Pre-Concentration and Analysis of Mycotoxins in Food Samples by Capillary Electrophoresis
Abstract
:1. Introduction
2. Sample Preparation for CE Analysis
2.1. SPE Procedures
2.1.1. Offline SPE
2.1.2. Online and Inline SPE
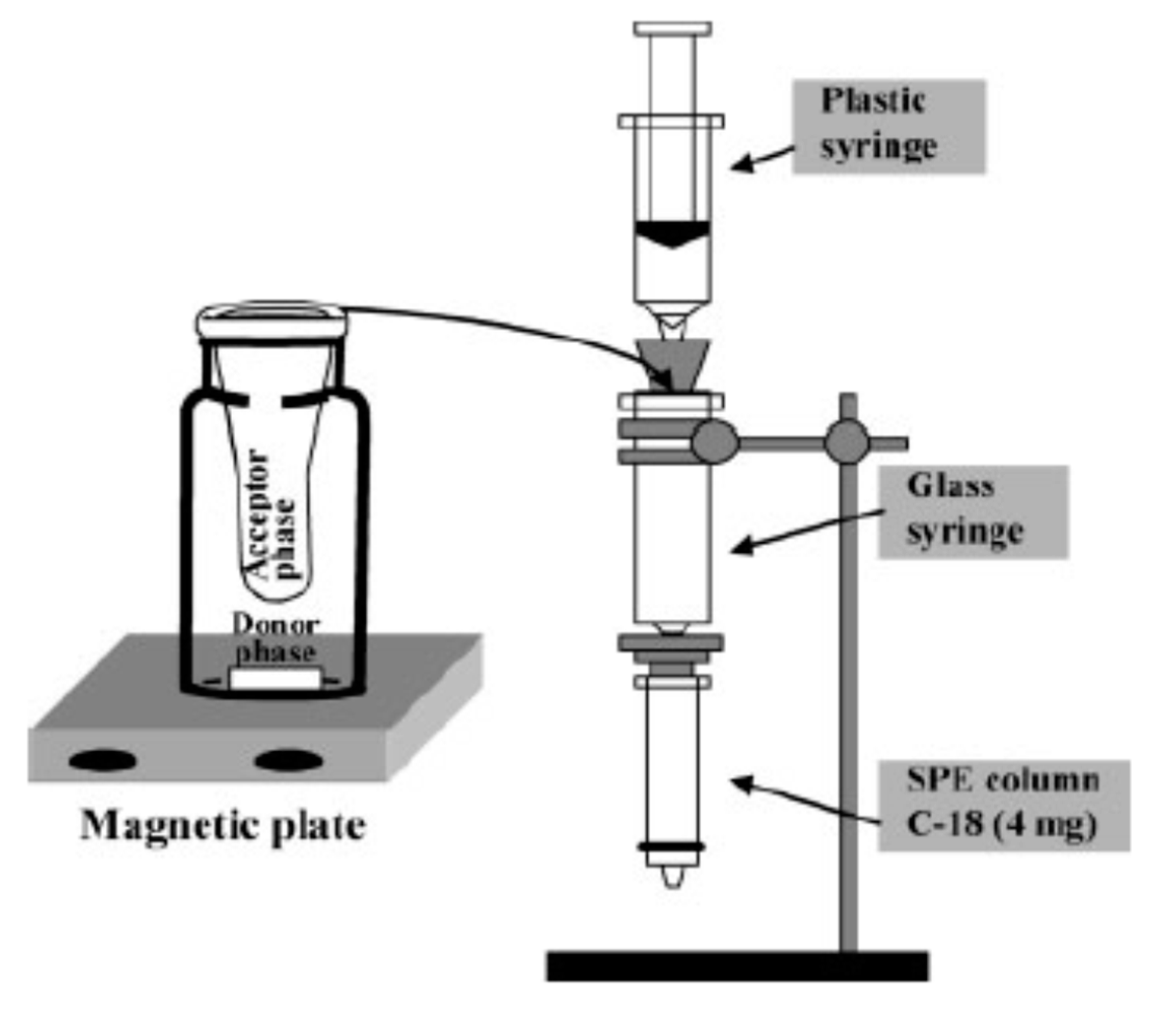
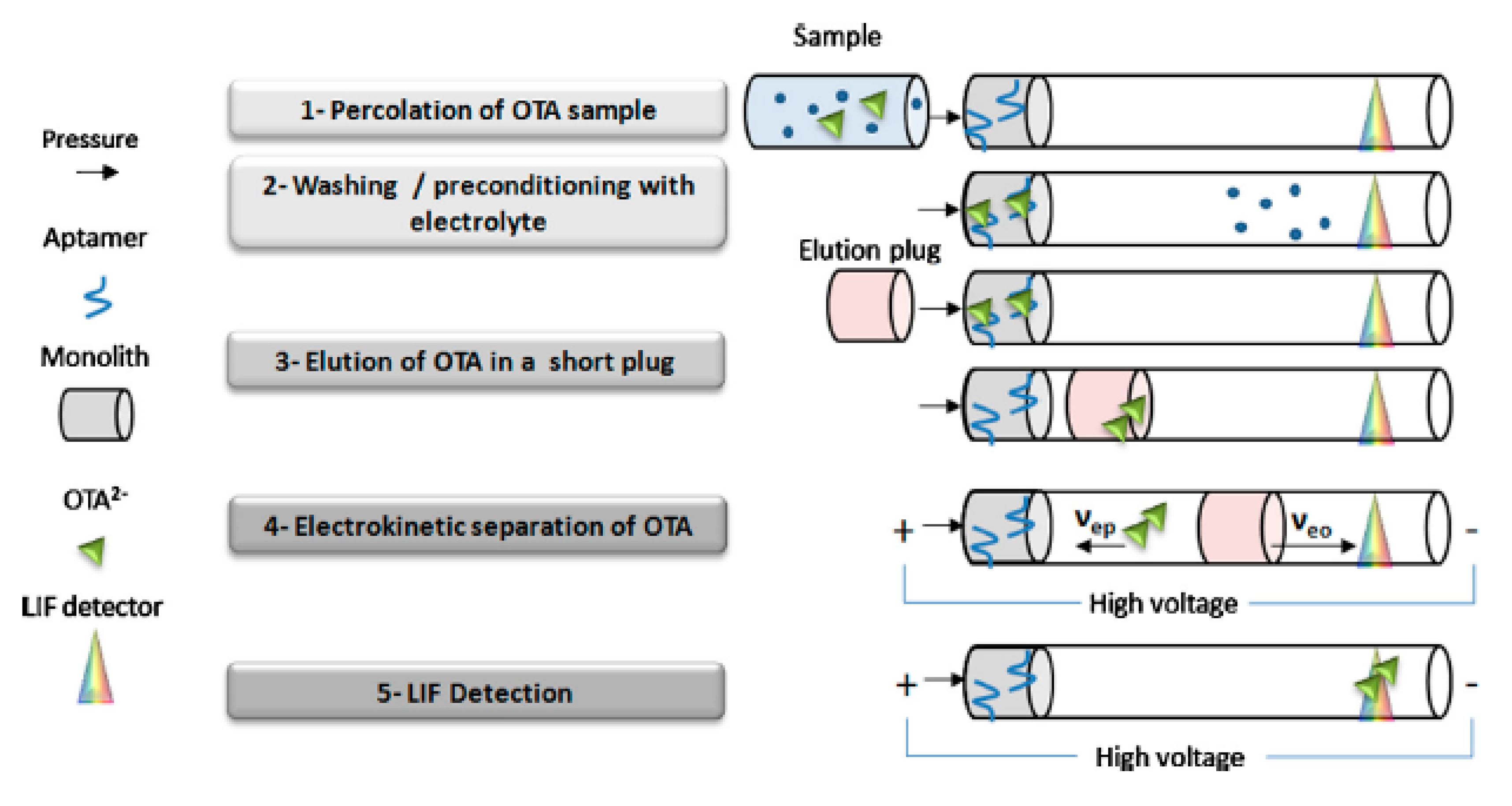
2.2. Immunoaffinity Capillary Columns (IACs)
2.3. Sweeping Techniques
2.4. Liquid–Liquid Extraction (LLE)
2.5. PCR Protocols
2.6. Cloud Point Extraction (CPE)
3. CE Analysis
Microchip-CE
4. CE Potentialities and Future Perspectives
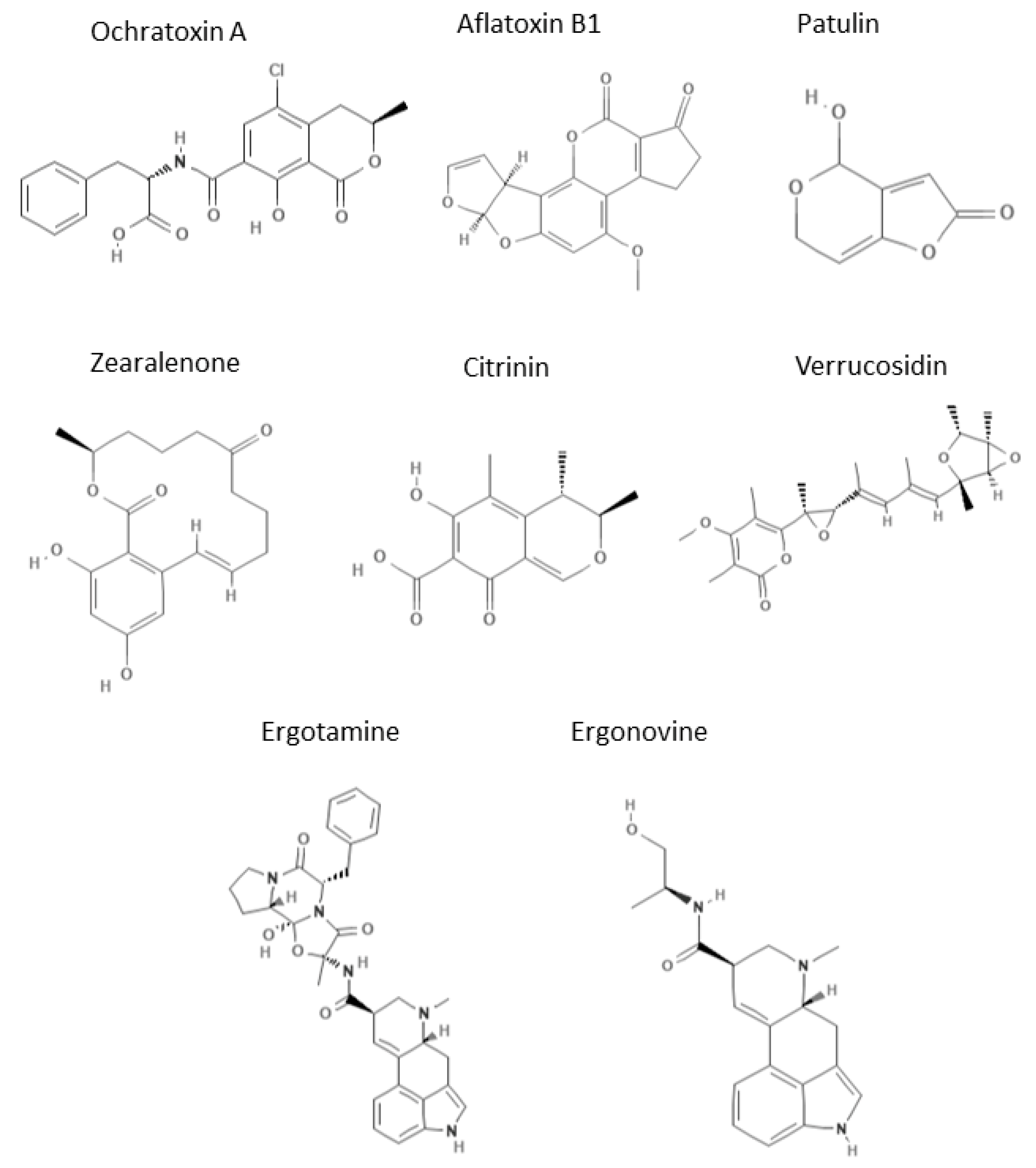
- Analyte solubility: Mycotoxin chemical structures are different, as is their water solubility, ranging from the water-soluble PAT to the water-insoluble CIT, with most mycotoxins (OTA, AFs, ZEA, verrucosidin, and ergot alkaloids) possessing a low water solubility. It is well known that the first parameter for which CE can be chosen is generally the water solubility/affinity of the analyte, as CE works in the presence of an aqueous buffer in the so-called free-solution approach. Considering OTA and AFs, OTA is a phenylalanine derivative and AFs have a tetrahydrocyclopenta[c]furo [3′,2′:4,5]furo[2,3-h]chromene skeleton. To supply to their low water solubility, mycotoxin solutions are prepared in organic solvents, such as acetonitrile [27,72] or methanol [56,58] or directly in neutral (pH 7.5) [42,67] and basic buffer (pH 8.5) [41].
