Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach
Abstract
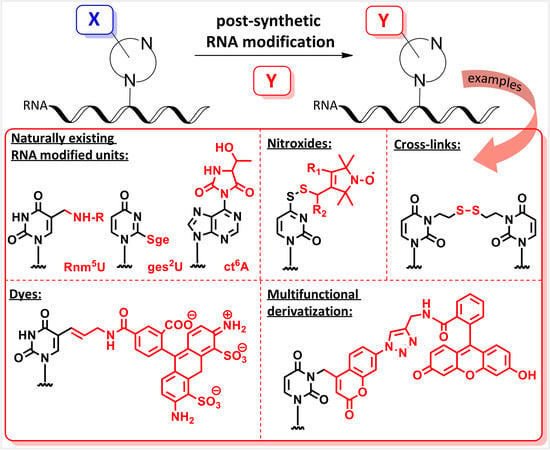
:1. Introduction
2. Solid-Phase Synthesis of Modified RNA Oligomers via Phosphoramidite Chemistry
3. Post-Synthetic Strategy for Nucleobase RNA Modifications
3.1. Nucleophilic Aromatic Substitution
3.2. Carbon–Carbon Bond-Forming Reaction via Sonogashira and Stille Couplings
3.3. Cycloaddition Reactions
3.4. Derivatization of Amino-Modified Oligoribonucleotides via Formation of Amide Linkage
3.5. Transformation of Ester Groups
3.6. Post-Synthetic Conversions of Sulfur-Containing RNA Oligomers
3.6.1. Post-Synthetic Conversion of 2-Thiouridine-Modified RNA Oligomers
3.6.2. Post-Synthetic Conversion of 4-Thiouridine-Prefunctionalized RNA Oligomers
3.6.3. Post-Synthetic Formation of Disulfide Crosslinks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ac | acetyl |
| ACE | bis(2-acetoxyethoxy)methyl |
| AMA | ammonia-methylamine solution |
| AZT | 3′-azidothymidine |
| Bz | benzoyl |
| BzH | benzhydryloxybis-(trimethylsiloxy)silyl |
| cmnm5ges2U | 5-carboxymethylaminomethyl-2-geranylthiouridine |
| cmnm5U | 5-carboxymethylaminomethyluridine |
| cnm5U | 5-cyanomethyluridine |
| CPG | controlled pore glass |
| ct6A | cyclic N6-threonylcarbamoyladenosine |
| dbf | dibutylaminomethylene |
| DBU | 1,8-diazabicyclo [5.4. 0]undec-7-ene |
| DCM | dichloromethane |
| DIPEA | N,N-diisopropylethylamine |
| dmf | dimethylaminomethylene |
| DMTr | 4,4′-dimethoxytrityl |
| DOD | bis(trimethylsiloxy)cyclododecyloxysilyl |
| DTT | dithiothreitol |
| EDC·HCl | 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride |
| EPR | electron paramagnetic resonance |
| ESI-MS | electrospray ionization mass spectrometry |
| FMN | flavin mononucleotide |
| Fpmp | 1-(2-fluorophenyl)-4-methoxypiperidin-4-yl |
| geBr | geranyl bromide |
| ges2U | 2-geranylthiouridine |
| H2U | 4-pyrimidinone nucleoside |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| HOBt | N-hydroxybenzotriazole |
| i6A | N6-isopentenyladenosine |
| iBu | isobutyryl |
| IE HPLC | ion-exchange high performance liquid chromatography |
| iEDDA | inverse electron demand Diels-Alder cycloaddition |
| inm5U | 5-(isopentenylaminomethyl)uridine |
| m5s2U | 5-methyl-2-thiouridine |
| m6A | N6-methyladenosine |
| MALDI-ToF-MS | matrix-assisted laser desorption/ionization-time of flight mass spectrometry |
| mcm5s2U | 5-metoxycarbonylmethyl-2-thiouridine, |
| mnm5ges2U | 5-methylaminomethyl-2-geranylthiouridine |
| mnm5s2U | 5-methylaminomethyl-2-thiouridine |
| mnm5U | 5-methylaminomethyluridine |
| ms2C | 2-methylthiocytidine |
| ms2ct6A | 2-methylthio cyclic N6-threonylcarbamoyladenosine |
| ms2i6A | 2-methylthio-N6-isopentenyladenosine |
| ms2m6A | 2-methylthio-N6-methyladenosine |
| ms2t6A | 2-methylthio-N6-threonylcarbamoyladenosine |
| msms2i6A | 2-methylthiomethylenethio-N6-isopentenyladenosine |
| NHS | N-hydroxysuccinimide |
| nm5U | 5-aminomethyluridine |
| NMP | N-methyl-2-pyrrolidone |
| NPE | 2-(2-nitrophenyl)ethyl |
| NPP | 2-(2-nitrophenyl)propyl |
| OPiv | pivaloyloxyl |
| P(furyl)3 | tri-2-furylphosphine |
| Pac | phenoxyacetyl |
| PAGE | polyacrylamide gel electrophoresis |
| Pd2(dba)3 | tris(dibenzylideneacetone)dipalladium(0) |
| PELDOR | pulsed electron-electron double resonance |
| Pivom5U | 5-pivaloyloxymethyluridine |
| R5H2U | 5-substituted 4-pyrimidinone nucleoside |
| R5s2U | 5-substituted 2-thiouridine |
| R5U | 5-substituted uridine |
| Rb | ribose |
| RP HPLC | reverse-phase high performance liquid chromatography |
| rt | room temperature |
| s2U | 2-thiouridine |
| s2Um | 2′-O-methyl-2-thiouridine |
| s4dU | 4-thio-2′-deoxyuridine |
| s4U | 4-thiouridine |
| SNAr | nucleophilic aromatic substitution |
| t6A | N6-threonylcarbamoyladenosine |
| Tac | 4-(tert-butylphenoxy)acetyl |
| TBAF | tetrabutylammonium fluoride |
| TBDMS | tert-butyldimethylsilyl |
| TBTA | tris[(1-benzy-1H-1,2,3-triazol-4-yl)methyl]amine |
| TC | 1,1-dioxo-1l6-thiomorpholine-4-carbothioate |
| TEA | triethylamine |
