High Carbon Dioxide Treatment Modulates Sugar Metabolism and Maintains the Quality of Fresh-Cut Pear Fruit
Abstract
:1. Introduction
2. Results
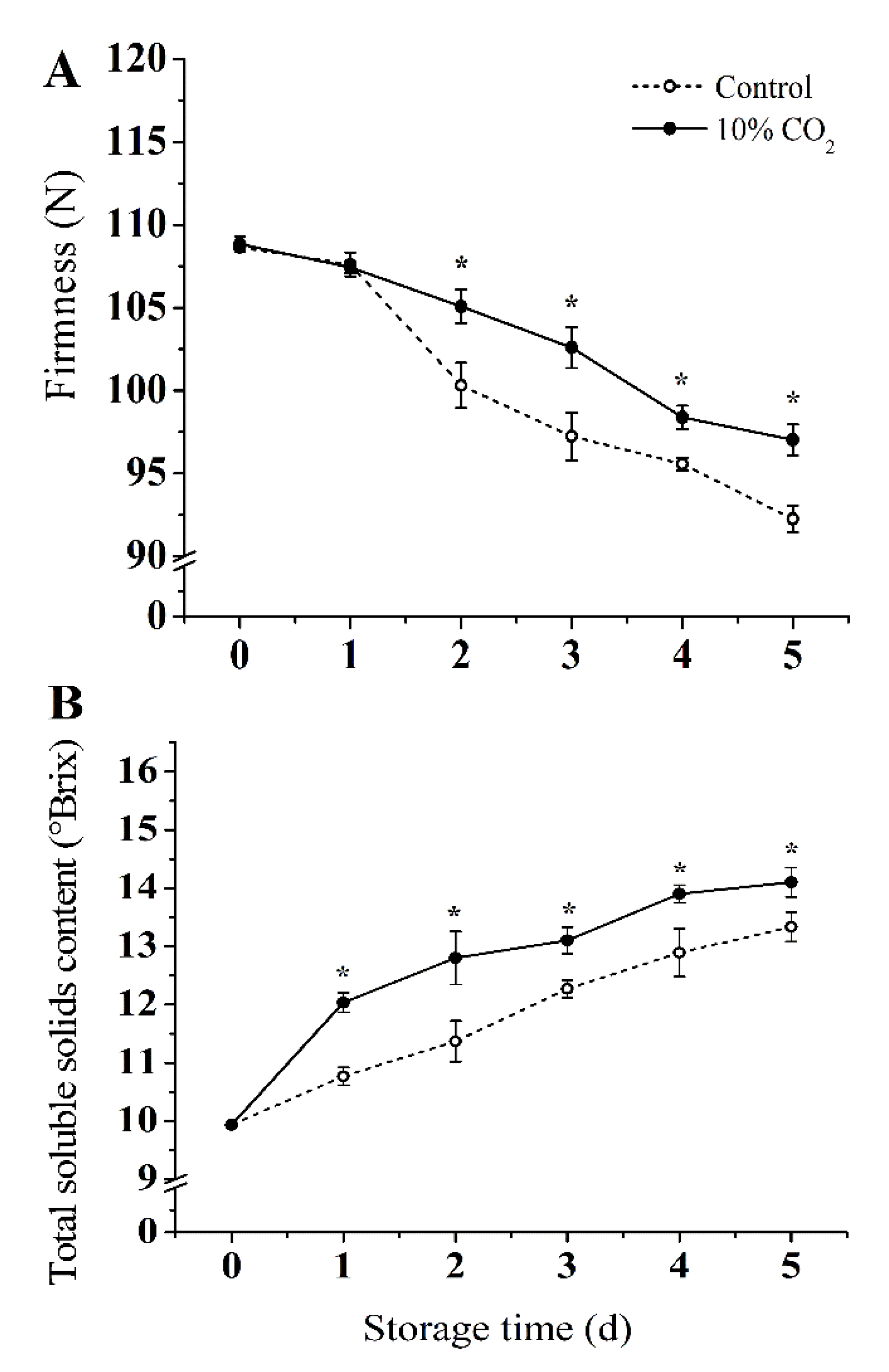
2.1. Firmness and Total Soluble Solids (TSS)
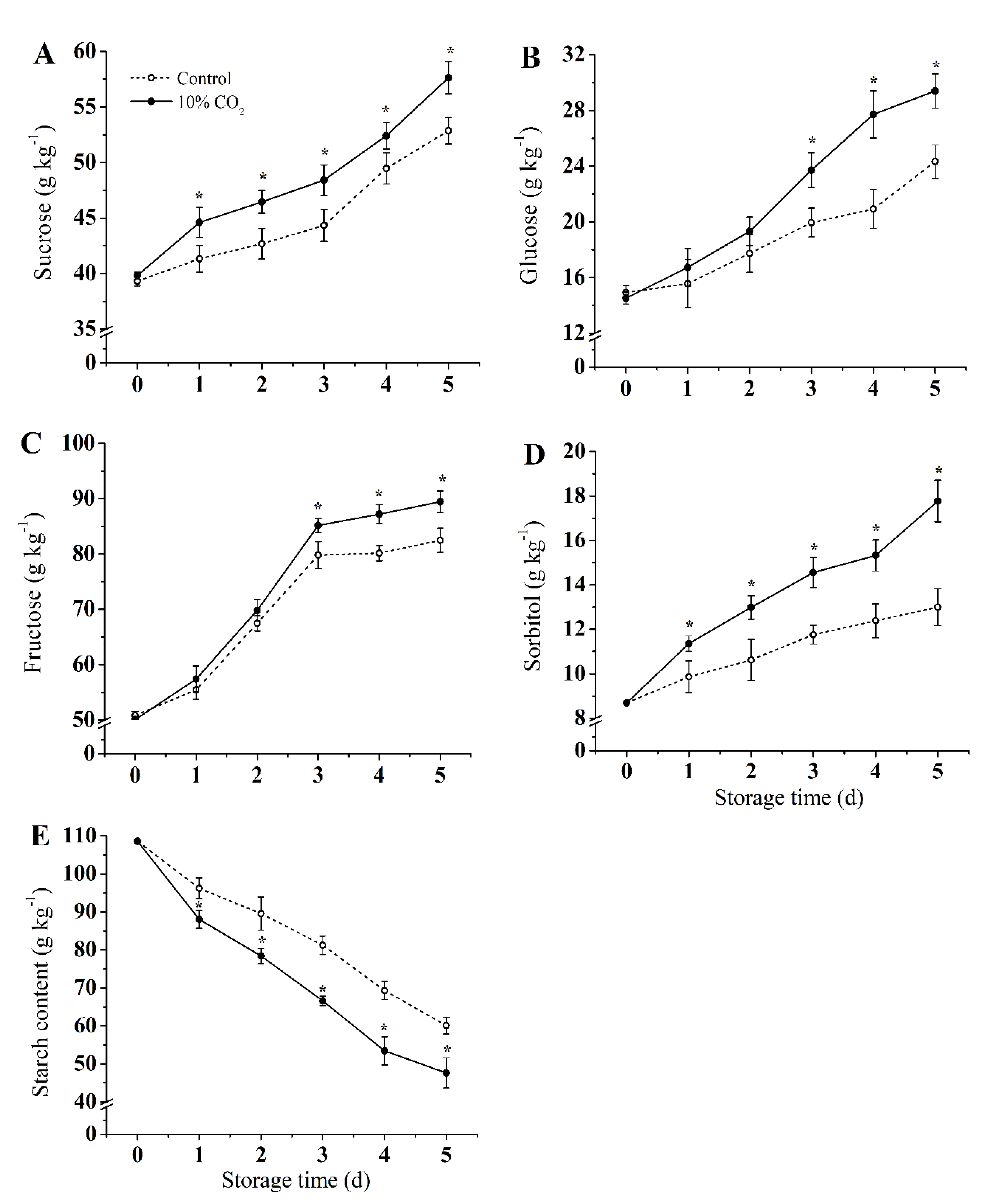
2.2. Sucrose, Glucose, Fructose, Sorbitol, and Starch Content
2.3. SS-Synthesis, SS-Cleavage, AI, NI, Amylase, SPS, HK, and FK Activities
2.4. SOX, NAD-SDH, and NADP-SDH Activities
2.5. Gene Expression of SS-Synthesis, AI, NI, Amylase, SPS, HK, and FK
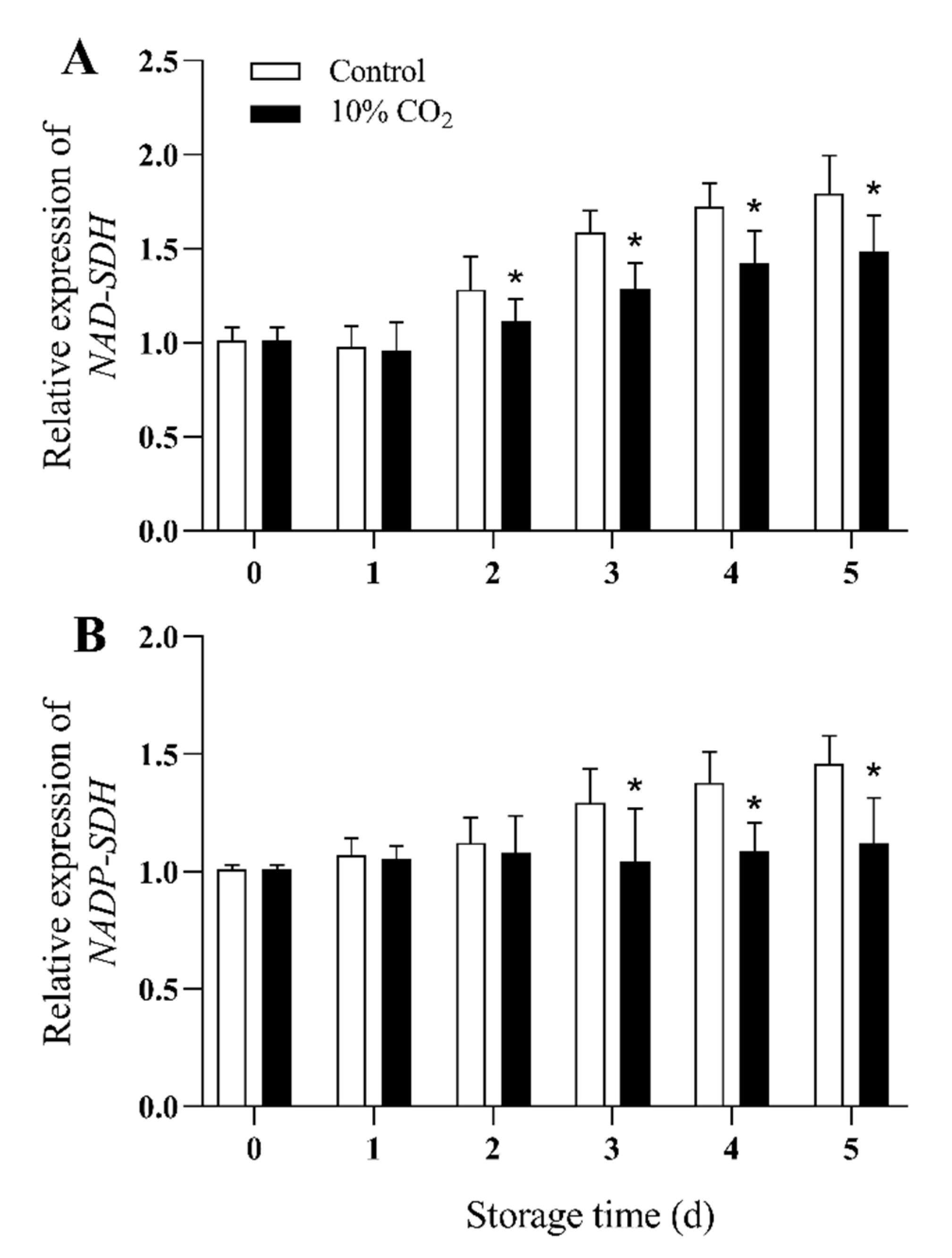
2.6. Gene Expression of NAD-SDH and NADP-SDH
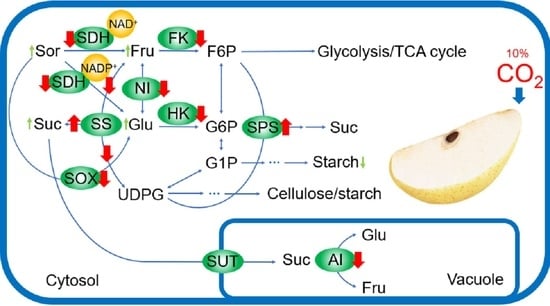
3. Discussion
4. Materials and Methods
4.1. Pear Fruit, Treatment, and Storage
4.2. Determination of Firmness and Total Soluble Solids (TSS)
4.3. Determination of Sucrose, Glucose, Fructose, and Sorbitol Content
4.4. Determination of Starch Content
4.5. Determination of SS-Synthesis, SS-Cleavage, AI, and NI Activities
4.6. Determination of Amylase and SPS Activities
4.7. Determination of FK and HK Activities
4.8. Determination of SOX, NAD-SDH, and NADP-SDH Activities
4.9. Determination of Related Gene Expression by Real-Time Quantitative PCR (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borsanie, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.A. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.J.; Liu, L.; Wang, Y.; Tao, H.X.; Fan, J.L.; Zhao, Z.Y.; Guo, Y.P. Effects of soil water stress on fruit yield, quality and their relationship with sugar metabolism in ‘Gala’ apple. Sci. Hortic. 2019, 258, 108753. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant. 2012, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.C.; Li, L.; Aghdam, M.S.; Luo, Z.S. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef]
