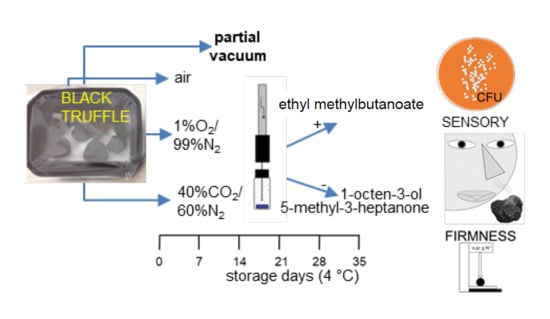
Hypobaric Packaging Prolongs the Shelf Life of Refrigerated Black Truffles (Tuber melanosporum)
Abstract
:1. Introduction
2. Results
2.1. Weight Loss, Firmness, Gas Composition, and Volatile Profile
2.2. Microbial Epiphytic Community
2.3. Principal Component Analysis
2.4. Sensory Analysis
3. Materials and Methods
3.1. Sampling Procedure
3.2. Atmosphere Composition, Weight Loss, and Firmness
3.3. Volatile Aroma Profile
3.4. Microbial Epiphytic Population
3.5. Sensory Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ar | aroma |
| C | atmospheric conditions |
| CAS# | Chemical Abstract Service number |
| CFU | colony forming units |
| CN | 40% CO2/60% N2 |
| FIRM | Instrumental Penetrometric Determination |
| Fl | flavor |
| GJ | global judgement (sensory) |
| MAP | modified atmosphere packaging |
| MW | molecular weight |
| ON | 1% O2/99% N2 |
| PDA | Potato Dextrose Agar |
| RI | retention index in gas chromatography |
| Rt | retention time in gas chromatography |
| SPME | solid phase microextraction |
| Tex | texture (sensory) |
| V | hypobaric packaging with air at 30 kPa (0.3 bar) |
| WL | weight loss |
References
- Mello, A.; Murat, C.; Bonfante, P. Truffles: Much more than a prized and local fungal delicacy. FEMS Microbiol. Lett. 2006, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Marco, P.; Oria, R.; Blanco, D.; Venturini, M.E. What is the best method for preserving the genuine black truffle (Tuber melanosporum) aroma? An olfactometric and sensory approach. LWT–Food Sci. Technol. 2017, 80, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Daba, G.M.; Elkhateeb, W.A.; Wen, T.C.; Thomas, P.W. The Continuous Story of Truffle-Plant Interaction. In Microbiome in Plant Health and Disease-Challenges and Opportunities; Kumar, V., Prasad, R., Kumar, M., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 375–383. [Google Scholar]
- Hajjar, S.E.; Massantini, R.; Botondi, R.; Kefalas, P.; Mencarelli, F. Influence of high carbon dioxide and low oxygen on the postharvest physiology of fresh truffles. Postharvest Biol. Technol. 2010, 58, 36–41. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Wang, G.; Li, H.-M.; Zhong, J.-J.; Tang, Y.-J. Volatile organic compounds from a Tuber melanosporum fermentation system. Food Chem. 2012, 135, 2628–2637. [Google Scholar] [CrossRef]
- Tsiaras, S.; Dragoslis, A. Selection of the Most Suitable Tree Species for Truffle Cultivation Using Fuzzy VIKOR and Fuzzy AHP: New Paths in Forest Policy Planning. Int. J. Environ. Sustain. Green Technol. (IJESGT) 2020, 11, 74–91. [Google Scholar] [CrossRef]
- Viganò, E.; Gori, F.; Amicucci, A. Enhancement of food production quality: The truffle case. Econ. Agroaliment. 2019, 21, 587–611. [Google Scholar] [CrossRef]
- Mencarelli, F.; Massantini, R.; Botondi, R. Physiological and textural response of truffles during low-temperature storage. J. Hortic. Sci. 1997, 72, 407–414. [Google Scholar] [CrossRef]
