Multilayer Gold-Silver Bimetallic Nanostructures to Enhance SERS Detection of Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Bimetallic Core-Shell Nanorods
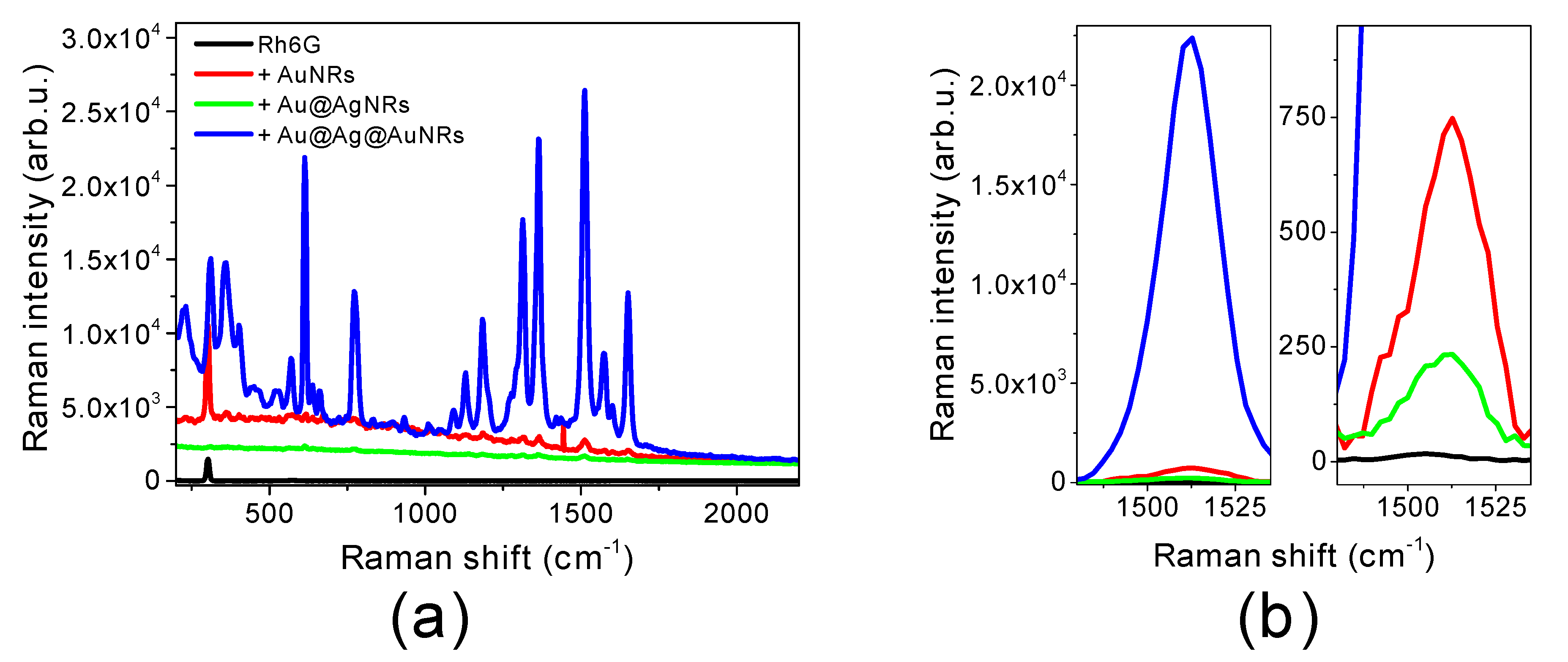
2.2. SERS Efficiency of Bimetallic Core-Shell Nanorods
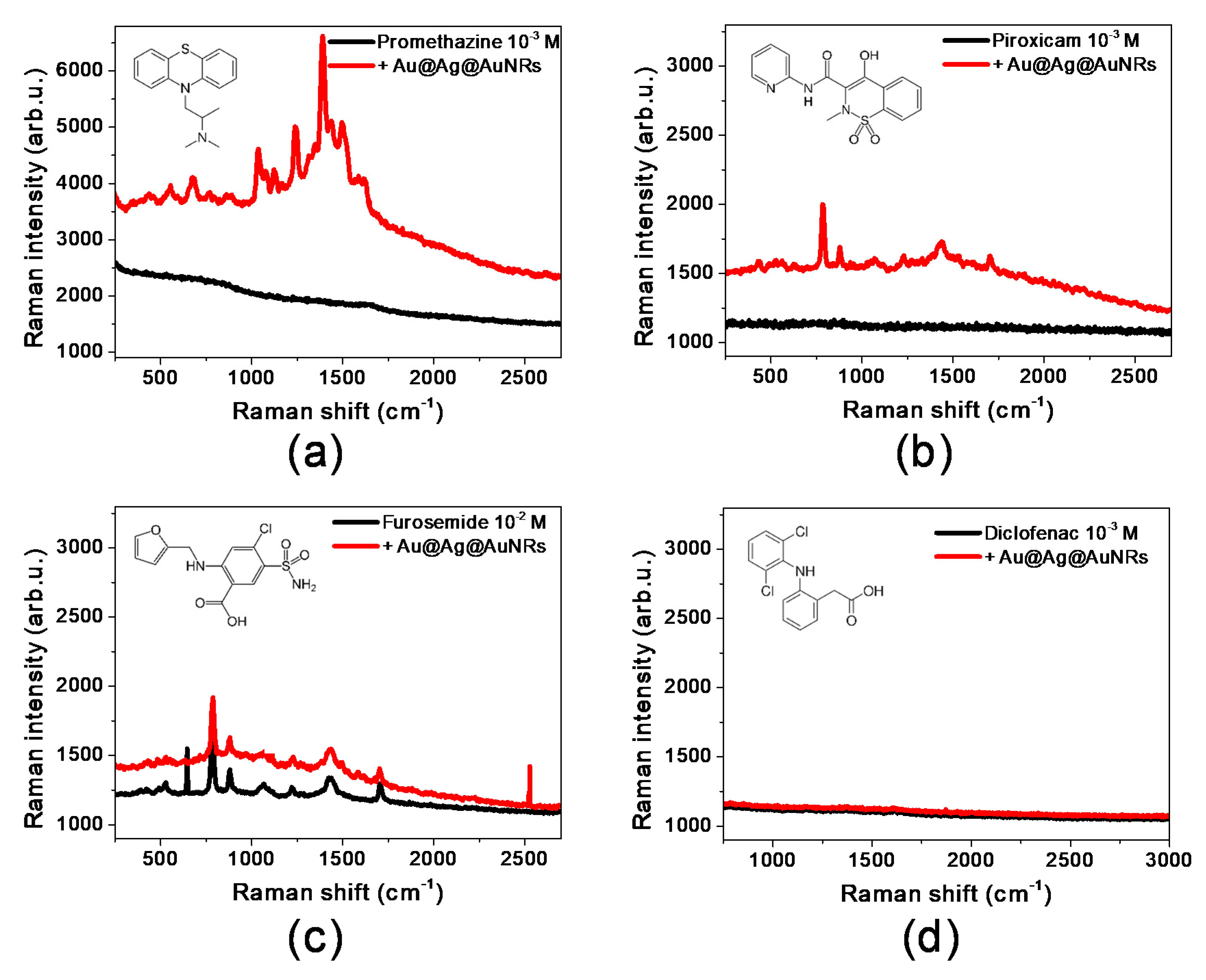
2.3. SERS Aetection of Selected Drugs
2.4. Concentration Effects on SERS Signals
3. Materials and Methods
3.1. Materials
3.2. Synthesis of AuNRs
3.3. Synthesis of Bimetallic Core-Shell Au@AgNRs and Au@Ag@AuNRs
3.4. Characterization of Nanomaterials
3.5. Raman Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aoki, P.H.B.; Furini, L.N.; Alessio, P.; Aliaga, A.E.; Constantino, C.J.L. Surface-enhanced Raman scattering (SERS) applied to cancer diagnosis and detection of pesticides, explosives, and drugs. Rev. Anal. Chem. 2013, 32, 55–76. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Uezono, S.; Toyouchi, S.; Umemoto, K.; Sekine, S.; Fujita, Y.; Ricci, M.; Lu, G.; Masuhara, A.; et al. In situ synthesis of Au-shelled Ag nanoparticles on PDMS for flexible, long-life, and broad spectrum-sensitive SERS substrates. Chem. Commun. 2017, 53, 11298–11301. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Majumder, S.; Senapati, D.; Banerjee, S.; Satpati, B. Core-shell gold@ silver hollow nanocubes for higher SERS enhancement and non-enzymatic biosensor. Mater. Chem. Phys. 2020, 239, 122113. [Google Scholar] [CrossRef]
- Joseph, D.; Kwak, C.H.; Huh, Y.S.; Han, Y.-K. Synthesis of AuAg@Ag core@ shell hollow cubic nanostructures as SERS substrates for attomolar chemical sensing. Sens. Actuators B Chem. 2019, 281, 471–477. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Surface-enhanced Raman scattering of core-shell Au@Ag nanoparticles aggregates for rapid detection of difenoconazole in grapes. Talanta 2019, 191, 449–456. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.