Partition Coefficients (logP) of Hydrolysable Tannins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrolysable Tannins Studied
2.2. Hydrophobicity of Ellagitannins from Partition Coefficient Measurements
2.2.1. Galloylglucoses and Gallotannins
2.2.2. Ellagitannins with 4C1 Glucopyranose Cores
2.2.3. Ellagitannins with 1C4 Glucopyranose Cores
2.2.4. C-glycosidic Ellagitannins
2.2.5. Oligomeric Ellagitannins
3. Materials and Methods
3.1. Chemicals
3.2. Isolation of Hydrolysable Tannins
3.3. Characterization of Isolated Hydrolysable Tannins
3.4. HPLC-DAD and UPLC-DAD Analyses
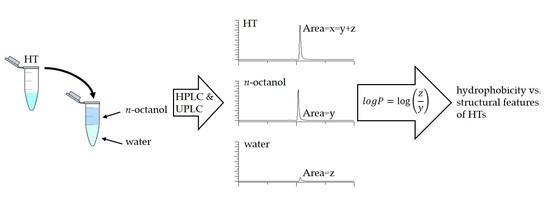
3.5. Partition Coefficient Measurements
3.6. Data Analysis and Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| # | rt(UPLC) /min | MW /Da | UPLC | HPLC | ||||
|---|---|---|---|---|---|---|---|---|
| logP1 | logP SD 2 | rec-% 3 | logP1 | logP SD 2 | rec-% 3 | |||
| 1 | 1.27 | 332.26 | −1.97 | 0.01 | 95.0% | −1.92 | 0.04 | 100.6% |
| 2 | 2.80 | 484.36 | −0.79 | 0.01 | 90.8% | −0.70 | 0.01 | 93.6% |
| 3 | 3.26 | 634.45 | −1.36 | 0.02 | 96.0% | −1.23 | 0.01 | 97.6% |
| 4 | 2.55 | 634.45 | −1.75 | 0.13 | 83.4% | −1.62 | 0.02 | 99.1% |
| 5 | 3.11 | 634.45 | −2.26 | 0.03 | 98.0% | −2.07 | 0.08 | 95.1% |
| 6 | 3.36 | 636.47 | 0.21 | 0.01 | 86.8% | 0.19 | 0.01 | 88.8% |
| 7 | 3.24 | 652.47 | −0.45 | 0.01 | 92.5% | −0.40 | 0.01 | 92.0% |
| 8 | 2.11 | 784.54 | - | - | 86.0% | - | - | 99.0% |
| 9 | 2.53/2.85 | 784.54 | - | - | 100.5% | - | - | 98.4% |
| 10 | 3.10/3.33 | 786.55 | −0.44 | 0.01 | 87.8% | −0.45 | 0.01 | 90.8% |
| 11 | 3.69 | 786.55 | −0.64 | 0.01 | 87.6% | −0.61 | 0.01 | 92.1% |
| 12a | 4.02 | 788.57 | 0.77 | 0.01 | 79.3% | 0.74 | 0.01 | 79.4% |
| 12b | 4.08 | 788.57 | 0.73 | 0.01 | 73.6% | 0.64 | 0.01 | 81.8% |
| 13 | 2.59 | 934.63 | - | - | 100.8% | - | - | 100.8% |
| 14 | 2.23 | 934.63 | - | - | 100.3% | - | - | 99.0% |
| 15 | 3.82 | 936.64 | −1.50 | 0.05 | 95.4% | −1.22 | 0.01 | 97.0% |
| 16 | 3.20 | 936.64 | −2.46 | 0.09 | 97.9% | - | - | 97.7% |
| 17 | 3.07 | 936.64 | - | - | 98.7% | - | - | 98.0% |
| 18 | 4.00 | 938.66 | 0.86 | 0.01 | 92.6% | 0.75 | 0.01 | 85.9% |
| 19 | 4.38 | 940.67 | 1.49 | 0.02 | 77.5% | 1.48 | 0.02 | 77.6% |
| 20 | 3.41 | 952.64 | −0.52 | 0.01 | 72.8% | −0.39 | 0.01 | 82.2% |
| 21 | 4.01/4.14 | 952.64 | −1.35 | 0.02 | 93.8% | −1.20 | 0.01 | 92.4% |
| 22 | 3.87 | 954.66 | −0.65 | 0.01 | 92.7% | −0.68 | 0.01 | 96.5% |
| 23 | 4.28 | 956.67 | 0.29 | 0.03 | 41.6% | 0.32 | 0.05 | 47.4% |
| 24 | 2.04 | 1066.74 | - | - | 98.0% | - | - | 100.3% |
| 25 | 2.72/3.05 | 1084.71 | - | - | 101.3% | - | - | 102.2% |
| 26 | 4.62 | 1092.78 | 0.86 | 0.03 | 67.7% | 0.97 | 0.03 | 80.2% |
| 27 | 2.21 | 1102.73 | - | - | 100.5% | - | - | 100.7% |
| 28 | 2.00 | 1102.73 | - | - | 100.2% | - | - | 98.8% |
| 29 | 3.18 | 1104.75 | −2.68 | 0.05 | 99.6% | −2.17 | 0.03 | 95.8% |
| 30 | 3.03 | 1104.75 | −3.20 | 0.06 | 98.2% | - | - | 94.7% |
| 31 | 4.90 | 1244.88 | −0.44 | 0.01 | 81.9% | −0.43 | 0.02 | 89.8% |
| 32 | 5.07 | 1396.99 | −0.59 | 0.01 | 81.2% | −0.43 | 0.06 | 78.8% |
| 33 | 5.51 | 1396.99 4 | −0.18 | 0.02 | 70.7% | −0.01 | 0.01 | 88.1% |
| 34 | 2.95/3.24 | 1569.08 | −3.30 | 0.09 | 103.6% | - | - | 98.5% |
| 35 | 3.05 | 1569.08 | −3.36 | 0.05 | 98.5% | - | - | 95.8% |
| 36 | 3.86/3.90 | 1723.20 | −0.82 | 0.00 | 91.0% | −0.69 | 0.01 | 91.9% |
| 37 | 3.32 | 1869.25 | - | - | 95.6% | - | - | 92.8% |