| TEA×3HF | triethylamine trihydrofluoride |
| TEAA | triethylammonium acetate |
| TEAF | triethylammonium fluoride |
| TEG | triethylene glycol |
| TEMED | N,N,N′,N′-tetramethylethylenediamine |
| TEMPO | 2,2,6,6-tetramethylpiperidin-1-oxyl |
| TEMPO-NH2 | 4-amino-2,2,6,6-tetramethylpiperidin-1-oxyl |
| TFP | tetrafluorophenol |
| THPTA | tris-[1-(3-hydroxypropyl)-1H-[1,2,3]triazol-4-yl)methyl]amine |
| TMS | trimethylsilyl |
| TMSE | 2-(trimethylsilyl)ethyl |
| TOM | triisopropylsilyloxymethyl |
| TPA | 2,2,5,5-tetramethylpyrrolin-1-yloxyl-3-acetylene |
| Tris-HCl | tris(hydroxymethyl)aminomethane hydrochloride |
| TSTU | N,N,N′,N′-tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate |
| τm5U | 5-taurinomethyluridine |
| τm5s2U | 5-taurinomethyl-2-thiouridine |
References
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Röthlisberger, P.; Berk, C.; Hall, J. RNA chemistry for RNA biology. Chimia 2019, 73, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-targeted therapeutics. Cell Metab. 2018, 27, 714–793. [Google Scholar] [CrossRef] [PubMed]
- Machnicka, M.A.; Olchowik, A.; Grosjean, H.; Bujnicki, J.M. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 2014, 11, 1619–1629. [Google Scholar] [CrossRef] [Green Version]
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piatkowski, P.; Baginski, B.; Wirecki, K.; de Crecy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018, 46, D303–D307. [Google Scholar] [CrossRef]
- Matuszewski, M.; Wojciechowski, J.; Miyauchi, K.; Gdaniec, Z.; Wolf, W.M.; Suzuki, T.; Sochacka, E. A hydantoin isoform of cyclic N6-threonylcarbamoyladenosine (ct6A) is present in tRNAs. Nucleic Acids Res. 2017, 45, 2137–2149. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.I.; Miyauchi, K.; Matuszewski, M.; D’Almeida, G.S.; Rubio, M.A.T.; Alfonzo, J.D.; Inoue, K.; Sakaguchi, Y.; Suzuki, T.; Sochacka, E.; et al. Identification of 2-methylthio cyclic N6-threonylcarbamoyladenosine (ms2ct6A) as a novel RNA modification at position 37 of tRNAs. Nucleic Acids Res. 2017, 45, 2124–2136. [Google Scholar] [CrossRef] [Green Version]
- Dal Magro, C.; Keller, P.; Kotter, A.; Werner, S.; Duarte, V.; Marchand, V.; Ignarski, M.; Freiwald, A.; Müller, R.U.; Dieterich, C.; et al. A vastly increased chemical variety of RNA modifications containing a thioacetal structure. Angew. Chem. 2018, 57, 7893–7897. [Google Scholar] [CrossRef]
- Dumelin, C.E.; Chen, Y.; Leconte, A.M.; Chen, Y.G.; Liu, D.R. Discovery and biological characterization of geranylated RNA in bacteria. Nat. Chem. Biol. 2012, 8, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Reichle, V.F.; Petrov, D.P.; Weber, V.; Jung, K.; Kellner, S. NAIL-MS reveals the repair of 2-methylthiocytidine by AlkB in E. coli. Nat. Commun. 2019, 10, 5600. [Google Scholar] [CrossRef]
- McCown, P.J.; Ruszkowska, A.; Kunkler, C.N.; Breger, K.; Hulewicz, J.P.; Wang, M.C.; Springer, N.A.; Brown, J.A. Naturally occurring modified ribonucleosides. Wiley Interdiscip. Rev. RNA 2020, e1595. [Google Scholar] [CrossRef]
- Sierant, M.; Leszczynska, G.; Sadowska, K.; Dziergowska, A.; Rozanski, M.; Sochacka, E.; Nawrtot, B. S-Gernayl-2-thiouridine wobble nucleosides of bacterial tRNAs; chemical and enzymatic synthesis of S-geranylated-RNAs and their physicochemical characterization. Nucleic Acids Res. 2016, 44, 10986–10998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierant, M.; Leszczynska, G.; Sadowska, K.; Komar, P.; Radzikowska-Cieciura, E.; Sochacka, E.; Nawrot, B. Escherichia coli tRNA 2-selenouridine synthase (SelU) converts S2U-RNA to Se2U-RNA via S-geranylated-intermediate. FEBS Lett. 2018, 592, 2248–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basanta-Sanchez, M.; Wang, R.; Liu, Z.; Ye, X.; Li, M.; Shi, X.; Agris, P.F.; Zhou, Y.; Huang, Y.; Sheng, J. TET1-mediated oxidation of 5-formylcytosine (5fC) to 5-carboxycytosine (5caC) in RNA. ChemBioChem 2017, 18, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sheng, J.; Hassan, A.E.A.; Jiang, S.; Gan, J.; Huang, Z. Novel RNA base pair with higher specificity using single selenium atom. Nucleic Acids Res. 2012, 40, 5171–5179. [Google Scholar] [CrossRef] [Green Version]
