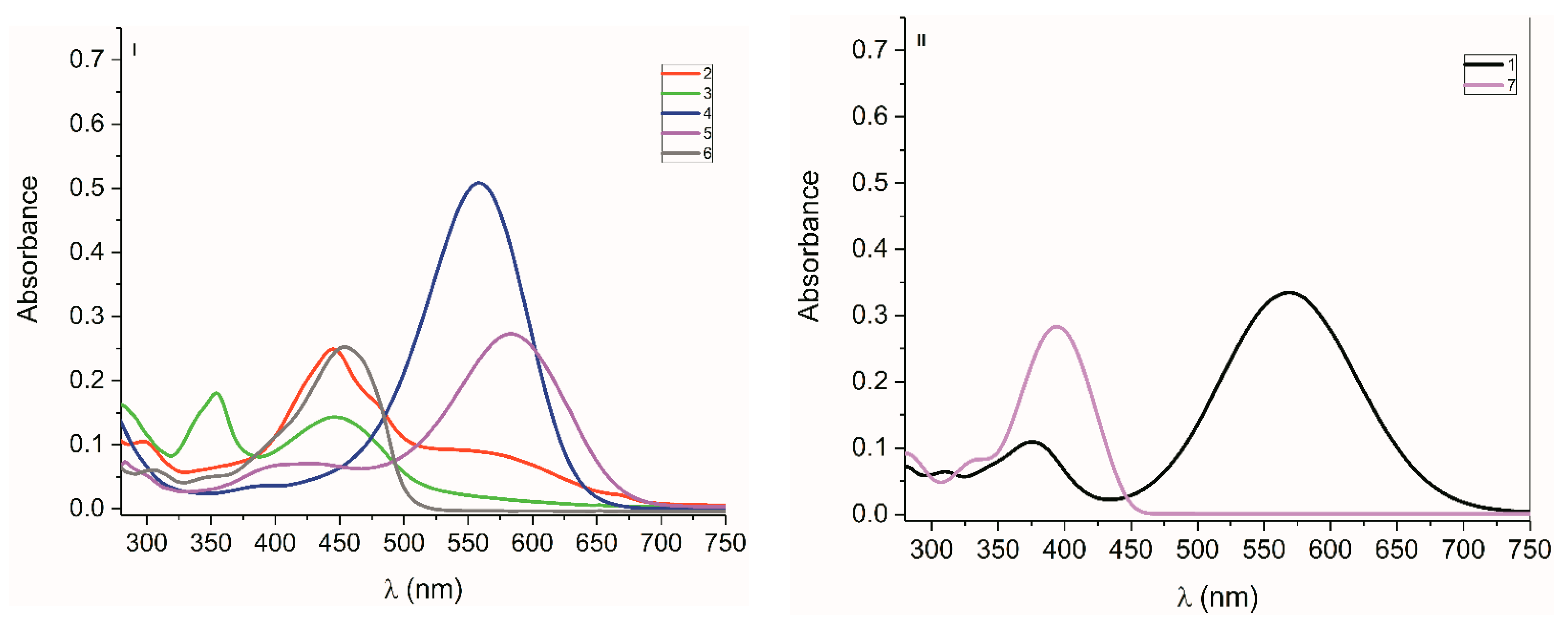
2.2. UV-Vis Spectra
2.2.1. Isatin Aryl Hydrazones
2.2.2. ((Arylamino)Methylene)Indolin-2-Ones 4 and 5
2.3. Photochemistry of Studied Compounds
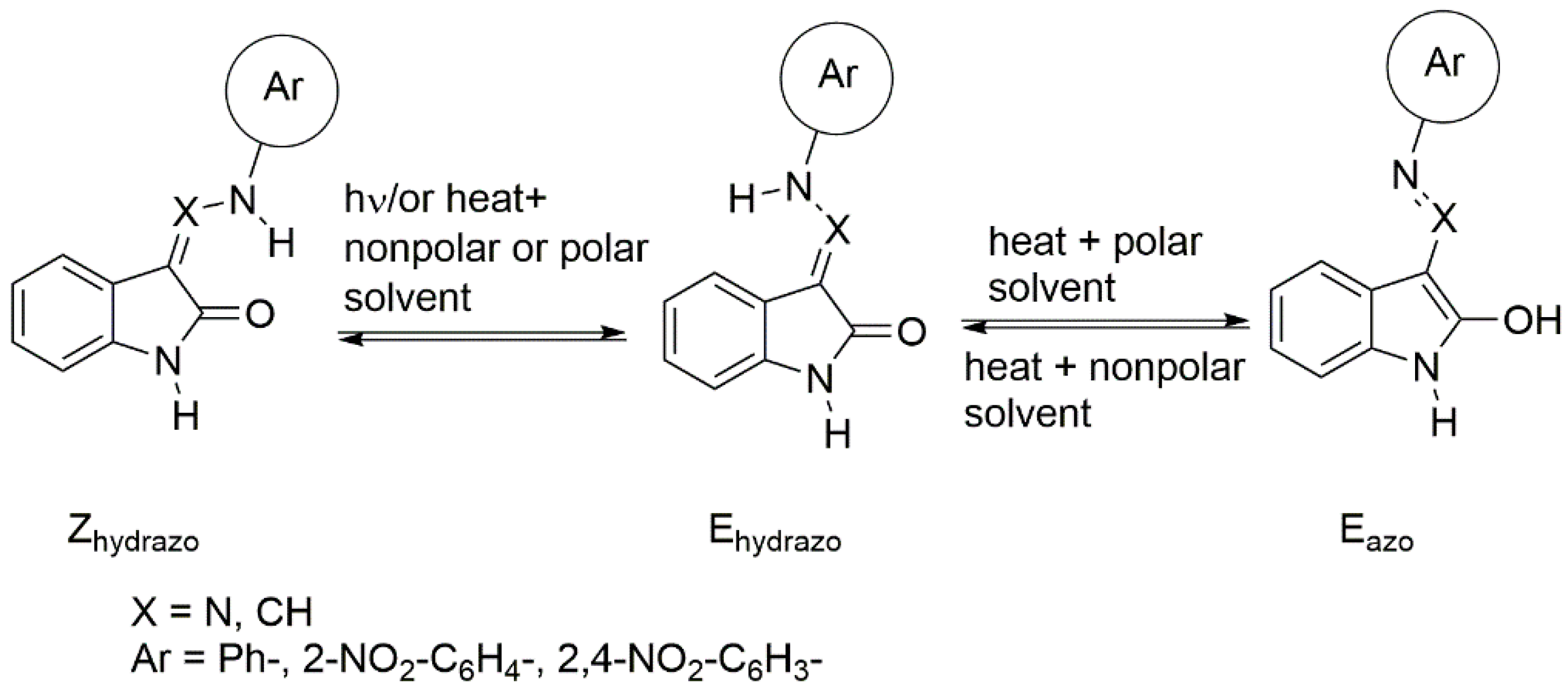
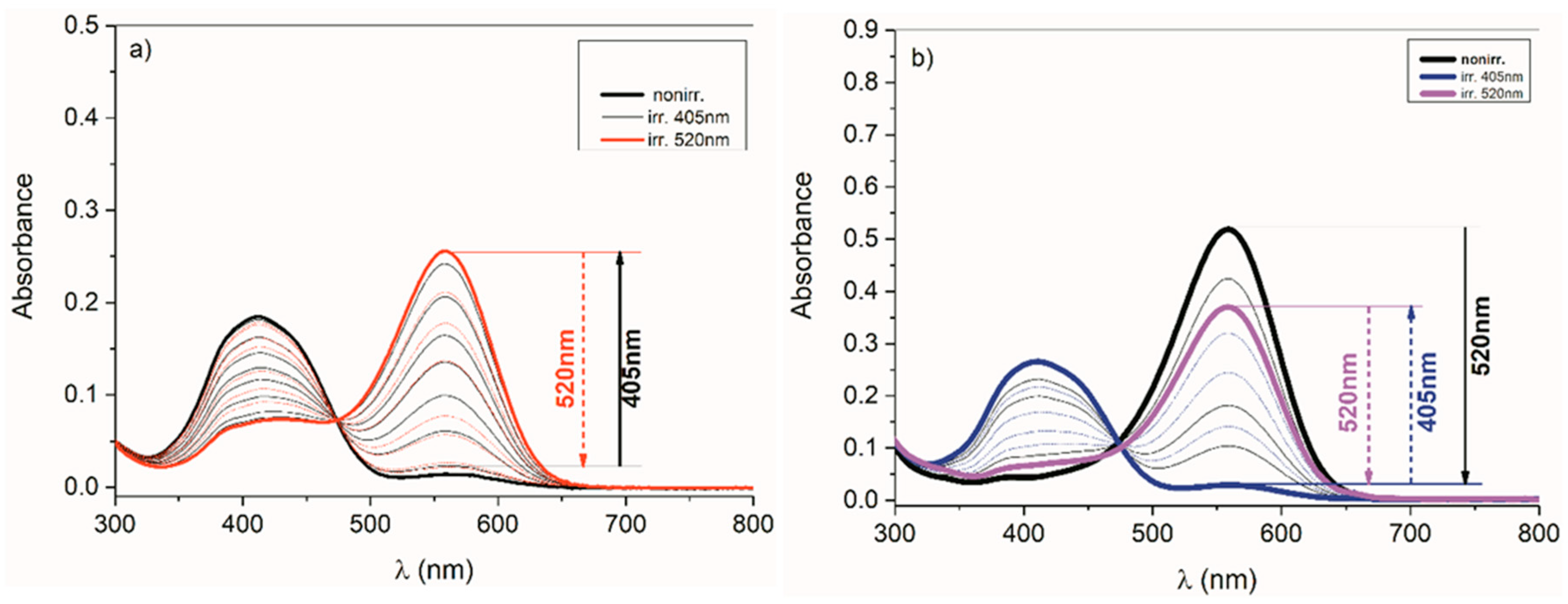
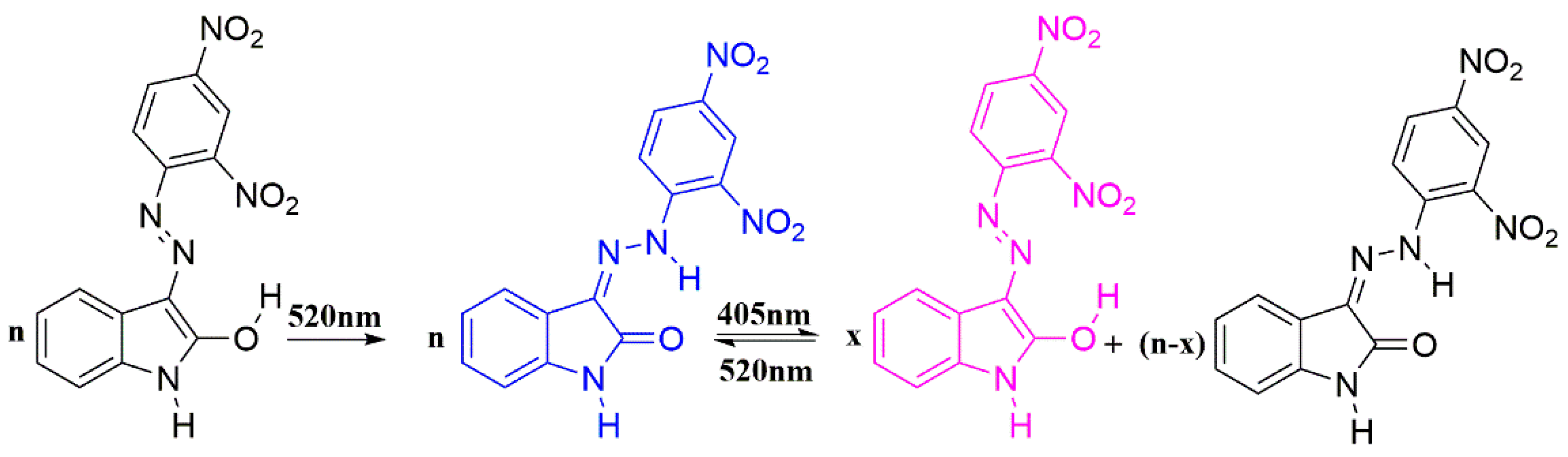
2.3.1. Photoisomerization of Isatin Arylhydrazones
2.3.2. Phototautomerization of Isatin Arylhydrazones
2.3.3. Photoisomerisation of ((Arylamino)Methylene)Indolin-2-Ones 4 and 5
2.3.4. Phototautomerization of ((Arylamino)Methylene)Indolin-2-Ones 4 and 5
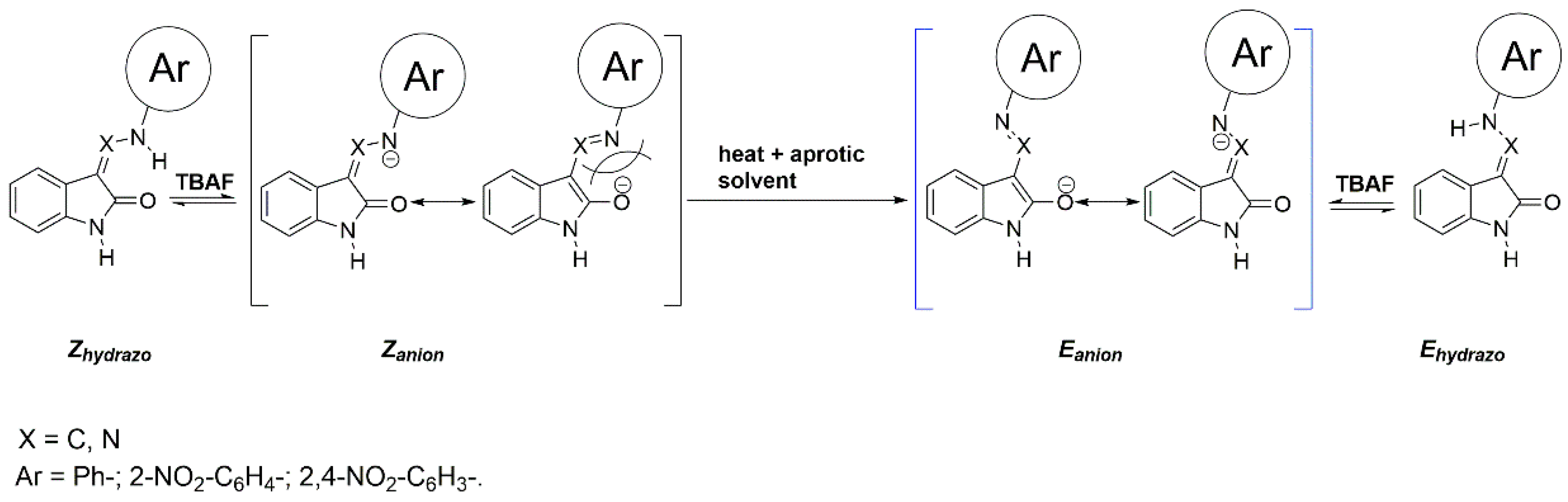
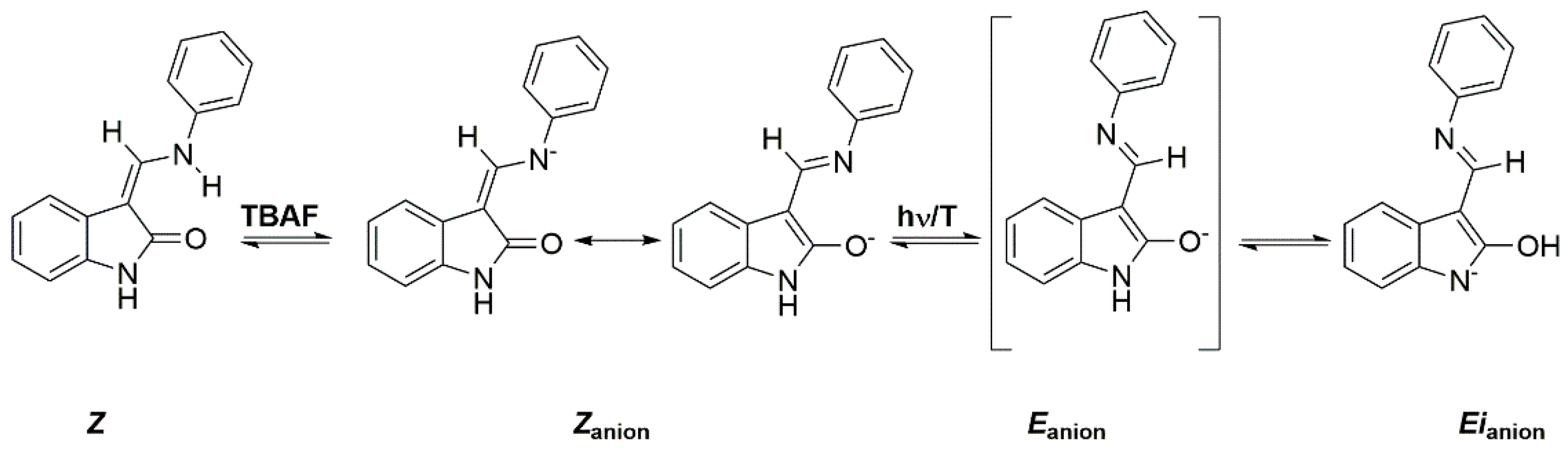
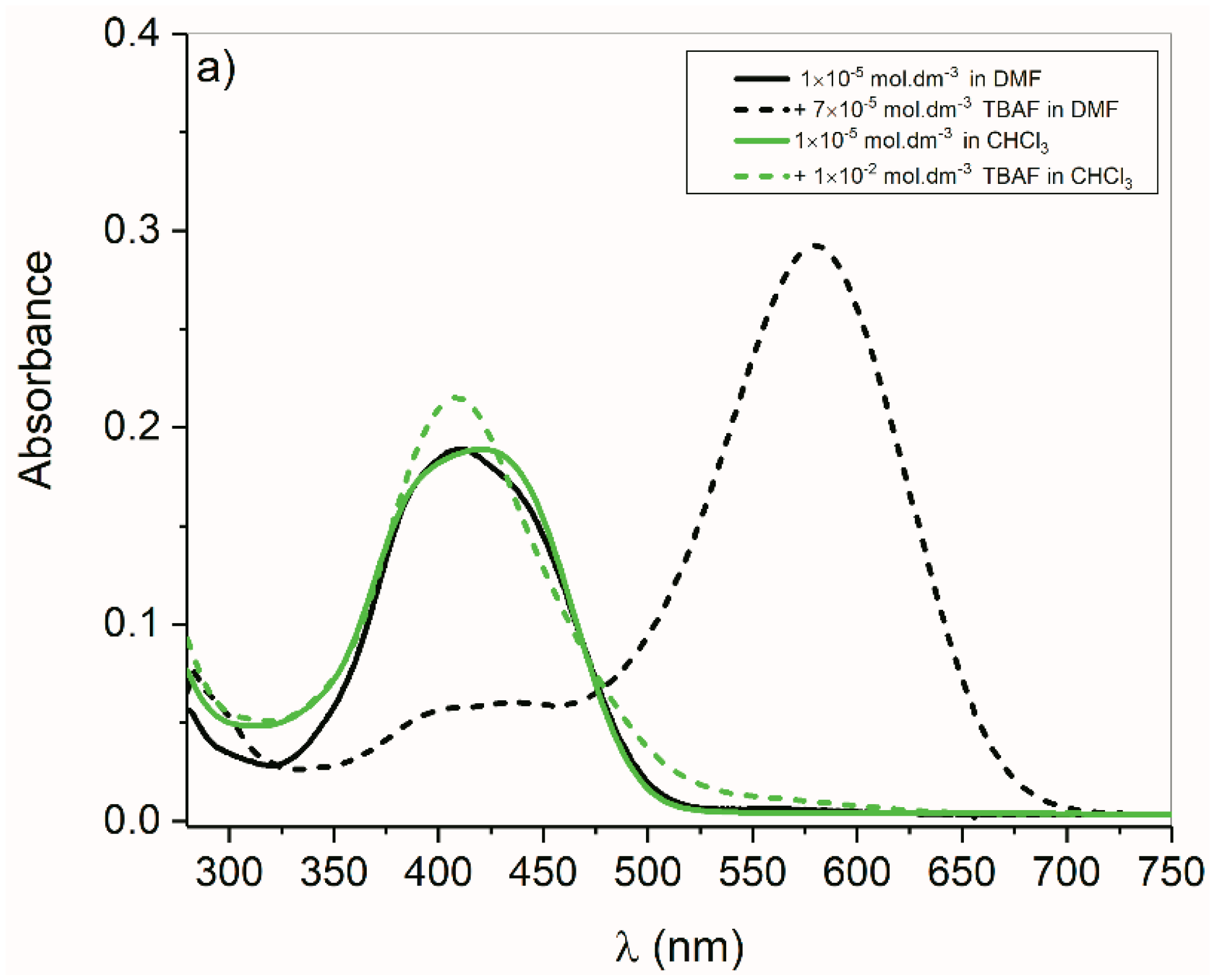
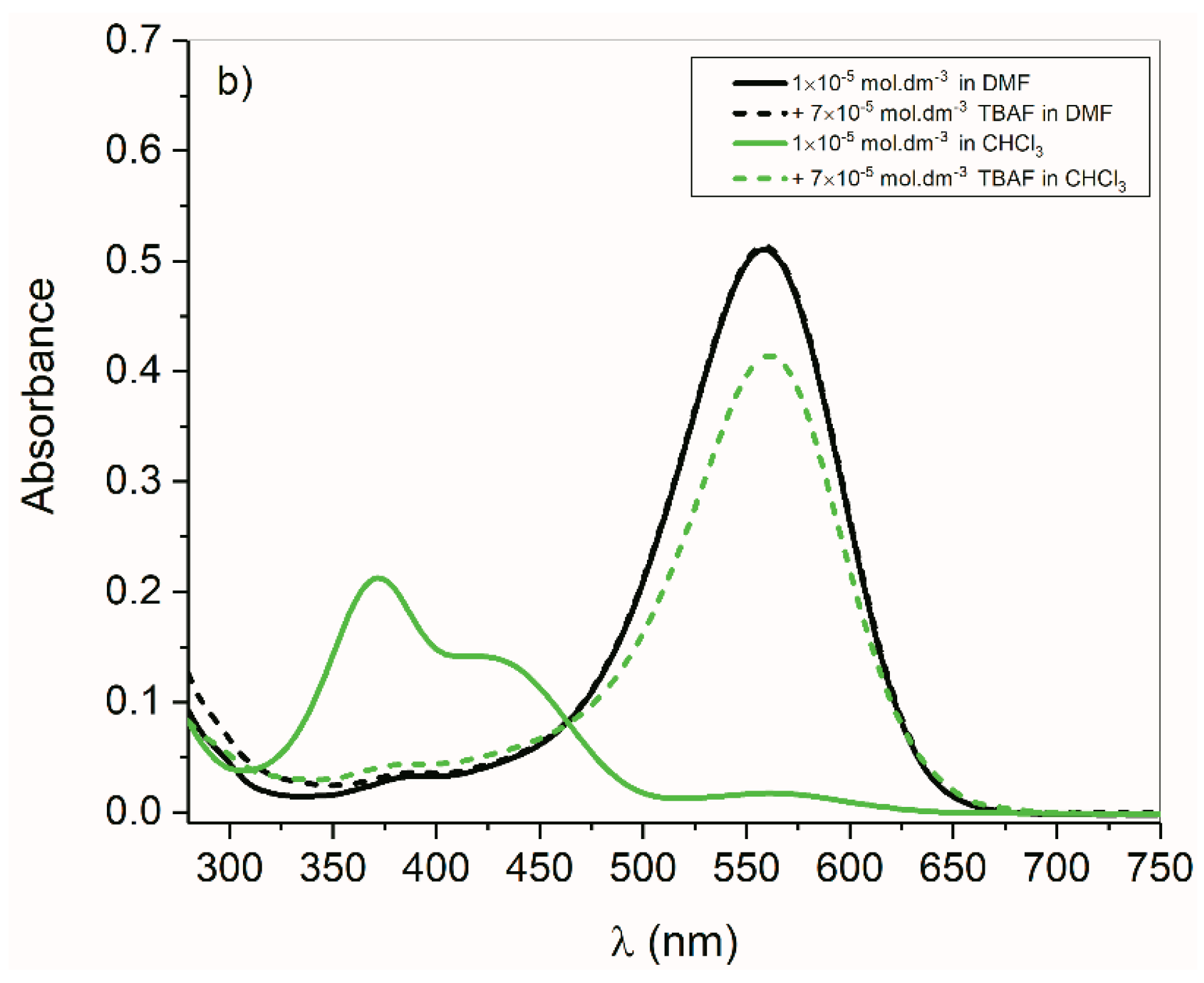
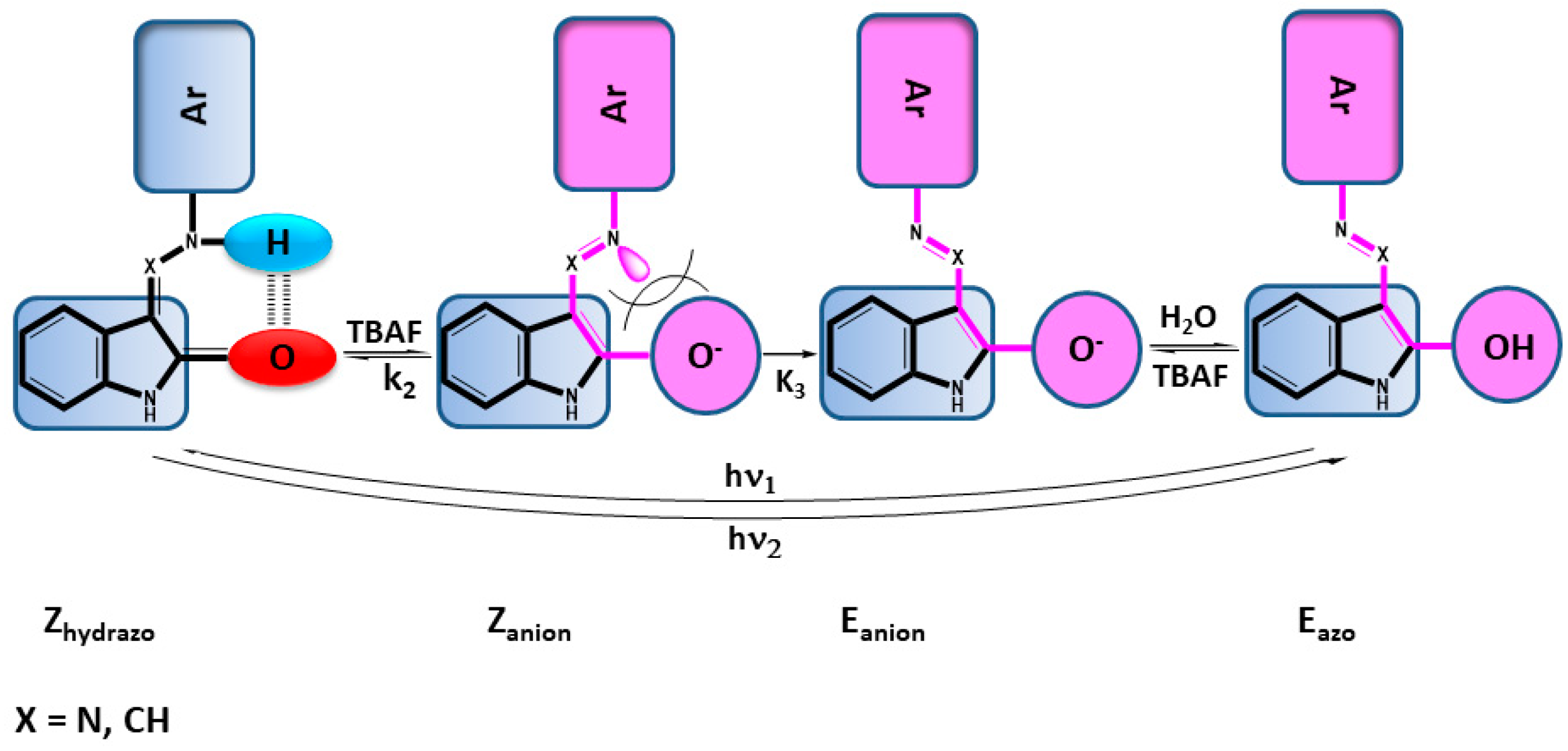
2.4. Anions of Studied Compounds
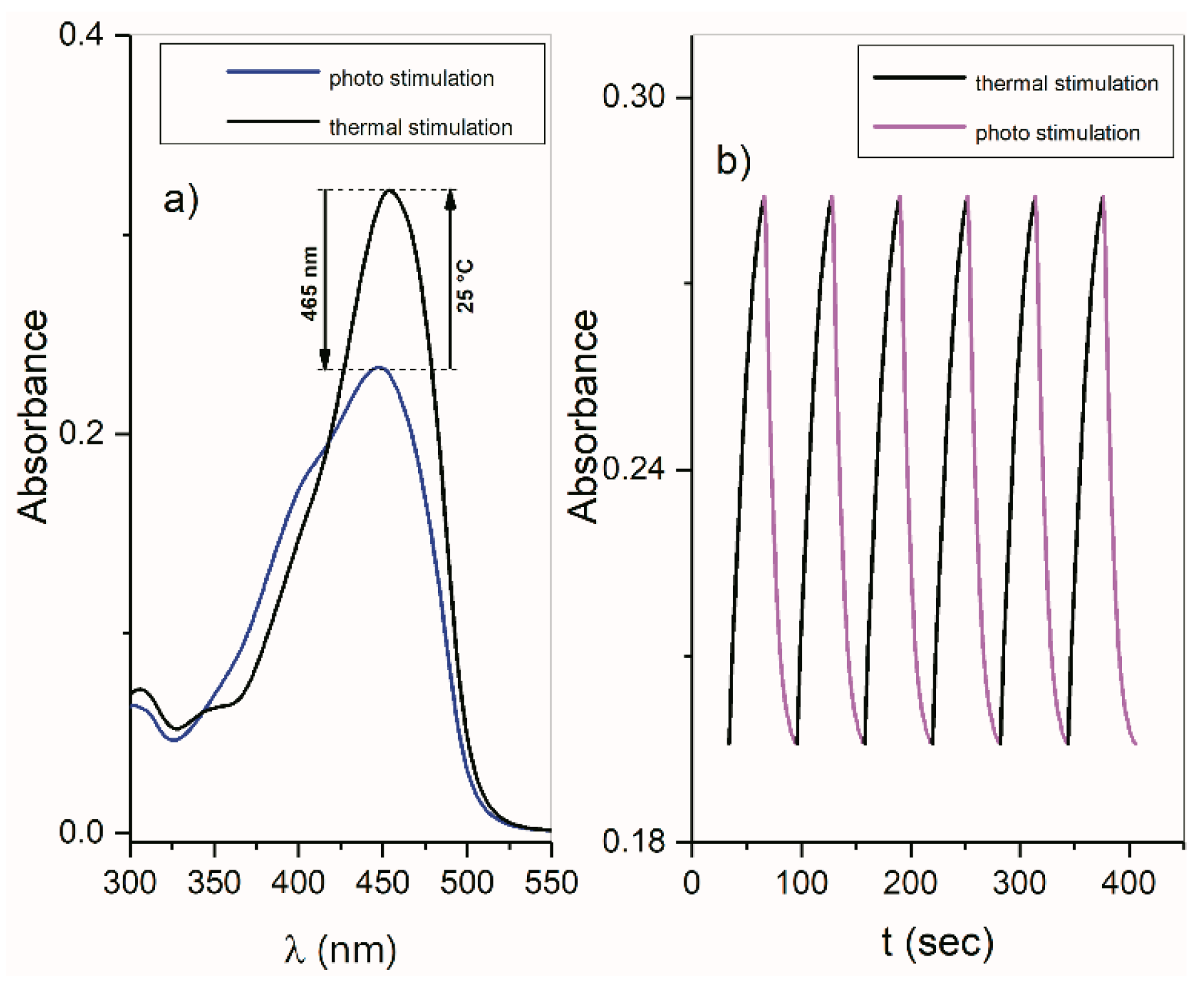