-W. Rapid detection of multiple organophosphorus pesticides (triazophos and parathion-methyl) residues in peach by SERS based on core-shell bimetallic Au@AgNPs. Food Addit. Contam. Part A 2019, 36, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.E.; Goryacheva, I.Y.; Markin, A.V. Sample pretreatment and SERS-based detection of ceftriaxone in urine. Anal. Bioanal. Chem. 2018, 410, 2221–2227. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Du, J.; Yang, M.; Shen, Y.; Li, X.; Han, X.; Yang, L.; Zhao, B. SERS investigation and high sensitive detection of carbenicillin disodium drug on the Ag substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 241–247. [Google Scholar] [CrossRef]
- Almehmadi, L.M.; Curley, S.M.; Tokranova, N.A.; Tenenbaum, S.A.; Lednev, I.K. Surface enhanced raman spectroscopy for single molecule protein detection. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, Y.; Jiang, X.; Dong, C.; Song, C.; Han, C.; Wang, L. Ultrasensitive SERS detection of nucleic acids via simultaneous amplification of target-triggered enzyme-free recycling and multiple-reporter. Biosens. Bioelectron. 2019, 141, 111402. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, S.H.; Lim, D.-K.; Suh, Y.D. SERS-based particle tracking and molecular imaging in live cells: Toward the monitoring of intracellular dynamics. Nanoscale 2019, 11, 21724–21727. [Google Scholar] [CrossRef]
- De Aberasturi, D.; Henriksen-Lacey, M.; Litti, L.; Langer, J.; Liz-Marzán, L.M. Using SERS tags to image the three-dimensional structure of complex cell models. Adv. Funct. Mater. 2020, 30, 1909655. [Google Scholar] [CrossRef] [Green Version]
- Wali, L.A.; Hasan, K.K.; Alwan, A.M. Rapid and highly efficient detection of ultra-low concentration of penicillin G by gold nanoparticles/porous silicon SERS active substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Wang, C.; Qiu, G.; Ye, W.; Li, Y.; Wang, D. ZnO/Ag nanorods as a prominent SERS substrate contributed by synergistic charge transfer effect for simultaneous detection of oral antidiabetic drugs pioglitazone and phenformin. Sens. Actuators B Chem. 2020, 307, 127634. [Google Scholar] [CrossRef]