- CE buffer: BGE, also named separation or electrophoretic buffer, represents a key issue in terms of buffer type, concentration, and (mainly) pH [36]. OTA is analyzed in neutral [56,58] or basic [27,41] BGE. The addition of βCD [56] can contribute to improving separation, exploiting its capacity to increase the analyte solubility and mobility, forming micellar-like structures. βCD proved to also be useful in the separation of ergot alkaloids [78]. For AFs, a basic buffer (borate) is the first choice BGE [67,72], with the addition of surfactants [67,72] and, eventually, organic solvents [67] to form hydrophobic/anionic interactions and maintain the analytes’ solubility, respectively [36].
- CE injection: A common hydrodynamic mode is used to inject the sample [36]. Often, prior to sample injection, a simple plug of methanol [27] or water-methanol [58] can be efficiently injected, contributing to a sample stacking and sensitivity improvement. Moreover, in aptamer affinity CE, a plug of desorbing buffer can be injected to a preconcentrate sampled, promoting analyte desorption from the aptamer [41].
- Multi-analyte detection: This is a very critical point in the mycotoxin analysis topic. The recent work of Xiao et al. can clarify the issue, as can future directions for CE in the analysis of multi-mycotoxins [44], in which HPLC-MS [19] and HPLC-MS/MS are the tools of choice [15,19,25]. The approach of microchip-CE can be considered the proper strategy to obtain a rapid analysis that is useful for detecting contaminants, especially when a rapid check is required in food control quality. In particular, aptamer-based microchip-CE represents an interesting solution for simultaneous determination. The