- Shi, K.K.; Liu, Z.C.; Wang, J.W.; Zhu, S.H.; Huang, D.D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 258, 108611. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, R.L.; Li, B.Q.; Tian, S.P. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef]
- Li, M.J.; Feng, F.J.; Cheng, L.J. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE. 2012, 7, e33055. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cao, J.K.; Jiang, W.B. Changes in sugar metabolism caused by exogenous oxalic acid related to chilling tolerance of apricot fruit. Postharvest Biol. Biotechnol. 2016, 114, 10–16. [Google Scholar] [CrossRef]
- Mao, L.C.; Que, F.; Wang, G.Z. Sugar metabolism and involvement of enzymes in sugarcane (Saccharum officinarum L.) stems during storage. Food Chem. 2006, 98, 338–342. [Google Scholar] [CrossRef]
- Lara, M.V.; Budde, C.O.; Porrini, L.; Borsani, J.; Murray, R.; Andreo, C.S.; Drincovich, M.F. Peach (Prunus Persica) fruit response to anoxia: Reversible ripening delay and biochemical changes. Plant. Cell Physiol. 2011, 52, 392–403. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, L.H.; Liu, M.P.; Wang, X.Y.; Zhao, G.Y.; Zong, W. Effect of poly-ε-lysine incorporated into alginate-based edible coatings on microbial and physicochemical properties of fresh-cut kiwifruit. Postharvest Biol. Biotechnol. 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; AI-Said, F.A.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences—A Review. Food Bioprocess. Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Ramayya, N.; Niranjan, K.; Duncan, E. Effects of modified atmosphere packaging on quality of Alphonso mangoes. J. Food Sci. Technol. 2012, 49, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Sellamuthu, P.S.; Mafune, M.; Sivakumar, D.; Soundy, P. Thyme oil vapour and modified atmosphere packaging reduce anthracnose incidence and maintain fruit quality in avocado. J. Sci. Food Agric. 2013, 93, 3024–3031. [Google Scholar] [CrossRef]