- Murphy IV, F.V.; Ramakrishnan, V.; Malkiewicz, A.; Agris, P.F. The role of modifications in codon discrimination by tRNALysUUU. Nat. Struct. Mol. Biol. 2004, 11, 1186–1191. [Google Scholar] [CrossRef]
- Cantara, W.A.; Bilbille, Y.; Kim, J.; Kaiser, R.; Leszczyńska, G.; Malkiewicz, A.; Agris, P.F. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNA(Arg1,2). J. Mol. Biol. 2012, 416, 579–597. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Ansari, G.; Guenther, R.; Sochacka, E.; Malkiewicz, A.; Agris, P.F. The uridine in “U-turn”: Contributions to tRNA-ribosomal binding. RNA 1999, 5, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Erlacher, M.D.; Chirkova, A.; Voegele, P.; Polacek, N. Generation of chemically engineered ribosomes for atomic mutagenesis studies on protein biosynthesis. Nat. Prot. 2011, 6, 580–592. [Google Scholar] [CrossRef]
- Koch, M.; Willi, J.; Pradere, U.; Hall, J.; Polacek, N. Critical 23S rRNA interactions for macrolide-dependent ribosome stalling on the ErmCL nascent peptide chain. Nucleic Acids Res. 2017, 45, 6717–6727. [Google Scholar] [CrossRef]
- Zhang, X.; Cekan, P.; Sigurdsson, S.T.; Qin, P.Z. Studying RNA using site-directed spin-labeling and continuous-wave electron paramagnetic resonance spectroscopy. Methods Enzymol. 2009, 469, 303–328. [Google Scholar] [PubMed] [Green Version]
- Wojczewski, C.; Stolze, K.; Engels, J.W. Fluorescent oligonucleotides – versatile tools as probes and primers for DNA and RNA analysis. Synlett 1999, 10, 1667–1678. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.Z.; Dieckmann, T. Application of EPR and NMR methods to the study of RNA. Curr. Opin. Struct. Biol. 2004, 14, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.T. Nitroxides and nucleic acids: Chemistry and electron paramagnetic resonance (EPR) spectroscopy. Pure Appl. Chem. 2011, 83, 677–686. [Google Scholar] [CrossRef]
- Haller, A.; Soulière, M.F.; Micura, R. The dynamic nature of RNA as key to understanding riboswitch mechanisms. Acc. Chem. Res. 2011, 44, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.A.; Sigurdsson, S.T. Site-directed spin labeling of nucleic acids. Eur. J. Org. Chem. 2012, 2012, 2291–2301. [Google Scholar] [CrossRef]
- Wachowius, F.; Höbartner, C. Chemical RNA modifications for studies of RNA structure and dynamics. ChemBioChem 2010, 11, 469–480. [Google Scholar] [CrossRef]
- Hengesbach, M.; Kobitski, A.; Voigts-Hoffmann, F.; Frauer, C.; Nienhaus, G.U.; Helm, M. RNA intramolecular dynamics by single-molecule FRET. Curr. Protoc. Nucleic Acid Chem. 2008, 34, 11.12.1–11.12.22. [Google Scholar] [CrossRef]
- Burnett, J.C.; Rossi, J.J. RNA-based therapeutics: Current progress and future prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Kurreck, J. Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem. 2003, 270, 1628–1644. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.B.; Seth, P.P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 2016, 59, 9645–9667. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.K.; Deleavey, G.F.; Damha, M.J. Chemically modified siRNA: Tools and application. Drug Discov. Today 2008, 13, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.; Fucini, R.V.; Jayalath, P.; Ibarra-Soza, J.M.; Haringsma, H.J.; Flanagan, W.M.; Willingham, A.; Beal, P.A. Nucleobase and ribose modifications control immunostimulation by a MicroRNA-122-mimetic RNA. J. Am. Chem. Soc. 2011, 133, 9200–9203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, H.; Fostvedt, E.; Beal, P.A. Minor-groove-modulating adenosine replacements control protein binding and RNAi activity in siRNA. ACS Chem. Biol. 2010, 5, 1115–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaucage, S.L.; Caruthers, M.H. Deoxynucleoside phosphoramidite. A new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett. 1981, 22, 1859–1862. [Google Scholar] [CrossRef]