high specificity of aptamers for each different mycotoxin, together with the microchip-CE advantage, combining, in one device, the whole procedure from sample preparation to detection can allow a good resolution in a short amount of time. In addition, the integrated detection system (LIF) contributes to the rapidity and good sensitivity of the analysis.
- In non-microchip-CE, a sample pre-treatment (such as SPE) carried out with highly specific sorbent materials (MIPs or aptamers) could represent a future avenue, mainly in the simultaneous analysis of different mycotoxins, but also for mycotoxins belonging to the same family with similar chemical structures. Nowadays, this strategy, combined with CE techniques, remains unexplored, and MEKC is already a powerful method through which to separate different AFs (AFB1, AFB2, AFG1, and AFG2). In fact, the addition of a surfactant and a basic pH buffer can efficiently resolve the four AFs in 20 min [67] or even in 6 min [72]. The use of highly specific sorbents can be fundamental for those mycotoxins such as verrucosidin, which is a powerful neurotoxin [39]; it has a pyrone-type polyketide structure and it can represent a risk in food safety as it is associated with molds found in fermented meats [87]. Recently, new derivatives (penicyrone, norpenicyrone, methyl norpenicyrone, and methyl penicyrone) [88] or conformational isomers [89] were isolated, for example, from the marine fungus Penicillium sp. Y-50-10 and the possibility to use highly specific sorbents could solve the resolution issue.