- Finnegan, E.; Mahajan, P.V.; O’Connell, M.; Francis, G.A.; Beirne, D. Modelling respiration in fresh-cut pineapple and prediction of gas permeability needs for optimal modified atmosphere packaging. Postharvest Biol. Biotechnol. 2013, 79, 47–53. [Google Scholar] [CrossRef]
- Colgecen, I.; Aday, M.S. The efficacy of the combined use of chlorine dioxide and passive modified atmosphere packaging on sweet cherry quality. Postharvest Biol. Biotechnol. 2015, 109, 10–19. [Google Scholar] [CrossRef]
- Antala, D.K.; Varshney, A.K.; Davara, P.R.; Sangani, V.P. Modified atmosphere packaging of guava fruit. Packag. Technol. Sci. 2015, 28, 557–564. [Google Scholar] [CrossRef]
- Blanch, M.; Álvarez, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Involvement of fatty acids in the response to high CO2 and low temperature in harvested strawberries. Postharvest Biol. Biotechnol. 2019, 147, 196–205. [Google Scholar] [CrossRef]
- Yang, M.Y.; Ban, Z.J.; Luo, Z.S.; Li, J.H.; Lu, H.Y.; Li, D.; Chen, C.K.; Li, L. Impact of elevated O2 and CO2 atmospheres on chemical attributes and quality of strawberry (Fragaria × ananassa Duch.) during storage. Food Chem. 2020, 307, 125550. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.C.; Qu, H.X.; Li, L.; Luo, Z.S. Delaying the biosynthesis of aromatic secondary metabolites in postharvest strawberry fruit exposed to elevated CO2 atmosphere. Food Chem. 2020, 306, 125611. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, D.; Li, L.; Belwal, T.; Luo, Z.S. Effects of elevated CO2 on pigment metabolism of postharvest mandarin fruit for degreening. Food Chem. 2020, 318, 126462. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Gilles, V.; Shaozhong, K.; Bertin, N.; Gautier, H.; Génard, M. Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit. J. Exp. Bot. 2020, 71, 5010–5026. [Google Scholar] [CrossRef]