- Matteucci, M.D.; Caruthers, M.H. Synthesis of deoxyoligonucleotides on a polymer support. J. Am. Chem. Soc. 1981, 103, 3185–3191. [Google Scholar] [CrossRef]
- Höbartner, C.; Wachowius, F. Chemical synthesis of modified RNA. In The Chemical Biology of Nucleic Acids; Mayer, B., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2010; pp. 1–37. [Google Scholar]
- Beaucage, S.L.; Iyer, R.P. The synthesis of modified oligonucleotides by the phosphoramidite approach and their applications. Tetrahedron 1993, 49, 6123–6194. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, K.K.; Theriault, N.; Sadana, K.L. Synthesis of oligoribonucleotides. J. Am. Chem. Soc. 1977, 99, 7741–7743. [Google Scholar] [CrossRef]
- Ogilvie, K.K.; Beaucagge, S.L.; Schifman, A.L.; Theriault, N.Y.; Sadana, K.L. The synthesis of oligoribonucleotides. II. The use of silyl protecting groups in nucleoside and nucleotide chemistry. VII. Can. J. Chem. 1978, 56, 2768–2780. [Google Scholar] [CrossRef] [Green Version]
- Pitsch, S.; Weiss, P.A.; Jenny, L.; Stutz, A.; Wu, X. Reliable chemical synthesis of oligoribonucleotides (RNA) with 2’-O-[(triisopropylsilyl)oxy]methyl(2’-O-tom)-protected phosphoramidites. Helv. Chim. Acta 2001, 84, 3773–3795. [Google Scholar] [CrossRef]
- Capaldi, D.C.; Reese, C.B. Use of the 1-(2-fluorophenyl)-4-methoxypiperidin-4-yl (Fpmp) and related groups in oligoribonucleotide synthesis: Stability of internucleotide linkages to aqueous acid. Nucleic Acids Res. 1994, 22, 2209–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellinger, D.J.; Timar, Z.; Myerson, J.; Sierzchala, A.B.; Turner, J.; Ferreira, F.; Kupihar, Z.; Dellinger, G.; Hill, K.W.; Powell, J.A.; et al. Streamlined process for the chemical synthesis of RNA using 2′-O-thionocarbamate-protected nucleoside phosphoramidites in the solid phase. J. Am. Chem. Soc. 2011, 133, 11540–11556. [Google Scholar] [CrossRef] [PubMed]
- Scaringe, S.A.; Wincott, F.E.; Caruthers, M.H. Novel RNA synthesis method using 5′-O-silyl-2′-O-orthoester protecting groups. J. Am. Chem. Soc. 1998, 120, 11820–11821. [Google Scholar] [CrossRef]
- Scaringe, S.A. RNA oligonucleotide synthesis method via 5′-silyl-2′-orthoester chemistry. Methods 2001, 23, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.C.; Pandey, D.; Srivastava, N.P.; Bajpai, S.P. RNA synthesis: Phosphoramidites for RNA synthesis in the reverse direction. Highly efficient synthesis and application to convenient introduction of ligands, chromophores and modifications of synthetic RNA at the 3′-end. Nucleic Acids Symp. Ser. 2008, 52, 103–104. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Pandey, D.; Srivastava, N.P.; Bajpai, S.P. RNA synthesis by reverse direction process: Phosphoramidites and high purity RNAs and introduction of ligands, chromophores, and modifications at 3′-end. Curr. Protoc. Nucleic Acids Chem. 2011, 45, 3.20.1–3.20.39. [Google Scholar] [CrossRef]
- Duss, O.; Yulikov, M.; Jeschke, G.; Allain, F.H.-T. EPR-aided approach for solution structure determination of large RNAs or protein-RNA complexes. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Kerzhner, M.; Matsuoka, H.; Wuebben, C.; Famulok, M.; Schiemann, O. High yield spin labeling of long RNAs for EPR spectroscopy. Biochemistry 2018, 57, 2923–2931. [Google Scholar] [CrossRef]
- Lang, K.; Micura, R. The preparation of site-specifically modified riboswitch domains as an example for enzymatic ligation of chemically synthesized RNA fragments. Nat. Protoc. 2008, 3, 1457–1466. [Google Scholar] [CrossRef]
- Büttner, L.; Seikowski, J.; Wawrzyniak, K.; Ochmann, A.; Höbartner, C. Synthesis of spin-labeled riboswitch RNAa using convertible nucleosides and DNA-catalyzed RNA ligation. Bioorg. Med. Chem. 2013, 21, 6171–6180. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, Y.S. Large-scale automated synthesis of therapeutic oligonucleotides: A status update. In Advances in Nucleic Acid Therapeutics; Agrawal, S., Gait, M.J., Eds.; Royal Society of Chemistry: London, UK, 2019; Chapter 19; pp. 453–473. [Google Scholar]