- Zanchi, C.; Giuliani, L.; Lucotti, A.; Pistaffa, M.; Trusso, S.; Neri, F.; Tommasini, M.; Ossi, P.M. On the performance of laser-synthesized, SERS-based sensors for drug detection. Appl. Surf. Sci. 2020, 507, 145109. [Google Scholar] [CrossRef]
- Maddipatla, D.; Janabi, F.; Narakathu, B.B.; Ali, S.; Turkani, V.S.; Bazuin, B.J.; Fleming, P.D.; Atashbar, M.Z. Development of a novel wrinkle-structure based SERS substrate for drug detection applications. Sens. Bio-Sens. Res. 2019, 24, 100281. [Google Scholar] [CrossRef]
- Liang, P.; Zhou, Y.; Xu, B.-J.; Xuan, Y.; Xia, J.; Wang, D.; Zhang, D.; Ye, J.; Yu, Z.; Jin, S. SERS-based vibration model and trace detection of drug molecules: Theoretical and experimental aspects. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 168–175. [Google Scholar] [CrossRef]
- Sun, F.; Hung, H.-C.; Sinclair, A.; Zhang, P.; Bai, T.; Galvan, D.D.; Jain, P.; Li, B.; Jiang, S.; Yu, Q. Hierarchical zwitterionic modification of a SERS substrate enables real-time drug monitoring in blood plasma. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Hassanain, W.A.; Izake, E.L.; Sivanesan, A.; Ayoko, G.A. Towards interference free HPLC-SERS for the trace analysis of drug metabolites in biological fluids. J. Pharm. Biomed. Anal. 2017, 136, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef]
- Baraldi, G.; Gonzalo, J.; Solis, J.; Siegel, J. Reorganizing and shaping of embedded near-coalescence silver nanoparticles with off-resonance femtosecond laser pulses. Nanotechnology 2013, 24, 255301. [Google Scholar] [CrossRef] [Green Version]
- Rodal-Cedeira, S.; Montes-García, V.; Polavarapu, L.; Solís, D.M.; Heidari, H.; La Porta, A.; Angiola, M.; Martucci, A.; Taboada, J.M.; Obelleiro, F.; et al. Plasmonic Au@ Pd nanorods with boosted refractive index susceptibility and SERS efficiency: A multifunctional platform for hydrogen sensing and monitoring of catalytic reactions. Chem. Mater. 2016, 28, 9169–9180. [Google Scholar] [CrossRef]