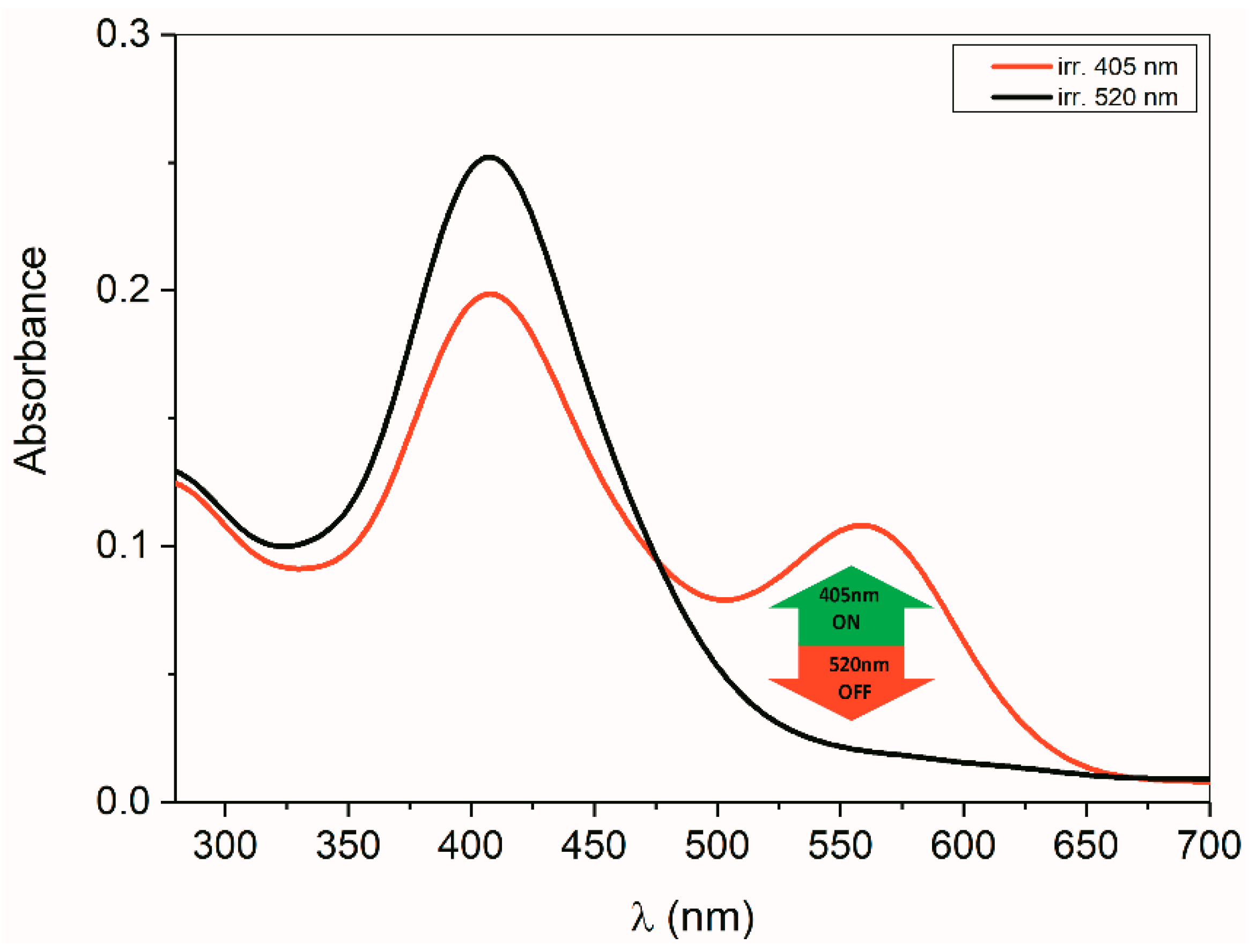
2.5. ON/OFF Switching
2.5.1. ON/OFF Switching of Isatin Arylhydrazones 1–3 and ((Arylamino)Methylene)Indolin-2-Ones 4 and 5
2.5.2. ON/OFF Switching of Anions of Arylhydrazone 1–3 and Compounds 4 and 5
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of Hydrazones 1–3
3.2.2. Synthesis of Compounds 5 and 6
3.3. Spectroscopic Measurements
3.4. HPLC Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Bertarelli, C.; Bianco, A.; Castagna, R.; Pariani, G. Photochromism into optics: Opportunities to develop light-triggered optical elements. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 106–125. [Google Scholar] [CrossRef]
- Yun, C.; You, J.; Kim, J.; Huh, J.; Kim, E. Photochromic fluorescence switching from diarylethenes and its applications. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 111–129. [Google Scholar] [CrossRef]