- MacMillan, A.M.; Verdine, G.L. Synthesis of functionally tethered oligodeoxynucleotides by the convertible nucleoside approach. J. Org. Chem. 1990, 55, 5931–5933. [Google Scholar] [CrossRef]
- MacMillan, A.M.; Verdine, G.L. Engineering tethered DNA molecules by the convertible nucleoside approach. Tetrahedron 1991, 47, 2603–2616. [Google Scholar] [CrossRef]
- Harris, C.M.; Zhou, L.; Strand, E.A.; Harris, T.M. New strategy for the synthesis of oligodeoxynucleotides bearing adducts at exocyclic amino sites of purine nucleosides. J. Am. Chem. Soc. 1991, 113, 4328–4329. [Google Scholar] [CrossRef]
- Ferentz, A.E.; Verdine, G.L. Disulfide cross-linked oligonucleotides. J. Am. Chem. Soc. 1991, 113, 4000–4002. [Google Scholar] [CrossRef]
- Ferentz, A.E.; Verdine, G.L. Aminolysis of 2-deoxyinosine aryl ethers: Nucleoside model studies for the synthesis of functionally tethered oligonucleotides. Nucleosides Nucleotides 1992, 11, 1749–1763. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Zheng, Q.; Swann, P.F. Synthesis of DNA containing modified bases by post-synthetic substitution. Synthesis of oligomers containing 4-substituted thymine: O4-alkylthymine, 5-methylcytosine, N4-dimethylamino-5-methylcytosine, and 4-thiothymine. J. Org. Chem. 1992, 57, 3839–3845. [Google Scholar] [CrossRef]
- Kierzek, E.; Kierzek, R. The synthesis of oligonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines via post-synthetic modification of precursor oligomers. Nucleic Acids Res. 2003, 31, 4461–4471. [Google Scholar] [CrossRef] [Green Version]
- Chwialkowska, A.; Wielgus, E.; Leszczynska, G.; Sobczak, M.; Mikolajczyk, B.; Sochacka, E.; Nawrot, B. An efficient approach for conversion of 5-substituted 2-thiouridines built in RNA oligomers into corresponding desulfured 4-pyrimidinone products. Bioorg. Med. Chem. Lett. 2015, 25, 3100–3104. [Google Scholar] [CrossRef]
- Bartosik, K.; Sochacka, E.; Leszczynska, G. Post-synthetic conversion of 5-pivaloyloxymethyluridine present in a support-bound RNA oligomer into biologically relevant derivatives of 5-methyluridine. Org. Biomol. Chem. 2017, 15, 2097–2103. [Google Scholar] [CrossRef] [Green Version]
- Debiec, K.; Matuszewski, M.; Podskoczyj, K.; Leszczynska, G.; Sochacka, E. Chemical synthesis of oligoribonucleotide (ASL of tRNALys T. brucei) containing a recently discovered cyclic form of 2-methylthio-N6-threonylcarbamoyladenosine (ct6A). Chem. Eur. J. 2019, 25, 13309–13317. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, M.; Debiec, K.; Sochacka, E. Efficient conversion of N6-threonylcarbamoyladenosine (t6A) into a tRNA native hydantoin cyclic form (ct6A) performed at nucleoside and oligoribonucleotide levels. Chem. Commun. 2017, 53, 7945–7948. [Google Scholar] [CrossRef] [PubMed]
- Piton, N.; Mu, Y.; Stock, G.; Prisner, T.F.; Schiemann, O.; Engels, J.W. Base-specific spin-labeling of RNA for structure determination. Nucleic Acids Res. 2007, 35, 3128–3143. [Google Scholar] [CrossRef]
- Allerson, C.R.; Verdine, G.L. Synthesis and biochemical evaluation of RNA containing an intrahelical disulfide crosslink. Chem. Biol. 1995, 2, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Allerson, C.R.; Chen, S.L.; Verdine, G.L. A chemical method for site-specific modification of RNA: The convertible nucleoside approach. J. Am. Chem. Soc. 1997, 119, 7423–7433. [Google Scholar] [CrossRef]
- MacMillan, A.M.; Chen, L.; Verdine, G.L. Synthesis of an oligonucleotide suicide substrate for DNA methyltransferases. J. Org. Chem. 1992, 57, 2989–2991. [Google Scholar] [CrossRef]
- Babaylova, E.S.; Ivanov, A.V.; Malygin, A.A.; Vorobjeva, M.A.; Venyaminova, A.G.; Polienko, Y.F.; Kirilyuk, I.A.; Krumkacheva, O.A.; Fedin, M.V.; Karpova, G.G.; et al. A versatile approach for site-directed spin labeling and structural EPR studies of RNA. Org. Biomol. Chem. 2014, 12, 3129–3136. [Google Scholar] [CrossRef]
- Sicoli, G.; Wachowius, F.; Bennati, M.; Höbartner, C. Probing secondary structure of spin-labeled RNA by pulsed EPR spectroscopy. Angew. Chem. Int. Ed. 2010, 49, 6443–6447. [Google Scholar] [CrossRef] [Green Version]