- Romanazzi, G.; Servili, A.; Murolo, S. Postharvest diseases of Tuber melanosporum. Acta Hortic. 2016, 1144, 129–131. [Google Scholar] [CrossRef]
- Vahdatzadeh, M.; Deveau, A.; Splivallo, R. Are bacteria responsible for aroma deterioration upon storage of the black truffle Tuber aestivum: A microbiome and volatilome study. Food Microbiol. 2019, 84, 103251. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Salvador, M.L.; Venturini, M.E. Shelf-life extension of fresh Tuber aestivum and Tuber melanosporum truffles by modified atmosphere packaging with microperforated films. J. Food Sci. 2010, 75, E225–E233. [Google Scholar] [CrossRef]
- Rivera, C.S.; Venturini, M.E.; Oria, R.; Blanco, D. Selection of a decontamination treatment for fresh Tuber aestivum and Tuber melanosporum truffles packaged in modified atmospheres. Food Control 2011, 22, 626–632. [Google Scholar] [CrossRef]
- Savini, S.; Loizzo, M.R.; Tundis, R.; Mozzon, M.; Foligni, R.; Longo, E.; Morozova, K.; Scampicchio, M.; Martin-Vertedor, D.; Boselli, E. Fresh refrigerated Tuber melanosporum truffle: Effect of the storage conditions on the antioxidant profile, antioxidant activity and volatile profile. Eur. Food Res. Technol. 2017, 243, 2255–2263. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Adhikari, B.; Gao, Z. Recent advances in pressure modification-based preservation technologies applied to fresh fruits and vegetables. Food Rev. Int. 2017, 33, 538–559. [Google Scholar] [CrossRef]
- Longo, E.; Morozova, K.; Loizzo, M.; Tundis, R.; Savini, S.; Foligni, R.; Mozzon, M.; Martin-Vertedor, D.; Scampicchio, M.; Boselli, E. High resolution mass approach to characterize refrigerated black truffles stored under different storage atmospheres. Food Res. Int. 2017, 102, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, S.; Su, Y.; Guo, Y. Analysis of volatile compounds in Radix bupleuri injection by GC-MS-MS. Chromatographia 2011, 74, 497–502. [Google Scholar] [CrossRef]
- Splivallo, R.; Bossi, S.; Maffei, M.; Bonfante, P. Discrimination of truffle fruiting body versus mycelial aromas by stir bar sorptive extraction. Phytochemistry 2007, 68, 2584–2598. [Google Scholar] [CrossRef]
- Harrison, B.M.; Priest, F.G. Composition of peats used in the preparation of malt for Scotch Whisky production-influence of geographical source and extraction depth. J. Agric. Food Chem. 2009, 57, 2385–2391. [Google Scholar] [CrossRef]
- Cao, H.; Li, Z.; Chen, X. QSRR study of GC retention indices of volatile compounds emitted from Mosla chinensis Maxim. by multiply linear regression. Chin. J. Chem. 2011, 29, 2187–2196. [Google Scholar] [CrossRef]
- Ruberto, G.; Biondi, D.; Barbagallo, C.; Meli, R.; Savoca, F. Constituents of stem and flower oils of Helichrysum litoreum Guss. Flavour Fragr. J. 2002, 17, 46–48. [Google Scholar] [CrossRef]