- Reguera, J.; de Aberasturi, D.J.; Winckelmans, N.; Langer, J.; Bals, S.; Liz-Marzán, L.M. Synthesis of Janus plasmonic--magnetic, star--sphere nanoparticles, and their application in SERS detection. Faraday Discuss. 2016, 191, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.; Teja, B.R.; Nagarjuna, R.; Ganesan, R.; Nag, A. Probing the surface composition effect of silver-gold alloy in SERS efficiency. Colloids Surf. A Phys. Eng. Asp. 2019, 578, 123638. [Google Scholar] [CrossRef]
- Asapu, R.; Ciocarlan, R.-G.; Claes, N.; Blommaerts, N.; Minjauw, M.; Ahmad, T.; Dendooven, J.; Cool, P.; Bals, S.; Denys, S.; et al. Plasmonic near-field localization of silver core--shell nanoparticle assemblies via wet chemistry nanogap engineering. ACS Appl. Mater. Interfaces 2017, 9, 41577–41585. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Lin, X.; Han, S.; He, L.; Zhao, H.; Zhang, J.; Hasi, W.; Wang, L. Width and length dependent SERS performance of core-shell Au@ Ag nanorod self-assembled monolayers. J. Alloy. Compd. 2019, 805, 318–326. [Google Scholar] [CrossRef]
- Bai, T.; Sun, J.; Che, R.; Xu, L.; Yin, C.; Guo, Z.; Gu, N. Controllable preparation of core-shell Au-Ag nanoshuttles with improved refractive index sensitivity and SERS activity. ACS Appl. Mater. Interfaces 2014, 6, 3331–3340. [Google Scholar] [CrossRef]
- Gambucci, M.; Aluigi, A.; Seri, M.; Sotgiu, G.; Zampini, G.; Donnadio, A.; Torreggiani, A.; Zamboni, R.; Latterini, L.; Posati, T. Effect of chemically engineered Au/Ag nanorods on the optical and mechanical properties of keratin based films. Front. Chem. 2020, 8, 158. [Google Scholar] [CrossRef]
- Liao, X.; Chen, Y.; Qin, M.; Chen, Y.; Yang, L.; Zhang, H.; Tian, Y. Au-Ag-Au double shell nanoparticles-based localized surface plasmon resonance and surface-enhanced Raman scattering biosensor for sensitive detection of 2-mercapto-1-methylimidazole. Talanta 2013, 117, 203–208. [Google Scholar] [CrossRef]