- Sample matrices (liquid or solid) and sampling: CE techniques are well known to be ideal for aqueous samples/analytes. Notwithstanding this, non-aqueous samples can also be easily analyzed. Solid food has to be prepared in order to enable homogeneous mycotoxin contamination. In fact, homogenization, treatment with organic solvents, mixing, and centrifugation are the usual procedures reported in the literature and these ensure the resolution of this apparently critical point, as reported in the analysis of AFs [67,72], CIT [64], ergot alkaloids [78], and verrucosidin [39] in cereals, cheese, sausages, and ham slices.
5. Conclusions
Funding
Conflicts of Interest
References
- Bennet, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Egbuta, M.A.; Mwanza, M.; Olubukola, O.B. Health risks associated with exposure to filamentous fungi. Int. J. Environ. Res. Public Health 2017, 14, 719. [Google Scholar] [CrossRef] [Green Version]
- Rogowska, A.; Pomastowskia, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 46–56. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Hajnal, E.J.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Monasterio, R.P.; Soto Vargas, V.C.; Silva, M.F. Analytical characterization of wine and its precursors by capillary electrophoresis. Electrophoresis 2012, 33, 2240–2252. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Witaszak, N.; Waśkiewicz, A.; Bocianowski, J.; Stępień, L. Contamination of pet food with mycobiota and fusarium mycotoxins-focus on dogs and cats. Toxins 2020, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. (Current Consolidated Version: 01/07/2020). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R1881 (accessed on 16 July 2020).
- Commission Regulation (EC) No. 401/2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. (Current Consolidated Version: 01/07/2014). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R0401 (accessed on 11 March 2020).
- Commission Regulation (EU) No 178/2010 of 2 March 2010 Amending Regulation (EC) No 401/2006 as Regards Groundnuts (Peanuts), Other Oilseeds, Tree Nuts, Apricot Kernel, Liquorice and Vegetable Oil. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32010R0178 (accessed on 11 March 2020).
- Lerda, D. Mycotoxins Factsheet, 2nd ed.; Technical notes (JRC 53699); Joint Research Centre, European Commission: Ispra, Italy, 2009. [Google Scholar]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, A.; Cabral-Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection methods for aflatoxin M1 in dairy products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). ACA 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Hu, X.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass Spectrom. Rev. 2013, 32, 420–452. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Stránská, M.; Václavíková, M.; Elliott, C.T.; Black, C.; Meneely, J.; Hajšlová, J.; Ezekiel, C.N.; Schuhmacher, R.; Krska, R. Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018, 410, 801–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campone, L.; Rizzo, S.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Labra, M.; Rastrelli, L. Determination of mycotoxins in beer by multi heart-cutting two-dimensional liquid chromatography tandem mass spectrometry method. Food Chem. 2020, 318, 126496. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, H.J. Development of an immunoaffinity chromatography and LC-MS/MS method for the determination of 6 zearalenones in animal feed. PLoS ONE 2018, 13, 0193584. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Metha, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef]
- Baggiani, C.; Giovannoli, C.; Anfossi, L. Man-made synthetic receptors for capture and analysis of ochratoxin A. Toxins 2015, 7, 4083–4098. [Google Scholar] [CrossRef] [Green Version]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Montone, C.M.; Chiozzi, R.Z.; Laganà, A. A rapid magnetic solid phase extraction method followed by liquid chromatography-tandem mass spectrometry analysis for the determination of mycotoxins in cereals. Toxins 2017, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.M.T.; Ciasca, B.; Powers, S.; Visconti, A. Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid-tandem mass spectrometry after multi-toxin immunoaffinity clean up. J. Chromatogr. A 2014, 1354, 139–143. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Tessiot, S.; Bessaire, T.; Racault, L.; Fiorese, E.; Urbani, A.; Chan, W.-C.; Cheng, P.; Mottier, P. Combining the quick, easy, cheap, effective, rugged and safe approach and clean-up by immunoaffinity column for the analysis of 15 mycotoxins by isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2014, 1337, 75–84. [Google Scholar] [CrossRef]