- Raymo, F.M.; Tomasulo, M. Electron and energy transfer modulation with photochromic switches. Chem. Soc. Rev. 2005, 34, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Deniz, E.; Raymo, F.M. Fluorescence modulation with photochromic switches in nanostructured constructs. Chem. Soc. Rev. 2009, 38, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaur, M.N.; Collado, D.; Lehn, J.M. Configurational and constitutional information storage: Multiple dynamics in systems based on pyridyl and acyl hydrazones. Chemistry 2011, 17, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 2014, 43, 1963–1981. [Google Scholar] [CrossRef] [Green Version]
- Vantomme, G.; Hafezi, N.; Lehn, J.-M. A light-induced reversible phase separation and its coupling to a dynamic library of imines. Chem. Sci. 2014, 5, 1475–1483. [Google Scholar] [CrossRef]
- Vantomme, G.; Lehn, J.M. Photo- and thermoresponsive supramolecular assemblies: Reversible photorelease of K+ ions and constitutional dynamics. Angew. Chem. Int. Ed. Engl. 2013, 52, 3940–3943. [Google Scholar] [CrossRef] [PubMed]
- Tisovský, P.; Horváth, M.; Csicsai, K.; Donovalová, J.; Filo, J.; Cigáň, M.; Sokolík, R.; Addová, G.; Gáplovský, A. Isatin-1,8-Naphthalimide Hydrazones: A Study of Their Sensor and ON/OFF Functionality. Molecules 2019, 24, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filo, J.; Tisovský, P.; Csicsai, K.; Donovalová, J.; Gáplovský, M.; Gáplovský, A.; Cigáň, M. Tautomeric photoswitches: Anion-assisted azo/azine-to-hydrazone photochromism. RSC Adv. 2019, 9, 15910–15916. [Google Scholar] [CrossRef] [Green Version]