- Thi Ngoc Anh, D.; Singh, P.; Shankar, C.; Mott, D.; Maenosono, S. Charge-transfer-induced suppression of galvanic replacement and synthesis of (Au@ Ag)@ Au double shell nanoparticles for highly uniform, robust and sensitive bioprobes. Appl. Phys. Lett. 2011, 99, 73107. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Saini, G.S.S.; Sharma, A.; Kaur, S.; Bindra, K.S.; Sathe, V.; Tripathi, S.K.; Mhahajan, C.G. Rhodamine 6G interaction with solvents studied by vibrational spectroscopy and density functional theory. J. Mol. Struct. 2009, 931, 10–19. [Google Scholar] [CrossRef]
- Jensen, L.; Schatz, G.C. Resonance Raman scattering of rhodamine 6G as calculated using time-dependent density functional theory. J. Phys. Chem. A 2006, 110, 5973–5977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, W.; Sui, H.; Kitahama, Y.; Ruan, W.; Ozaki, Y.; Zhao, B. Exploring the effect of intermolecular H-bonding: A study on charge-transfer contribution to surface-enhanced raman scattering of P-mercaptobenzoic acid. J. Phys. Chem. C 2014, 118, 10191–10197. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.-J.; Kim, H.; Yi, G.-C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in nonplasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- Manzur, M.E.; Brandán, S.A. S (−) and R (+) species derived from antihistaminic promethazine agent: Structural and vibrational studies. Heliyon 2019, 5, e02322. [Google Scholar] [CrossRef]
- Hernández, M.; Corda, E.; Garcia-Ramos, J.V.; Domingo, C.; Sevilla, P. SERS of the anti-inflammatory drug piroxicam adsorbed on the surface of silver or gold colloids as nanocarrier model. J. Raman Spectrosc. 2016, 47, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Bolukbasi, O.; Yilmaz, A. X-ray structure analysis and vibrational spectra of Furosemide. Vib. Spectrosc. 2012, 62, 42–49. [Google Scholar] [CrossRef]