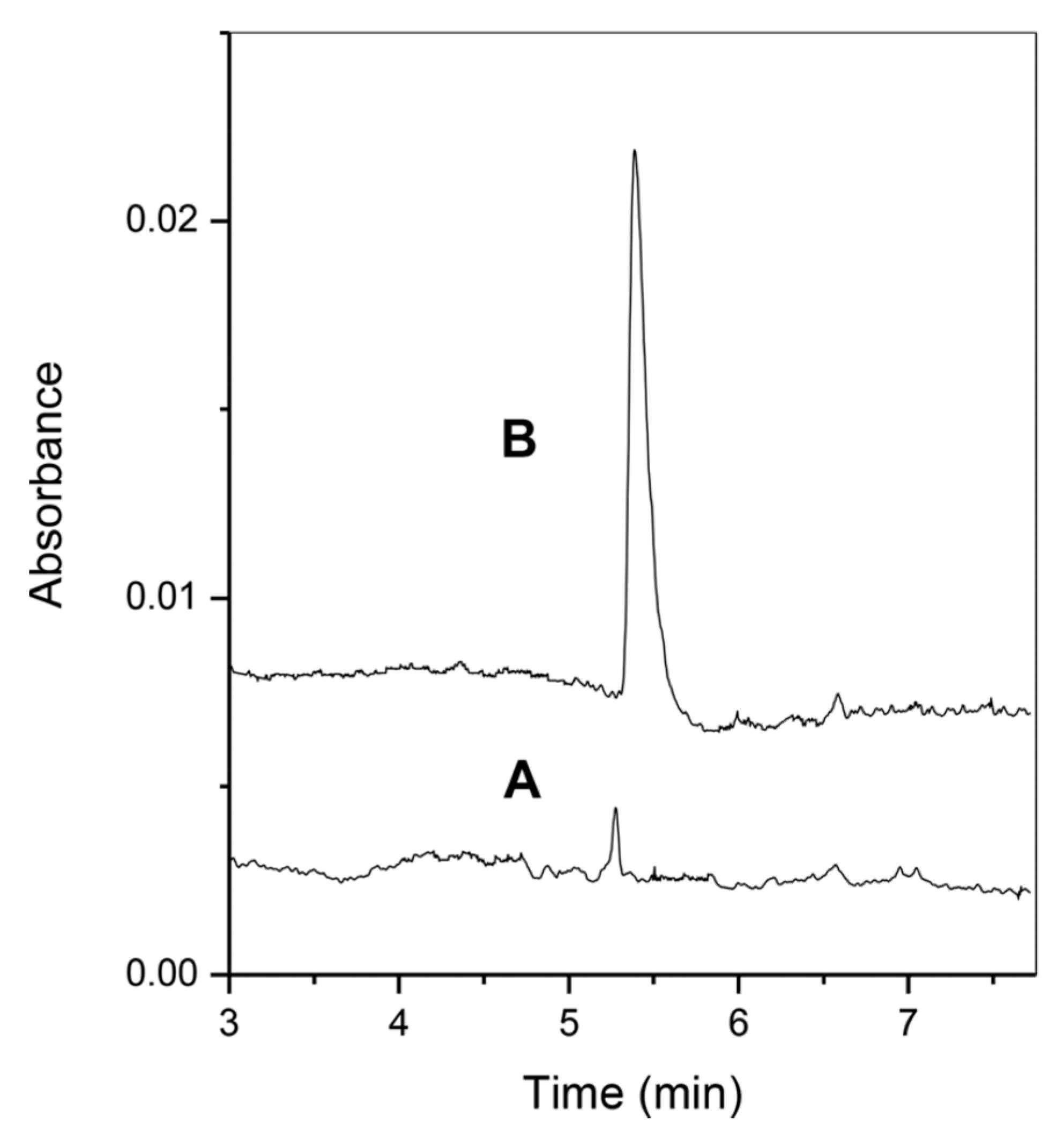
- Chamieh, J.; Faye, C.; Dugas, V.; Moreau, T.; Vandenabeele-Trambouze, O.; Demesmay, C. Preparation and full characterization of a micro-immunoaffinity monolithic column and its in-line coupling with capillary zone electrophoresis with Ochratoxin A as model solute. J. Chromatogr. A. 2012, 1232, 93–100. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. pH-controlled dispersive liquid-liquid microextraction for the analysis of ionisable compounds in complex matrices: case study of ochratoxin A in cereals. Anal. Chim. Acta 2012, 754, 61–66. [Google Scholar] [CrossRef]
- Zinedine, A.; Blesa, J.; Mahnine, N.; El Abidi, A.; Montesano, D.; Mañes, J. Pressurized liquid extraction coupled to liquid chromatography for the analysis of ochratoxin A in breakfast and infants cereals from Morocco. Food Control 2010, 21, 132–135. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Rastrelli, L. Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-tandem mass spectrometry for the identification and the accurate quantification by isotope dilution assay of ochratoxin A in wine samples. Anal. Bioanal. Chem. 2011, 399, 1279–1286. [Google Scholar] [CrossRef]
- Antep, H.M.; Merdivan, M. Development of new dispersive liquid-liquid microextraction technique for the identification of zearalenone in beer. Anal. Methods 2012, 4, 4129–4134. [Google Scholar] [CrossRef]
- Camilleri, P. Capillary Electrophoresis: Theory and Practice; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Weinberger, R. Practical Capillary Electrophoresis; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Karlinsey, J.M. Electrophoresis. In Chemical Analysis of Food: Techniques and Applications, 1st ed.; Picó, Y., Ed.; Academic Press/Elsevier: Waltham, MA, USA, 2012; pp. 375–405. [Google Scholar]
- Piňero, m.-Y.; Bauza, R.; Arce, L. Thirty years of capillary electrophoresis in food analysis laboratories: Potential applications. Electrophoresis 2011, 32, 1379–1393. [Google Scholar] [CrossRef]
- Papetti, A.; Colombo, R. High-performance capillary electrophoresis for food quality evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Elsevier: Cambridge, UK, 2019; pp. 301–377. [Google Scholar]
- Luque, M.I.; Rodríguez, A.; Andrade, M.J.; Martín, A.; Córdoba, J.J. Development of a pcr protocol to detect aflatoxigenic molds in food products. J. Food Prot. 2012, 75, 85–94. [Google Scholar] [CrossRef]
- Rodríguez, A.; Isabel Luque, M.; Andrade, M.J.; Rodríguez, M.; Asensio, M.A.; Córdoba, J.J. Development of real-time PCR methods to quantify patulin-producing molds in food products. Food Microbiol. 2011, 28, 1190–1199. [Google Scholar] [CrossRef]
- Rodríguez, A.; Córdoba, J.J.; Werning, M.L.; Andrade, M.J.; Rodríguez, M. Duplex real-time PCR method with internal amplification control for quantification of verrucosidin producing molds in dry-ripened foods. Int. J. Food Microbiol. 2012, 153, 85–91. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques. Molecules 2019, 24, 4617. [Google Scholar] [CrossRef] [Green Version]
- Marechal, A.; Jarrosson, F.; Randon, J.; Dugas, V.; Demesmay, C. In-line coupling of an aptamer based miniaturized monolithic affinity preconcentration unit with capillary electrophoresis and Laser Induced Fluorescence detection. J. Chromatogr. A. 2015, 1406, 109–117. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Wang, H.; Zhao, Q. An aptamer assay for aflatoxin B1 detection using Mg2+ mediated free zone capillary electrophoresis coupled with laser induced fluorescence. Talanta 2019, 204, 182–188. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Zhang, Q.; Zhang, N.; Zhang, W.; Ding, X.; Li, R. Current development of microfluidic immunosensing approaches for mycotoxin detection via capillary electromigration and lateral flow technology. Electrophoresis 2012, 33, 2253–2265. [Google Scholar] [CrossRef]