| 38 | 2.28 | 1869.25 | - | - | 100.0% | - | - | 98.0% |
| 39 | 2.63 | 1869.25 | −3.45 | 0.10 | 98.5% | - | - | 102.3% |
| 40 | 4.16 | 1871.27 | −3.42 | 0.05 | 98.5% | - | - | 99.4% |
| 41 | 3.86 | 1871.27 | −3.75 | 0.11 | 101.4% | - | - | 101.0% |
| 42 | 4.07 | 1873.28 | −2.67 | 0.12 | 103.3% | - | - | 98.3% |
| 43 | 4.22 | 1875.30 | −0.48 | 0.01 | 91.3% | −0.49 | 0.01 | 91.6% |
| 44 | 3.28 | 2353.62 | −2.64 | 0.06 | 102.7% | - | - | 100.3% |
| 45 | 3.81 | 2805.90 | −1.50 | 0.01 | 96.2% | −1.33 | 0.02 | 97.3% |
| 46 | 4.18 | 2811.94 | −0.80 | 0.01 | 78.8% | −0.54 | 0.01 | 82.7% |
References
- Moilanen, J.; Salminen, J.-P. Ecologically neglected tannins and their biologically relevant activity: Chemical structures of plant ellagitannins reveal their in vitro oxidative activity at high pH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.P.; Green, R.J. Ellagitannins with Glucopyranose Cores Have Higher Affinities to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Pellikaan, W.F.; Karonen, M.; Salminen, J.-P.P. A study of the structure-activity relationship of oligomeric ellagitannins on ruminal fermentation in vitro. J. Dairy Sci. 2016, 99, 8041–8052. [Google Scholar] [CrossRef] [Green Version]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in Vitro Activity Against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Conradi, R.A.; Burton, P.S.; Borchardt, R.T. Physico-Chemical and Biological Factors that Influence a Drug’s Cellular Permeability by Passive Diffusion. Lipophilicity Drug Action Toxicol. 2008, 4, 233–252. [Google Scholar] [CrossRef]
- Tanaka, T.; Zhang, H.; Jiang, Z.H.; Kouno, I. Relationship between hydrophobicity and structure of hydrolyzable tannins, and association of tannins with crude drug constituents in aqueous solution. Chem. Pharm. Bull. 1997, 45, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Harvey, I.; Mlambo, V.; Sikosana, J.L.N.; Smith, T.; Owen, E.; Brown, R.H. Octanol-Water Partition Coefficients for Predicting the Effects of Tannins in Ruminant Nutrition. J. Agric. Food Chem. 2007, 55, 5436–5444. [Google Scholar] [CrossRef]
- Haslam, E. Plant polyphenols (syn. vegetable tannins) and chemical defense-A reappraisal. J. Chem. Ecol. 1988, 14, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kuriyama, K. Circular dichroism of hydrolysable tannins-I ellagitannins and gallotannins. Tetrahedron Lett. 1982, 23, 3937–3940. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kuriyama, K. Circular dichroism of hydrolysable tannins-II dehydroellagitannins. Tetrahedron Lett. 1982, 23, 3941–3944. [Google Scholar] [CrossRef]
- Donovan, J.L.; Luthria, D.L.; Stremple, P.; Waterhouse, A.L. Analysis of (+)-catechin, (−)-epicatechin and their 3′- and 4′-O-methylated analogs. A comparison of sensitive methods. J. Chromatogr. B Biomed. Sci. Appl. 1999, 726, 277–283. [Google Scholar] [CrossRef]
- Quideau, S. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Singapore, Singapore, 2009; ISBN 9789812797414. [Google Scholar]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol-Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Salminen, J.P.; Lempa, K. Effects of hydrolysable tannins on a herbivorous insect: Fate of individual tannins in insect digestive tract. Chemoecology 2002, 12, 203–211. [Google Scholar] [CrossRef]