- Li, X.W.; Liu, P.; Zhou, J.Y.; Su, M.S.; Ye, Z.W. Effects of exogenous application of GA4+7 and NAA on sugar accumulation and related gene expression in peach fruits during developing and ripening stages. J. Plant. Growth Regul. 2020, 4, 1–12. [Google Scholar]
- Li, M.J.; Li, P.M.; Ma, F.W.; Dandekar, A.M.; Cheng, L.L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 60, 2–11. [Google Scholar]
- Lin, Q.; Xie, Y.J.; Guan, W.Q.; Duan, Y.Q.; Wang, Z.D.; Sun, C.D. Combined transcriptomic and proteomic analysis of cold stress induced sugar accumulation and heat shock proteins expression during postharvest potato tuber storage. Food Chem. 2019, 297, 124991. [Google Scholar] [CrossRef]
- Pandey, R.; Zinta, G.; AbdElgawad, H.; Ahmad, A.; Jain, V.; Janssens, I.A. Physiological and molecular alterations in plants exposed to high CO2 under phosphorus stress. Biotechnol. Adv. 2015, 33, 303–316. [Google Scholar]
- Cortellino, G.; Piazza, L.; Spinelli, L.; Torricelli, A.; Rizzolo, A. Influence of maturity degree, modified atmosphere and anti-browning dipping on texture changes kinetics of fresh-cut apples. Postharvest Biol. Biotechnol. 2016, 124, 137–146. [Google Scholar]
- Thewes, F.R.; Brackmann, A.; Neuwald, D.A. Dynamics of sugars, anaerobic metabolism enzymes and metabolites in apples stored under dynamic controlled atmosphere. Sci. Hortic. 2019, 255, 145–152. [Google Scholar] [CrossRef]
- Teixeira, G.H.A.; Júnior, L.C.C.; Ferraudo, A.S.; Durigan, J.F. Quality of guava (Psidium guajava L. cv. Pedro Sato) fruit stored in low-O2 controlled atmospheres is negatively affected by increasing levels of CO2. Postharvest Biol. Biotechnol. 2016, 111, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Davarynejad, G.H.; Aryanpooya, Z.; Persely, S.Z. Effect of modified atmosphere packaging on fresh sour cherry fruit quality. Acta Hortic. 2010, 877, 449–453. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Vries, F.; Merwe, K.V.D.; Crouch, E.; Opara, U.L. Repeated application of dynamic controlled atmospheres reduced superficial scald incidence in ‘Granny Smith’ apples. Sci. Hortic. 2017, 220, 168–175. [Google Scholar] [CrossRef]