- Xiao, M.-W.; Bai, X.-L.; Liu, Y.-M.; Yang, L.; Liao, X. Simultaneous determination of trace Aflatoxin B1 and Ochratoxin A by aptamer-based microchip capillary electrophoresis in food samples. J. Chromatogr. A 2018, 1569, 222–228. [Google Scholar] [CrossRef]
- Martín, A.; Vilela, D.; Escarpa, A. Food analysis on microchip electrophoresis: An updated review. Electrophoresis 2012, 33, 2212–2227. [Google Scholar] [CrossRef]
- Wilkes, J.G.; Sutherland, J.B. Sample preparation and high-resolution separation of mycotoxins possessing carboxyl groups. J. Chromatogr. B 1998, 717, 135–156. [Google Scholar] [CrossRef]
- Maragos, C.M.; Appell, M.; Lippolis, V.; Visconti, A.; Catucci, L.; Pascale, M. Use of cyclodextrins as modifiers of fluorescence in the detection of mycotoxins. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2008, 25, 164–171. [Google Scholar] [CrossRef]
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and Applications in Food Samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. ACA 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Sajid, M. Porous membrane protected micro-solid-phase extraction: A review features, advancements and applications. ACA 2017, 965, 36–53. [Google Scholar] [CrossRef]
- Huertaz Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gmiz-Gracia, L. Solid phase extraction as sample treatment for the determination of Ochratoxin A in foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3405–3420. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice, 1st ed.; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Boyacı, E.; Rodríguez-Lafuente, A.; Gorynski, K.; Mirnaghi, F.; Souza-Silva, E.A.; Hein, D.; Pawliszyn, J. 5 Sample preparation with solid phase microextraction and exhaustive extraction approaches: Comparison for challenging cases. Anal. Chim. Acta 2015, 11, 14–30. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, J.; Wang, Y.; Chen, X. Developments and trends of molecularly imprinted solid-phase microextraction. J. Chromatogr. Sci. 2013, 5, 577–586. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans. 1993. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono56.pdf (accessed on 26 March 2020).
- Almeda, S.; Arce, L.; Valcárcel, M. Combined use of supported liquid membrane and solid-phase extraction to enhance selectivity and sensitivity in capillary electrophoresis for the determination of ochratoxin A in wine. Electrophoresis 2008, 29, 1573–1581. [Google Scholar] [CrossRef]
- Rostami, S.; Zór, K.; Zhai, D.S.; Viehrig, M.; Morelli, L.; Mehdinia, A.; Smedsgaard, J.; Rindzevicius, T.; Boisen, A. High-throughput label-free detection of Ochratoxin A in wine using supported liquid membrane extraction and Ag-capped silicon nanopillar SERS substrates. Food Control 2020, 113, 107183. [Google Scholar] [CrossRef]
- Almeda, S.; Arce, L.; Benavente, F.; Sanz-Nebot, V.; Barbosa, J.; Valcárcel, M. Comparison of off- and in-line solid-phase extraction for enhancing sensitivity in capillary electrophoresis using ochratoxin as a model compound. Anal. Bioanal. Chem. 2009, 394, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.C.; Luiz, A.L.; Maciel, S.C.F.; Maciel, E.V.S.; Lanças, F.M. Recent approaches for on-line analysis of residues and contaminants in food matrices: A review. J. Sep. Sci. 2015, 38, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Di Sanzo, R.; Carabetta, S.; .Russo, M.; Rastrelli, L. Occurrence of aflatoxin M1 in milk samples from Italy analysed by online-SPE UHPLC-MS/MS. Nat. Prod. Res. 2018, 32, 1803–1808. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Rapid and automated analysis of aflatoxin M1 in milk and dairy products by online solid phase extraction coupled to ultra-high-pressure-liquid-chromatography tandem mass spectrometry. J. Chromatogr. A. 2016, 1428, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Rapid and automated on-line solid phase extraction HPLC-MS/MS with peak focusing for the determination of ochratoxin A in wine samples. Food Chem. 2018, 244, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pont, L.; Pero-Gascon, R.; Gimenez, E.; Sanz-Nebot, V.; Benavente, F. A critical retrospective and prospective review of designs and materials in in-line solid-phase extraction capillary electrophoresis. ACA 2019, 1079, 1–19. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, H.; Bing, X. Preparation of an immunoaffinity column for the clean-up of fermented food samples contaminated with citrinin. Food Addit. Contam. -Part A 2013, 30, 389–394. [Google Scholar] [CrossRef]