- Larsen, T.O.; Frisvad, J.C. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol. Res. 1995, 99, 1153–1166. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Bücking, M.; Muresan, S.; Blaas, J.; Wietsma, W.A. Identification of the volatile component(s) causing the characteristic foxy odor in various cultivars of Fritillaria imperialis L. (Liliaceae). J. Agric. Food Chem. 2006, 54, 5087–5091. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid Extraction of Volatile Compounds Using a New Simultaneous Microwave Distillation: Solvent Extraction Device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U. Volatile substances of Chinese traditional Jinhua ham and Cantonese sausage. J. Food Sci. 2001, 66, 827–831. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Yi, G.; Li, W.; Zhang, G. A comparison of aroma components of pineapple fruits ripened in different seasons. Afr. J. Agric. Res. 2011, 6, 1771–1778. [Google Scholar]
- Díaz, P.; Ibáñez, E.; Señoráns, F.J.; Reglero, G. Truffle aroma characterization by headspace solid-phase microextraction. J. Chromatogr. A 2003, 1017, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.-L.; Beelman, R.B.; Ziegler, G.R. Factors affecting 1-octen-3-ol in mushrooms at harvest and during postharvest storage. J. Food Sci. 1993, 58, 331–334. [Google Scholar] [CrossRef]
- Menotta, M.; Gioacchini, A.M.; Amicucci, A.; Buffalini, M.; Sisti, D.; Stocchi, V. Headspace solid-phase microextraction with gas chromatography and mass spectrometry in the investigation of volatile organic compounds in an ectomycorrhizae synthesis system. Rapid Comm. Mass Spectrom. 2004, 18, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, E.; Landi, L.; Romanazzi, G. Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carb. Polym. 2015, 132, 111–117. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Di Venere, D.; Salerno, M. Effects of pre and postharvest chitosan treatments to control storage grey mould of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Beckley, J.H.; Resurreccion, A.V. Texture profile method. In Sensory and Consumer Research in Food Product Design and Development; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 290. [Google Scholar]
- Yasar, S.; Boselli, E.; Rossetti, F.; Gok, M.S. Effect of Fermented Cereals, Probiotics, and Phytase on the Sensory Quality of Poultry Meat. Sci. Agric. Bohem. 2018, 49, 225–235. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the volatile compounds are available from the authors. |



| T | Sample 1 | CFU/g | FIRM 2 | 1 3 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | WL% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | C 2 | 3.8 × 10⁵ a | 3.7 a | 35.1 a | 2.1 a | 4.6 a | 4.6 a | 4.8 a | 1.2 a | 2.1 a | ND | 29.5 a | 9.2 a | 2.9 a | 3.8 a | 0.0 a |
| V | 2.6 × 10⁵ a | 3.0 a | 41.3 a | 3.6 a | 9.8 a | 8.7 a | 9.8 a,b | 3.3 a,c | 1.9 a | 2.1 a | 4.2 b | 3.0 b | 2.7 a | 9.7 a | 1.0 a | |
| ON | 3.4 × 10⁵ a | 3.2 a | 27.7 a | 3.2 a | 4.4 a | 8.0 a | 14.9 b | 9.5 b,c | 3.5 a | 7.1 a | 6.8 b | 2.7 b | 5.5 a | 6.7 a | 1.0 a | |