- Marian, J.M.; Lindsay, A.G.; Julian, A.H.; Paul, L.H. Sugar metabolism and compartmentation in asparagus and broccoli during controlled atmosphere storage. Postharvest Biol. Biotechnol. 2004, 32, 45–56. [Google Scholar]
- Ge, Y.H.; Wei, M.L.; Li, C.Y.; Chen, Y.R.; Duan, B.; Li, X.; Tang, Q.; Li, X.H. Changes in the sucrose metabolism in apple fruit following postharvest acibenzolar-S-methyl treatment. J. Sci. Food Agric. 2019, 99, 1519–1524. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Roy, S.; Das, R.; Sengupta, D.N. Differential transcriptional regulation of banana sucrose phosphate synthase gene in response to ethylene, auxin, wounding, low temperature and different photoperiods during fruit ripening and functional analysis of banana SPS gene promoter. Planta 2008, 229, 207–223. [Google Scholar] [CrossRef]
- Yu, L.N.; Shao, X.F.; Wei, Y.Y.; Xu, F.; Wang, H.F. Sucrose degradation is regulated by 1-methycyclopropene treatment and is related to chilling tolerance in two peach cultivars. Postharvest Biol. Biotechnol. 2017, 124, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, I.; Ricard, B.; Rolin, D.; Maucourt, M.; Andrieu, M.; Bizid, E.; Smiti, S.; Brouquisse, R. Effect of hexokinase activity on tomato root metabolism during prolonged hypoxia. Plant. Cell Environ. 2007, 30, 508–517. [Google Scholar] [CrossRef]
- Mustroph, A.; Albrecht, G. Tolerance of crop plants to oxygen deficiency stress: Fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol. Plant. 2003, 117, 508–520. [Google Scholar] [CrossRef]
- Yu, C.Y.; Cheng, H.Y.; Cheng, R.; Qi, K.J.; Gu, C.; Zhang, S.L. Expression analysis of sorbitol transporters in pear tissues reveals that PbSOT6/20 is associated with sorbitol accumulation in pear fruits. Sci. Hortic. 2019, 243, 595–601. [Google Scholar] [CrossRef]