- Quirino, J.P.; Terabe, S. Approaching a million-fold sensitivity increase in capillary electrophoresis with direct ultraviolet detection: Cation-selective exhaustive injection and sweeping. Anal. Chem. 2000, 72, 1023–1030. [Google Scholar] [CrossRef]
- Quirino, J.P.; Kim, J.-B.; Terabe, S. Sweeping: Concentration mechanism and applications to high-sensitivity analysis in capillary electrophoresis. J. Chromatogr. A 2002, 965, 357–373. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M.; Soto-Chinchilla, J.J.; Enrique García-Ayuso, L. On-line preconcentration for the determination of aflatoxins in rice samples by micellar electrokinetic capillary chromatography with laser-induced fluorescence detection. Electrophoresis 2010, 31, 2180–2185. [Google Scholar] [CrossRef]
- Hanson, C. (Ed.) Recent Advances in Liquid-Liquid Extraction, 1st ed.; Elsevier: London, UK, 1971. [Google Scholar]
- Murillo-Arbizou, M.; González-Peñas, E.; Amézqueta, S. Comparison between capillary electrophoresis and high performance liquid chromatography for the study of the occurrence of patulin in apple juice intended for infants. Food Chem. Toxicol. 2010, 48, 2429–2434. [Google Scholar] [CrossRef]
- Güray, T.; Tuncel, M.; Uysal, U.D. A Rapid determination of patulin using capillary zone electrophoresis and its application to analysis of apple juices. J. Chromatogr. Sci. 2013, 51, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Somsubsin, S.; Seebunrueng, K.; Boonchiangma, S.; Srijaranai, S. A simple solvent based microextraction for high performance liquid chromatographic analysis of aflatoxins in rice samples. Talanta 2018, 176, 172–177. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Wu, C.; Hou, F.; Chang, S.; Wang, Z.; Pu, Q.; Guo, D.; Fu, H. Fast screening of aflatoxins in dairy cattle feeds with CE-LIF method combined with preconcentration technique of vortex assisted low density solvent-microextraction. Electrophoresis 2019, 40, 499–507. [Google Scholar] [CrossRef]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [Green Version]
- Arribas, A.S.; Bermejo, E.; Zapardiel, A.; Téllez, H.; Rodríguez-Flores, J.; Zougagh, M.; Ríos, Á.; Chicharro, M. Screening and confirmatory methods for the analysis of macrocyclic lactone mycotoxins by CE with amperometric detection. Electrophoresis 2009, 30, 499–506. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Moreno-Cordero, B.; Pérez-Pavón, J.L.; García-Pinto, C.; Fernández Laespada, E. Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 902, 251–265. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-forming organic ligands in cloud-point extraction of metal ions: A review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Felici, E.; Wang, C.C.; Fernández, L.P.; Gomez, M.R. Simultaneous separation of ergot alkaloids by capillary electrophoresis after cloud point extraction from cereal samples. Electrophoresis 2015, 36, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Li, Z.; Wu, T. A review of biosensing techniques for detection of trace carcinogen contamination in food products. Anal. Bioanal. Chem. 2015, 407, 2711–2726. [Google Scholar] [CrossRef]
- Hervás, M.; López, M.A.; Escarpa, A. Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 2011, 136, 2131–2138. [Google Scholar] [CrossRef]
- Maragos, C.M.; Appell, M. Capillary electrophoresis of the mycotoxin zearalenone using cyclodextrin-enhanced fluorescence. J. Chromatogr. A. 2007, 1143, 252–257. [Google Scholar] [CrossRef]
- Sauceda-Friebe, J.C.; Karsunke, X.Y.Z.; Vazac, S.; Biselli, S.; Niessner, R.; Knoppa, D. Regenerable immuno-biochip for screening ochratoxin A in green coffee extract using an automated microarray chip reader with chemiluminescence detection. ACA 2011, 689, 234–242. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, M.M.; Zhang, H.Q.; Wen, W.; Zhang, X.H.; Wang, S.F. Selective and sensitive determination of ochratoxin A based on a molecularly imprinted electrochemical luminescence sensor. Anal. Methods 2015, 7, 10224–10228. [Google Scholar] [CrossRef]