- Rodriques-Correia, A.; Stoess, T.; Wilhelm, J.; Berlin, B.; Heckel, A. Rapid synthetic access to caged RNA. Indian J. Chem. 2013, 52A, 1014–1018. [Google Scholar]
- Adams, C.J.; Murray, J.B.; Arnold, J.R.P.; Stockley, P.G. A convenient synthesis of S-cyanoethyl-protected 4-thiouridine and its incorporation into oligoribonucleotides. Tetrahedron Lett. 1994, 35, 765–768. [Google Scholar] [CrossRef]
- McGregor, A.; Rao, M.V.; Duckworth, G.; Stockley, P.G.; Connolly, B.A. Preparation of oligoribonucleotides containing 4-thiouridine using Fpmp chemistry. Photo-crosslinking to RNA binding proteins using 300 nm irradiation. Nucleic Acids Res. 1996, 24, 3173–3180. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Wu, H.; Rana, T.M. Synthesis of uridine phosphoramidite analogs: Reagents for site-specific incorporation of photoreactive sites into RNA sequences. Bioconjugate Chem. 1994, 5, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Davis, D.R. Synthesis and studies on the effect of 2-thiouridine and 4-thiouridine on sugar conformation and RNA duplex stability. Nucleic Acids Res. 1997, 25, 1272–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avino, A.; Garcia, R.G.; Eritja, R. Synthesis of oligoribonucleotides containing 4-thiouridine using the convertible nucleoside approach and the 1-(2-fluorophenyl)-4-methoxypiperidin-4-yl group. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, Y.; Kumagai, I.; Ohtsuka, E. Investigation of the recognition of an important uridine in an internal loop of a hairpin ribozyme prepared using post-synthetically modified oligonucleotides. Nucleic Acids Res. 1999, 27, 4314–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guennewig, B.; Stoltz, M.; Menzi, M.; Dogar, M.; Hall, J. Properties of N4-methylated cytidines in miRNA mimics. Nucleic Acid Ther. 2012, 22, 109–116. [Google Scholar] [CrossRef]
- Khan, S.I.; Grinstaff, M.W. Palladium (0)-catalyzed modification of oligonucleotides during automated solid-phase synthesis. J. Am. Chem. Soc. 1999, 121, 4704–4705. [Google Scholar] [CrossRef]
- Strube, T.; Schiemann, O.; MacMillan, F.; Prisner, T.; Engels, J.W. A new facile method for spin-labeling of oligonucleotides. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1271–1274. [Google Scholar] [CrossRef]
- Piton, N.; Schiemann, O.; Mu, Y.; Stock, G.; Prisner, T.; Engels, J.W. Synthesis of spin-labeled RNAs for long range distance measurements by PELDOR. Nucleosides Nucleotides Nucleic Acids 2005, 24, 771–775. [Google Scholar] [CrossRef]
- Schiemann, O.; Piton, N.; Plackmeyer, J.; Bode, B.E.; Prisner, T.F.; Engels, J.W. Spin labeling of oligonucleotides with the nitroxide TPA and use of PELDOR, a pulse EPR method, to measure intramolecular distances. Nat. Protoc. 2007, 2, 904–923. [Google Scholar] [CrossRef]
- Kwon, T.; Piton, N.; Grünewald, C.; Engels, J.W. Synthesis of pyrene labeled RNA for fluorescence measurements. Nucleosides Nucleotides Nucleic Acids 2007, 26, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, C.; Kwon, T.; Piton, N.; Förster, U.; Wachtveitl, J.; Engels, J.W. RNA as scaffold for pyrene excited complexes. Bioorg. Med. Chem. 2008, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wicke, L.; Engels, J.W. Postsynthetic on column RNA labeling via Stille coupling. Bioconjugate Chem. 2012, 23, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloaddition of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Ibarra-Soza, J.M.; Morris, A.A.; Jayalath, P.; Peacock, H.; Conrad, W.E.; Donald, M.B.; Kurth, M.J.; Beal, P.A. 7-Substituted 8-aza-7-deazaadenosines for modification of the siRNA major Groove. Org. Biomol. Chem. 2012, 10, 6491–6497. [Google Scholar] [CrossRef] [Green Version]
- Kerzhner, M.; Abdullin, D.; Więcek, J.; Matsuoka, H.; Hagelueken, G.; Schiemann, O.; Famulok, M. Post-synthetic spin-labeling of RNA through click chemistry for PELDOR measurements. Chem. Eur. J. 2016, 22, 12113–12121. [Google Scholar] [CrossRef]