- Salminen, J.P.; Ossipov, V.; Loponen, J.; Haukioja, E.; Pihlaja, K. Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 1999, 864, 283–291. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.P.; Green, R.J. Binding of an Oligomeric Ellagitannin Series to Bovine Serum Albumin (BSA): Analysis by Isothermal Titration Calorimetry (ITC). J. Agric. Food Chem. 2015, 63, 10647–10654. [Google Scholar] [CrossRef]
- Baert, N.; Karonen, M.; Salminen, J.P. Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yarnes, C.T.; Boecklen, W.J.; Tuominen, K.; Salminen, J.P. Defining phytochemical phenotypes: Size and shape analysis of phenolic compounds in oaks (Fagaceae, Quercus) of the Chihuahuan Desert. Can. J. Bot. 2006, 84, 1233–1248. [Google Scholar] [CrossRef]
- Tuominen, A.; Toivonen, E.; Mutikainen, P.; Salminen, J.P. Defensive strategies in Geranium sylvaticum. Part 1: Organ-specific distribution of water-soluble tannins, flavonoids and phenolic acids. Phytochemistry 2013, 95, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, J.; Tähtinen, P.; Valkamaa, S.; Engström, M.T.; Karonen, M.; Salminen, J.P. Variability in Foliar Ellagitannins of Hippophaë rhamnoides L. and Identification of a New Ellagitannin, Hippophaenin C. J. Agric. Food Chem. 2018, 66, 613–620. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Yoshida, T.; Hatano, T.; Yazaki, K.; Ashida, M. Ellagitannins of the casuarinaceae, stachyuraceae and myrtaceae. Phytochemistry 1980, 21, 2871–2874. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I.; Nagasawa, T.; Oura, H. Tannins and Related Compounds. I. Rhubarb (1). Chem. Pharm. Bull. 1981, 29, 2862–2870. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Wen, L.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Structural identification of isomallotusinin and other phenolics in Phyllanthus emblica L. fruit hull. Food Chem. 2012, 132, 1527–1533. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Casuariin, Stachyurin and Strictinin, New Ellagitannins from Casuarina Stricta and Stachyurus Praecox. Chem. Pharm. Bull. 1982, 30, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Haddock, E.A.; Gupta, R.K.; Al-Shafi, S.M.K.; Haslam, E.; Magnolato, D. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 1. Introduction. Naturally occurring galloyl esters. J. Chem. Soc. Perkin Trans. 1 1982, 2515–2524. [Google Scholar] [CrossRef]
- Hervé Du Penhoat, C.L.M.; Michon, V.M.F.; Peng, S.; Viriot, C.; Scalbert, A.; Gage, D. Structural Elucidation of New Dimeric Ellagitannins from Quercus robur L. Roburins A-E. J. Chem. Soc. Perkin Trans. 1 1991, 53, 1689–1699. [Google Scholar] [CrossRef]
- Ito, H.; Yamaguchi, K.; Kim, T.H.; Khennouf, S.; Gharzouli, K.; Yoshida, T. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J. Nat. Prod. 2002, 65, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Hatano, T.; Ogawa, N. Rugosin D, E, F and G, dimeric and trimeric hydrolyzable tannins. Chem. Pharm. Bull. 1982, 30, 4234–4237. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtanen, V.; Karonen, M. Partition Coefficients (logP) of Hydrolysable Tannins. Molecules 2020, 25, 3691. https://doi.org/10.3390/molecules25163691
Virtanen V, Karonen M. Partition Coefficients (logP) of Hydrolysable Tannins. Molecules. 2020; 25(16):3691. https://doi.org/10.3390/molecules25163691
Chicago/Turabian StyleVirtanen, Valtteri, and Maarit Karonen. 2020. "Partition Coefficients (logP) of Hydrolysable Tannins" Molecules 25, no. 16: 3691. https://doi.org/10.3390/molecules25163691