| CN | 1.1 × 106 a | 3.2 a | 39.1 a | 2.6 a | 3.1 a | 5.6 a | 10.5 a,b | 6.4 b,c | 3.8 a | 2.2 a | 9.2 b | 4.9 a | 4.0 a | 8.5 a | 1.3 a | |
| T7 | C | 1.2 × 10⁵ a | 2.8 a | 29.6 a | 7.6 a | 10.0 a | 11.8 a | 4.1 a | 1.8 a | 4.1 a | 2.3 a | 6.0 a | 1.9 a | 5.9 a | 15.0 a | 0.7 a |
| V | 4.8 × 10⁵ a | 2.5 a | 33.9 a | 5.3 a | 10.4 a | 9.4 a | 6.7 a | 3.5 a | 2.4 a,b | 2.1 a | 8.3 a | 3.5 a | 5.2 a | 9.5 a | 2.0 a | |
| ON | 4.1 × 10⁵ a | 3.2 a | 38.3 a | 6.7 a | 7.9 a | 8.1 a | 3.8 a | 1.0 a | 1.3 b | 13.4 a | 5.3 a | 1.5 a | 3.5 a | 9.3 a | 1.7 a | |
| CN | 2.2 × 106 a | 3.2 a | 44.7 a | 5.7 a | 11.5 a | 7.5 a | 4.4 a | 1.8 a | 1.4 a,b | 3.9 a | 4.4 a | 5.2 a | 2.7 a | 6.7 a | 1.7 a | |
| T14 | C | 2.9 × 106 a | 3.7 a | 28.0 a | 15.6 a | 15.8 a | 9.7 a | 1.6 a | 1.1 a | 1.9 a | 1.7 a | 4.2 a | 3.8 a | 10.0 a | 6.7 a | 3.0 a |
| V | 3.6 × 106 a | 3.8 a | 27.2 a | 14.9 a | 10.0 a | 9.3 a | 5.4 a | 3.2 a | 1.4 a | 2.9 a | 5.5 a | 3.0 a | 8.3 a | 8.8 a | 3.3 a | |
| ON | 5.0 × 106 a | 3.2 a | 34.8 a | 11.3 a | 15.4 a | 8.3 a | 2.1 a | ND | 2.8 a | 3.2 a | 5.7 a | 3.3 a | 6.3 a | 6.7 a | 3.4 a | |
| CN | 6.9 × 106 a | 2.9 a | 28.8 a | 10.3 a | 16.4 a | 10.9 a | 3.4 a | 0.7 a | 2.2 a | 5.1 a | 5.5 a | 2.6 a | 7.8 a | 6.3 a | 3.6 a | |
| T21 | C | 4.8 × 106 a | 3.2 a | 29.3 a | 17.6 a | 10.0 a | 11.3 a | 2.4 a | 1.3 a | 2.7 a | 1.3 a | 1.9 a | 0.8 a | 8.9 a | 12.3 a | 4.3 a |
| V | 1.8 × 106 a | 3.6 a | 39.6 a | 15.3 a | 10.3 a | 8.1 a | 2.1 a | 0.6 a | 1.9 a | 1.1 a | 2.8 a | 0.8 a | 9.8 a | 7.4 a | 4.6 a,b | |
| ON | 1.1 × 106 a | 3.5 a | 18.9 a | 11.3 a | 13.1 a | 8.0 a | 1.9 a | 0.6 a | 2.6 a | 19.5 b | 6.6 a | 1.7 a | 5.9 a | 9.9 a | 7.3 a,b | |
| CN | 2.4 × 106 a | 3.2 a | 21.5 a | 20.7 a | 14.2 a | 10.3 a | 4.6 a | 1.7 a | 2.6 a | 3.0 a | 4.1 a | 1.3 a | 6.8 a | 9.3 a | 3.4 a,c | |
| T28 | C | 8.9 × 106 a | 2.9 a | 24.5 a | 23.6 a | 6.3 a | 10.6 a | 3.5 a | 2.0 a | 1.7 a | 2.6 a | 4.4 a | 1.2 a | 9.8 a | 9.6 a | 4.8 a |
| V | 5.9 × 106 a,b | 2.4 a | 28.6 a | 12.4 a | 16.6 a | 9.6 a | 2.6 a | 0.8 a | 0.9 a,b | 8.6 a,b | 3.1 a | 1.9 a | 4.4 a | 10.4 a | 3.0 a,b | |
| ON | 6.1 × 106 a,b | 2.9 a | 16.3 a | 15.7 a | 6.7 a | 9.7 a | 0.7 a | 0.2 a | 2.2 a,b,c | 20.0 b | 4.9 a | 2.3 a | 11.7 a | 9.6 a | 5.8 a,b | |
| CN | 3.5 × 106 b | 2.7 a | 19.3 a | 23.3 a | 10.5 a | 8.7 a | 2.2 a | 0.6 a | 4.4 a,c | 3.5 a | 5.5 a | 1.4 a | 6.4 a | 14.2 a | 7.9 a,c | |
| T35 | C | 7.8 × 106 a | 2.2 a | 29.3 a | 23.9 a | 6.2 a | 9.4 a | 2.1 a | 0.8 a | 1.7 a | 5.8 a | 3.3 a | 6.4 a | 5.5 a | 5.1 a | 9.9 a |
| V | 3.8 × 106 a | 2.5 a,b | 15.0 a | 29.4 a | 10.6 a | 7.9 a | 2.7 a | 0.7 a | 3.6 a | 3.8 a | 3.8 a | 1.6 a | 12.1 a | 8.3 a | 9.3 a | |
| ON | 4.1 × 106 a | 3.4 b | 25.3 a | 20.6 a | 8.4 a | 13.1 a | 1.5 a | 0.4 a | 2.8 a | 2.5 a | 3.3 a | 1.2 a | 13.1 a | 7.4 a | 7.0 a | |
| CN | 4.3 × 106 a | 2.4 a,b | 22.5 a | 24.1 a | 11.1 a | 9.9 a | 1.5 a | 0.3 a | 2.2 a | 1.0 a | 3.1 a | 4.6 a | 9.3 a | 10.1 a | 6.7 a | |