- Peacock, H.; Maydanovych, O.; Beal, P.A. N2-Modified 2-aminopurine ribonucleosides as minor-groove-modulating adenosine replacements in duplex RNA. Org. Lett. 2010, 12, 1044–1047. [Google Scholar] [CrossRef] [Green Version]
- Kellner, S.; Seidu-Larry, S.; Burhenne, J.; Motorin, Y.; Helm, M. A multifunctional bioconjugate module for versatile photoaffinity labeling and click chemistry of RNA. Nucleic Acids Res. 2011, 39, 7348–7360. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K.; Akao, Y.; Ueno, Y. Diazirine-containing tag-free RNA probes for efficient RISC-loading and photoaffinity labeling of microRNA targets. Bioorg. Med. Chem. Lett. 2018, 28, 2906–2909. [Google Scholar] [CrossRef] [PubMed]
- Pyka, A.M.; Domnick, C.; Braun, F.; Kath-Schorr, S. Diels-Alder cycloadditions on synthetic RNA in mammalian cells. Bioconjugate Chem. 2014, 25, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron demand Diels-Alder reactivity. J. Am. Chem Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, N.K.; Weissleder, R.; Hilderbrand, S.A. Tetrazine-based cycloadditions: Application to pretargeted live cell imaging. Bioconjugate Chem. 2008, 19, 2297–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rublack, B.; Nguyen, H.; Appel, B.; Springstubbe, D.; Strohbach, D.; Müller, S. Synthesis of specifically modified oligonucleotides for application in structural and functional analysis of RNA. J. Nucleic Acids 2011, 80525. [Google Scholar] [CrossRef] [Green Version]
- Arguello, A.E.; DeLiberto, A.N.; Kleiner, R.E. An RNA chemical proteomics approach reveals the N6-methyladenosine (m6A)-regulated protein-RNA interactome. J. Am. Chem. Soc. 2017, 139, 17249–17252. [Google Scholar] [CrossRef] [PubMed]
- Zenkova, M.; Ehresmann, C.; Caillet, J.; Springer, M.; Karpova, G.; Ehresmann, B.; Romby, P. A Novel Approach to introduce site-directed specific cross-links within RNA-protein complexes. Eur. J. Biochem. 1995, 231, 726–735. [Google Scholar] [CrossRef]
- Leszczynska, G.; Leonczak, P.; Wozniak, K.; Malkiewicz, A. Chemical synthesis of the 5-taurinomethyl(-2-thio)uridine modified anticodon arm of the human mitochondrial tRNALeu(UUR) and tRNALys. RNA 2014, 20, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Leszczynska, G.; Pięta, J.; Wozniak, K.; Malkiewicz, A. Site-selected incorporation of 5-carboxymethyl aminomethyl(-2-thio)uridine into RNA sequences by phosphoramidite chemistry. Org. Biomol. Chem. 2014, 12, 1052–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosik, K.; Leszczynska, G. Synthesis of various substituted 5-methyluridines (xm5U) and 2-thiouridines (xm5s2U) via nucleophilic substitution of 5-pivaloyloxymethyluridine/2-thiouridine. Tetrahedron Lett. 2015, 56, 6593–6597. [Google Scholar] [CrossRef]
- Kumelis, R.G.; Nambiar, K.P. Efficient desulfurization of 2-thiopyrimidine nucleosides to the corresponding 4-pyrimidinones. Tetrahedron Lett. 1993, 34, 3813–3816. [Google Scholar] [CrossRef]
- Sochacka, E.; Kraszewska, K.; Sochacki, M.; Sobczak, M.; Janicka, M.; Nawrot, B. The 2-thiouridine unit in the RNA strand is desulfured predominantly to 4-pyrimidinone nucleoside under in vitro oxidative stress conditions. Chem. Commun. 2011, 47, 4914–4916. [Google Scholar] [CrossRef]
- Sochacka, E.; Bartos, P.; Kraszewska, K.; Nawrot, B. Desulfuration of 2-thiouridine with hydrogen peroxide in the physiological pH range 6.6–7.6 is pH-dependent and results in two distinct products. Bioorg. Med. Chem. Lett. 2013, 23, 5803–5805. [Google Scholar] [CrossRef]
- Nawrot, B.; Sochacka, E. Preparation of short interfering RNA containing the modified nucleosides 2-thiouridine, pseudouridine, or dihydrouridine. Curr. Protoc. Nucleic Acid Chem. 2009, 37, 16.2.1–16.2.16. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, G.; Pięta, J.; Leonczak, P.; Tomaszewska, A.; Malkiewicz, A. Site-specific incorporation of 5-methylaminomethyl-2-thiouridine and 2-thiouridine(s) into RNA sequence. Tetrahedron Lett. 2012, 53, 1214–1217. [Google Scholar] [CrossRef]