- Horváth, M.; Cigáň, M.; Filo, J.; Jakusová, K.; Gáplovský, M.; Šándrik, R.; Gáplovský, A. Isatin pentafluorophenylhydrazones: Interesting conformational change during anion sensing. RSC Adv. 2016, 6, 109742–109750. [Google Scholar] [CrossRef]
- Tisovský, P.; Šandrik, R.; Horváth, M.; Donovalová, J.; Jakusová, K.; Cigáň, M.; Sokolík, R.; Gáplovský, A.; Gáplovský, M.; Filo, J. Effect of Structure on Charge Distribution in the Isatin Anions in Aprotic Environment: Spectral Study. Molecules 2017, 22, 1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrik, R.; Tisovsky, P.; Csicsai, K.; Donovalova, J.; Gaplovsky, M.; Sokolik, R.; Filo, J.; Gaplovsky, A. ON/OFF Photostimulation of Isatin Bipyridyl Hydrazones: Photochemical and Spectral Study. Molecules 2019, 24, 2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salado, I.G.; Zaldivar-Diez, J.; Sebastian-Perez, V.; Li, L.; Geiger, L.; Gonzalez, S.; Campillo, N.E.; Gil, C.; Morales, A.V.; Perez, D.I.; et al. Leucine rich repeat kinase 2 (LRRK2) inhibitors based on indolinone scaffold: Potential pro-neurogenic agents. Eur. J. Med. Chem. 2017, 138, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.D. Synthesis of potential antineoplastic agents. XX. Compounds related to the 3-o-nitrophenylhydrazone of isatin. J. Med. Chem. 1969, 12, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.K.; Saravanan, M.; Ramesh, A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur. J. Med. Chem. 2001, 36, 615–625. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Eine effiziente Synthese von Aminoalkylidenderivaten fnfringcyclischer methylenaktiver Verbindungen. Mon. Chem. 1981, 112, 369–383. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |






| Compound | cZhydrazo × 106 [mol·dm−3] | cEhydrazo × 106 [mol·dm−3] | cZhydrazo/cEhydrazo | cEazo |
|---|---|---|---|---|
| 1 | 7.30 | 2.70 | 2.70 | - |
| 2Z | 6.64 | 3.36 | 1.98 | - |
| 3Z | 1.68 | 8.31 | - | 0.20 |
| 4 | 5.68 | 4.32 | 1.31 | - |
| 5 | 9.06 | 0.94 | 9.67 | - |
| cTBAF [mol·dm−3] | k [s−1] |
|---|---|
| 1 × 10−4 | 0.008 |
| 2.5 × 10−4 | 0.009 |
| 5 × 10−4 | 0.025 |
| 1 × 10−3 | 0.040 |
| Compound | CTBAF [mol·dm−3] | kZanion [s−1] | kZanion-Eanion [s−1] |
|---|---|---|---|
| 4 | 1 × 10−2 | / | 0.002 |
| 5 | 2 × 10−4 | 1.025 | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisovský, P.; Csicsai, K.; Donovalová, J.; Šandrik, R.; Sokolík, R.; Gáplovský, A. Effect of a =X-NH-Fragment, (X = C, N), on Z/E Isomerization and ON/OFF Functionality of Isatin Arylhydrazones, ((Arylamino)Methylene)Indolin-2-Ones and Their Anions. Molecules 2020, 25, 3082. https://doi.org/10.3390/molecules25133082
Tisovský P, Csicsai K, Donovalová J, Šandrik R, Sokolík R, Gáplovský A. Effect of a =X-NH-Fragment, (X = C, N), on Z/E Isomerization and ON/OFF Functionality of Isatin Arylhydrazones, ((Arylamino)Methylene)Indolin-2-Ones and Their Anions. Molecules. 2020; 25(13):3082. https://doi.org/10.3390/molecules25133082
Chicago/Turabian StyleTisovský, Pavol, Klaudia Csicsai, Jana Donovalová, Róbert Šandrik, Róbert Sokolík, and Anton Gáplovský. 2020. "Effect of a =X-NH-Fragment, (X = C, N), on Z/E Isomerization and ON/OFF Functionality of Isatin Arylhydrazones, ((Arylamino)Methylene)Indolin-2-Ones and Their Anions" Molecules 25, no. 13: 3082. https://doi.org/10.3390/molecules25133082