| Pooled standard deviation | 4.5 × 106 | 1.1 | 30.7 | 17.7 | 10.7 | 7.0 | 5.9 | 4.3 | 2.8 | 15.5 | 11.2 | 5.6 | 10.1 | 9.4 | 3.6 | |
| Peak# | Compound Identified | Formula | MW | CAS# a | Rt | RI | Ref. b |
|---|---|---|---|---|---|---|---|
| 1 | hexanal | C6H12O | 100 | 66-25-1 | 6.84 | 807 | [17] |
| 2 | ethyl methylbutanoate | C7H14O2 | 130 | 7452-79-1 c/108-64-5 d | 8.78 | 839 c/844 d | [18] |
| 3 | heptanal | C7H14O | 114 | 111-71-7 | 10.80 | 906 | [17] |
| 4 | methoxybenzene (alias anisol) | C7H8O | 108 | 100-66-3 | 11.40 | 918 | [19] |
| 5 | 1-octen-3-ol | C8H16O | 128 | 3391-86-4 | 14.29 | 985 | [20] |
| 6 | 5-methyl-3-heptanone | C8H16O | 128 | 541-85-5 | 14.58 | 954 | [21] |
| 7 | octanal | C8H16O | 128 | 124-13-0 | 15.19 | 1007 | [17] |
| 8 | 1-methoxy-3-methylbenzene | C8H10O | 122 | 100-84-5 | 15.82 | 1028 | [22] |
| 9 | 5-Ethylcyclopent-1-enecarboxaldehyde | C8H12O | 124 | 36431-60-4 | 16.24 | 1026 | [18] |
| 10 | (E)-2-octenal | C8H14O | 126 | 2548-87-0 | 17.33 | 1063 | [17] |
| 11 | 2-nonen-1-ol | C9H18O | 142 | 22104-79-6 | 19.22 | 1105 | [23] |
| 12 | 1,2-dimethoxybenzene (alias veratrol) | C8H10O2 | 138 | 91-16-7 | 20.83 | 1143 | [24] |
| Storage Time (days) | Texture 1 | Aroma | Flavor | Global Judgement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C 2 | V | ON | CN | C | V | ON | CN | C | V | ON | CN | C | V | ON | CN | |
| 0 | 9.0 | 4.0 | 9.0 | 9.0 | 4.5 | 5.0 | 4.5 | 5.0 | 5.0 | 4.0 | 5.0 | 5.0 | 5.0 | 4.0 | 5.0 | 5.0 |
| 7 | 8.0 | 8.5 | 9.0 | 9.0 | 5.0 | 4.0 | 3.0 | 3.0 | 5.0 | 4.0 | 4.0 | 3.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| 14 | 7.0 | 7.0 | 8.0 | 8.0 | 3.0 | 3.0 | 3.0 | 3.0 | 4.0 | 4.0 | 3.0 | 3.0 | 4.0 | 4.0 | 4.0 | 3.5 |
| 21 | 6.0 | 9.0 | 8.0 | 8.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 4.0 | 3.0 | 2.0 | 4.0 | 4.0 | 4.0 | 3.0 |
| 28 | 6.0 | 8.0 | 8.0 | 8.0 | 2.5 | 3.0 | 2.0 | 3.0 | 3.0 | 3.0 | 2.0 | 2.0 | 3.5 | 3.0 | 2.0 | 3.0 |
| Pooled standard deviation | 1.30 | 1.99 | 0.55 | 0.55 | 1.08 | 0.89 | 0.89 | 0.89 | 1.0 | 0.45 | 1.14 | 1.22 | 0.55 | 0.45 | 1.10 | 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savini, S.; Longo, E.; Servili, A.; Murolo, S.; Mozzon, M.; Romanazzi, G.; Boselli, E. Hypobaric Packaging Prolongs the Shelf Life of Refrigerated Black Truffles (Tuber melanosporum). Molecules 2020, 25, 3837. https://doi.org/10.3390/molecules25173837
Savini S, Longo E, Servili A, Murolo S, Mozzon M, Romanazzi G, Boselli E. Hypobaric Packaging Prolongs the Shelf Life of Refrigerated Black Truffles (Tuber melanosporum). Molecules. 2020; 25(17):3837. https://doi.org/10.3390/molecules25173837
Chicago/Turabian StyleSavini, Sara, Edoardo Longo, Andrea Servili, Sergio Murolo, Massimo Mozzon, Gianfranco Romanazzi, and Emanuele Boselli. 2020. "Hypobaric Packaging Prolongs the Shelf Life of Refrigerated Black Truffles (Tuber melanosporum)" Molecules 25, no. 17: 3837. https://doi.org/10.3390/molecules25173837