- Leszczynska, G.; Sadowska, K.; Sierant, M.; Sobczak, M.; Nawrot, B.; Sochacka, E. Reaction of S-geranyl-2-thiouracil modified oligonucleotides with alkyl amines leads to the N2-alkyl isocytidine derivatives. Org. Biomol. Chem. 2017, 15, 5332–5336. [Google Scholar] [CrossRef]
- Schmid, K.; Adobes-Vidal, M.; Helm, M. Alkyne-functionalized coumarin compound for analytic and preparative 4-thiouridine labeling. Bioconjugate Chem. 2017, 28, 1123–1134. [Google Scholar] [CrossRef]
- Hara, H.; Horiuchi, T.; Saneyoshi, M.; Nishimura, S. 4-Thiouridine-specific spin-labeling of E. coli transfer RNA. Biochem. Biophys. Res. Commun. 1970, 38, 305–311. [Google Scholar] [CrossRef]
- Coleman, R.S.; Kesicki, E.A. Synthesis and postsynthetic modification of oligodeoxynucleotides containing 4-thio-2′-deoxyuridine (dS4U). J. Am. Chem. Soc. 1994, 116, 11636–11642. [Google Scholar] [CrossRef]
- Ramos, A.; Varani, G. A new method to detect long-range protein-RNA contacts: NMR detection of electron-proton relaxation induced by nitroxide spin-labeled RNA. J. Am. Chem. Soc. 1998, 120, 10992–10993. [Google Scholar] [CrossRef]
- Qin, P.Z.; Hideg, K.; Feigon, J.; Hubbell, W.L. Monitoring RNA base structure and dynamics using site-directed spin labeling. Biochemistry 2003, 42, 6772–6783. [Google Scholar] [CrossRef]
- Borbat, P.P.; Davis, J.H.; Butcher, S.E.; Freed, J.H. Measurement of large distance in biomolecules using double-quantum filtered refocused electron spin-echoes. J. Am. Chem. Soc. 2004, 126, 7746–7747. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.Z.; Feigon, J.; Hubbell, W.L. Site-directed spin labeling studies reveal solution conformational changes in a GAAA tetraloop receptor upon Mg2+-dependent docking of a GAAA tetraloop. J. Mol. Biol. 2005, 351, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.Z.; Iseri, J.; Oki, A. A model system for investigating lineshape/structure correlations in RNA site-directed spin labeling. Biochem. Biophys. Res. Commun. 2006, 343, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsett, M.N. The isolation of 4-thiouridylate disulfide from oxidized transfer ribonucleic acid of Escherichia coli. J. Biol. Chem. 1967, 242, 4067–4071. [Google Scholar]
- Goodwin, J.T.; Glick, G.D. Synthesis of disulfide stabilized RNA hairpin. Tetrahedron Lett. 1994, 35, 1647–1650. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, J.T.; Osborne, S.E.; Scholle, E.J.; Glick, G.D. Design, synthesis, and analysis of yeast tRNAPhe analogs possessing intra- and interhelical disulfide cross-links. J. Am. Chem. Soc. 1996, 118, 5207–5215. [Google Scholar] [CrossRef]
- MacMillan, A.M.; Query, C.C.; Allerson, C.R.; Chen, S.; Verdine, G.L.; Sharp, P.A. Dynamic association of proteins with the pre-mRNA branch region. Genes Dev. 1994, 8, 3008–3020. [Google Scholar] [CrossRef] [Green Version]
- Carell, T.; Vrabel, M. Chapter 1: Bioorthogonal chemistry – introduction and overview. Top. Curr. Chem. 2016, 374, 1–21. [Google Scholar]
- Merkel, M.; Peewasan, K.; Arndt, S.; Ploschik, D.; Wagenknecht, H.-A. Copper-free postsynthetic labeling of nucleic acids by means of bioorthogonal reactions. ChemBioChem 2015, 16, 1541–1553. [Google Scholar] [CrossRef]
- Krell, K.; Harijan, D.; Ganz, D.; Doll, L.; Wagenknecht, H.-A. Postsynthetic modifications of DNA and RNA by means of copper-free cycloaddittions as bioorthogonal reactions. Bioconjugate Chem. 2020, 31, 990–1011. [Google Scholar] [CrossRef] [PubMed]
































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosik, K.; Debiec, K.; Czarnecka, A.; Sochacka, E.; Leszczynska, G. Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach. Molecules 2020, 25, 3344. https://doi.org/10.3390/molecules25153344
Bartosik K, Debiec K, Czarnecka A, Sochacka E, Leszczynska G. Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach. Molecules. 2020; 25(15):3344. https://doi.org/10.3390/molecules25153344
Chicago/Turabian StyleBartosik, Karolina, Katarzyna Debiec, Anna Czarnecka, Elzbieta Sochacka, and Grazyna Leszczynska. 2020. "Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach" Molecules 25, no. 15: 3344. https://doi.org/10.3390/molecules25153344