- Perrotta, P.R.; Arévalo, F.J.; Vettorazzi, N.R.; Zón, M.A.; Fernández, H. Development of a very sensitive electrochemical magneto immunosensor for the direct determination of ochratoxin A in red wine. Sen. Actuators B-Chem. 2012, 162, 327–333. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, A.; Liu, J.; Guan, Z.; Zhou, Y.; Xu, S.; Yang, S.; Li, C. A simple and sensitive approach for ochratoxin a detection using a label-free fluorescent aptasensor. PLoS ONE 2014, 9, 85968. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Nijmeijer, S.; Fink-Gremmels, J. Genotoxicity assessment of five tremorgenic mycotoxins (Fumitremorgen B, Paxilline, Penitrem A, Verruculogen, and Verrucosidin) produced by molds isolated from fermented meats. J. Food Prot. 2003, 66, 2123–2129. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Auckloo, B.M.; Chen, C.-T.A.; Chen, X.; Wu, X.; Wu, B. Four verrucosidin derivatives isolated from the hydrothermal vent sulfur-derived fungus penicillium sp. Y-50-10. Chem. Nat. Comp. 2018, 54, 253–256. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Auckloo, B.M.; Chen, X.; Chen, C.-T.A.; Tao, X.; Wu, B. An unusual conformational isomer of verrucosidin backbone from a hydrothermal vent fungus, penicillium sp. Y-50-10. Mar. Drugs 2016, 14, 156. [Google Scholar] [CrossRef] [Green Version]


| Mycotoxin | Sample | Pre-Concentration Procedure | CE Mode | Refs |
|---|---|---|---|---|
| OTA | Wine | Offline SPE | CZE-UV | [56] |
| River water | Offline/Inline SPE | CZE-UV | [58] | |
| Wine and beer | Inline aptamer-based μSPE Inline μIAC | CZE-LIF | [41] | |
| Model solute | Offline SPE | CZE–UV/LIF | [27] | |
| AFs | Agricultural products | VALDS-ME | CZE-LIF | [72] |
| (cereals) | - | - | - | |
| Rice | Sweeping | MEKC-LIF | [67] | |
| AFB1 | Corn flour | Aptamer probe addition | CZE-LIF | [42] |
| PAT | Infant apple juices | LLE | MEKC-UV | [69] |
| LLE | CZE-UV | [70] | ||
| ZEA | Maize flour | SFE | CZE-AD | [74] |
| CIT | Red yeast rice and Monascus color | IAC | CZE-UV | [64] |
| Verrucosidin | Cheese, sausages, ham slices | TaqMan RTi-PCR | MEKC-UV | [39] |
| Ergot alkaloids | Cereals (grains and flours) | CPE | CZE-UV | [78] |
| Mycotoxin | Sample | Microchip | Immobilization | Detection | Refs |
|---|---|---|---|---|---|
| OTA | Green coffee extracts | Functionalized glass | Peptide-OTA conjugate covalently immobilized | CLD | [83] |
| Foodstuffs (rice and corn) | Aptamer-based | / | LIF | [44] | |
| Corn | MIP | Ru(bpy)32+ | ECL | [84] | |
| AFB1 | Foodstuffs (rice and corn) | Aptamer-based | / | LIF | [44] |
| ZEA | Infant cereal milkshakes | Double-T glass | Magnetic beads | ECL | [81] |
| Mycotoxin | Sample | EC Maximum Levels [11] | CE-Methods Sensitivity Values |
|---|---|---|---|
| OTA | Wine | ≤2 μg/Kg | LOD: 30 μg/L [56] |
| Wine | ≤2 μg/Kg | LOQ: 0.1 pg [41] | |
| Beer | not set | LOQ: 0.1 pg [41] | |
| Green coffee extracts | not set | LOQ: 7 μg/Kg [83] | |
| Foodstuffs (rice and corn) | ≤5 μg/Kg | LOD: 0.021 μg/L [44] | |
| AFs | Corn | ≤5 μg/Kg | LOD: 0.03 μg/L [84] |
| Agricultural products (cereals) | ≤4 μg/Kg | LOQs: 0.007–0.300 μg/L [72] | |
| Rice | ≤4 μg/Kg | LOQs: 0.13–1.74 μg/L [67] | |
| AFB1 | Corn flour | ≤2 μg/Kg | LOQ: 0.156 μg/L (0.5 nM) [42] |
| Foodstuffs (rice and corn) | ≤2 μg/Kg | LOD: 0.026 μg/L [44] | |
| PAT | Infant apple juices | ≤10 μg/Kg | LOQ: 2.5 μg/L [69] LOQ: 17.9 μg/L [70] |
| ZEA | Maize flour | ≤75 μg/Kg | LOD: 0.25 μg/L [74] |
| Infant cereal milkshakes | ≤20 μg/Kg | LOD: 0.4 μg/L [81] | |
| CIT | Red yeast rice and Monascus color | ≤100 μg/Kg | not present [64] |
| Verrucosidin | Cheese, sausages, ham slices | not set | LOD: 0.1 pg [39] |
| Ergot alkaloids | Cereals (grains and flours) | not set | LODs: 2.2–2.6 μg/Kg [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, R.; Papetti, A. Pre-Concentration and Analysis of Mycotoxins in Food Samples by Capillary Electrophoresis. Molecules 2020, 25, 3441. https://doi.org/10.3390/molecules25153441
Colombo R, Papetti A. Pre-Concentration and Analysis of Mycotoxins in Food Samples by Capillary Electrophoresis. Molecules. 2020; 25(15):3441. https://doi.org/10.3390/molecules25153441
Chicago/Turabian StyleColombo, Raffaella, and Adele Papetti. 2020. "Pre-Concentration and Analysis of Mycotoxins in Food Samples by Capillary Electrophoresis" Molecules 25, no. 15: 3441. https://doi.org/10.3390/molecules25153441