- Iliescu, T.; Baia, M.; Kiefer, W. FT-Raman, surface-enhanced Raman spectroscopy and theoretical investigations of diclofenac sodium. Chem. Phys. 2004, 298, 167–174. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Latterini, L.; Tarpani, L. Photothermal effect of gold nanostructures for application in bioimaging and therapy. In Bio- and Bioinspired Nanomaterials; Daniel, R.-M., Fernando, N., Claudio, R., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2015. [Google Scholar]
Sample Availability: Samples of the nanorods are available from the authors. |
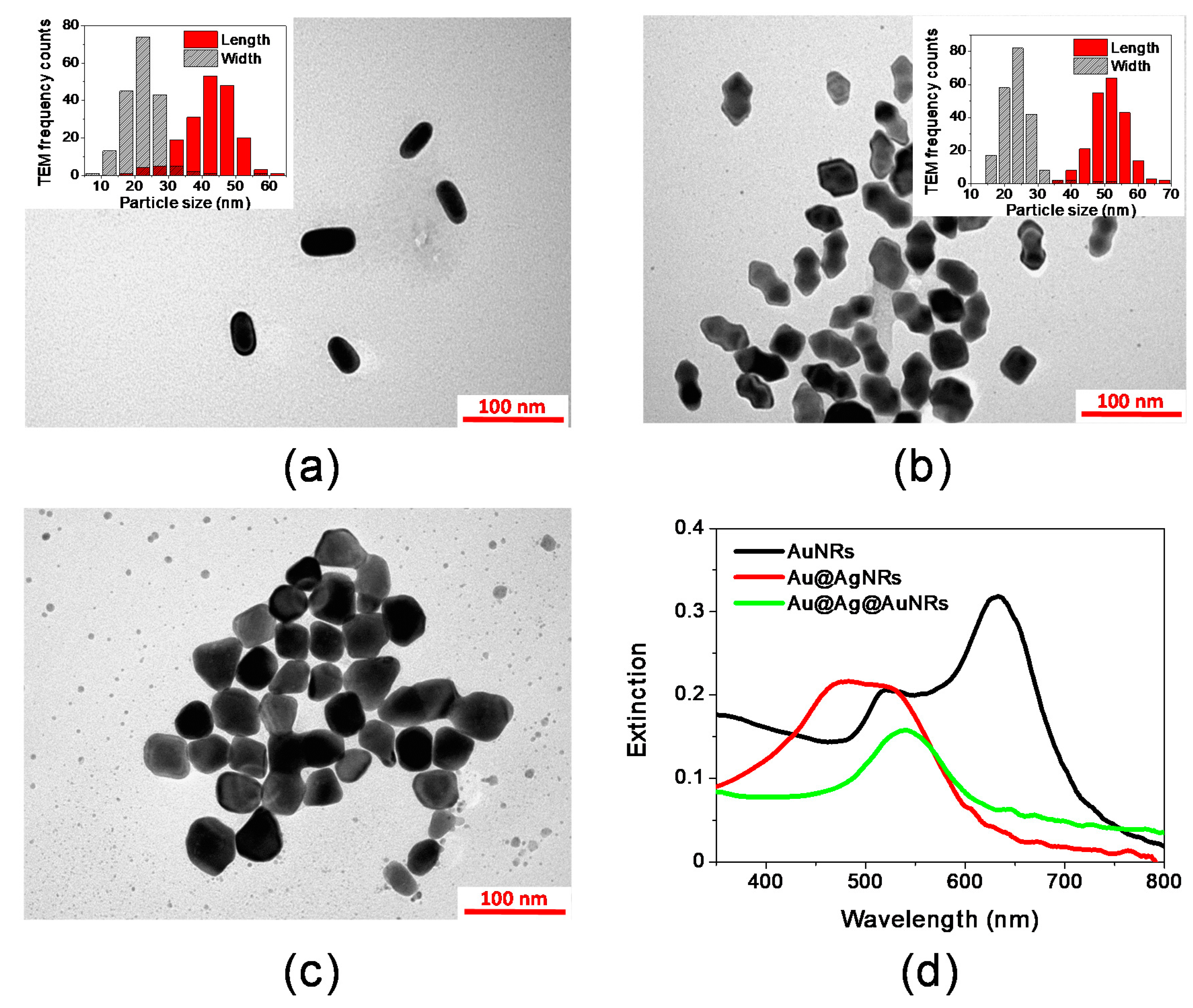

| Sample | Length (nm) | Width (nm) | Aspect Ratio | Δlenght (nm) |
|---|---|---|---|---|
| AuNRs | 43.5 (σ = 6.4) | 22.2 (σ = 4.7) | 2.00 (σ = 0.30) | --- |
| Au@AgNRs | 51.2 (σ = 4.9) | 23.3 (σ = 4.0) | 2.22 (σ = 0.48) | 7.7 |
| Au@Ag@AuNRs | ≈55 | ≈42 | ≈1.3 | ≈2.8 |
| Sample | AEF |
|---|---|
| Rh6G | --- |
| Rh6G + AuNRs | 18 ± 2 |
| Rh6G + Au@AgNRs | 4.9 ± 0.5 |
| Rh6G + Au@Ag@AuNRs | 3650 ± 370 |
| Sample | Raman Shift (cm−1) | Raman Intensity of Reference Signal |
|---|---|---|
| Promethazine | 1389 | 55 |
| + Au@Ag@AuNRs | 3170 | |
| Piroxicam | 786 | 32 |
| + Au@Ag@AuNRs | 441 | |
| Furosemide | 786 | 294 |
| + Au@Ag@AuNRs | 416 | |
| Diclofenac | [Not detectable] | [Not detectable] |
| + Au@Ag@AuNRs | [Not detectable] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambucci, M.; Cambiotti, E.; Sassi, P.; Latterini, L. Multilayer Gold-Silver Bimetallic Nanostructures to Enhance SERS Detection of Drugs. Molecules 2020, 25, 3405. https://doi.org/10.3390/molecules25153405
Gambucci M, Cambiotti E, Sassi P, Latterini L. Multilayer Gold-Silver Bimetallic Nanostructures to Enhance SERS Detection of Drugs. Molecules. 2020; 25(15):3405. https://doi.org/10.3390/molecules25153405
Chicago/Turabian StyleGambucci, Marta, Elena Cambiotti, Paola Sassi, and Loredana Latterini. 2020. "Multilayer Gold-Silver Bimetallic Nanostructures to Enhance SERS Detection of Drugs" Molecules 25, no. 15: 3405. https://doi.org/10.3390/molecules25153405