- Liu, D.F.; Ni, J.B.; Wu, R.Y.; Teng, Y.W. High temperature alters sorbitol metabolism in pyrus pyrifolia leaves and fruit flesh during late stages of fruit enlargement. J. Am. Soc. Hortic. Sci. 2013, 138, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Ge, Y.H.; Li, C.Y.; Gao, X.N.; Tang, Q.; Li, X.; Wei, M.L.; Chen, Y.R. Effect of exogenous ATP treatment on sucrose metabolism and quality of ‘Nanguo’ pear fruit. Sci. Hortic. 2019, 249, 71–76. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) |
|---|---|---|---|
| Actin | TCCCACATGCCATCCTTCGTTTG | GTCCCTCACAATTTCCCGCTCAG | 122 |
| SPS | GGCGGGAAATGACTGGGTGAAC | CGGAGGAGCAGCGATGATTTGG | 90 |
| SS-synthesis | AGCAGCACCAACCTTAGCAATCC | CGGAGGAGCAGCGATGATTTGG | 119 |
| NI | CAAGCACGCCTGTTCCAGACC | GCAGGAGAAGGCATTCACGAGTTC | 129 |
| AI | CGCTACTCCCTGACGGTAACATTG | ATACGCTAGGCATTGCACTTGGAC | 80 |
| Amylase | AACCGAACCCAGATCAAGATGCAC | CGAGTCCTTCAGCCTCCAAAGC | 146 |
| NAD-SDH | ATGGCTGCTTGGCTCGTTGATG | TCACTTCCGCAAATGCCGACAG | 116 |
| NAD-MDH | TCCGTCCCCGAGCGTAAAGTC | ACCAACATCAGCAGCAACTCCAG | 147 |
| FK | ACAAACTGAGAGATGCGCTCG | GCAATGCCGGAATAGCACCT | 81 |
| HK | TCCTTGAGTTTGCTCCCGAC | TGGAGTGGGGTAACTTTCGC | 292 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ma, Q.; Belwal, T.; Li, D.; Li, W.; Li, L.; Luo, Z. High Carbon Dioxide Treatment Modulates Sugar Metabolism and Maintains the Quality of Fresh-Cut Pear Fruit. Molecules 2020, 25, 4261. https://doi.org/10.3390/molecules25184261
Wang D, Ma Q, Belwal T, Li D, Li W, Li L, Luo Z. High Carbon Dioxide Treatment Modulates Sugar Metabolism and Maintains the Quality of Fresh-Cut Pear Fruit. Molecules. 2020; 25(18):4261. https://doi.org/10.3390/molecules25184261
Chicago/Turabian StyleWang, Di, Quan Ma, Tarun Belwal, Dong Li, Wenxuan Li, Li Li, and Zisheng Luo. 2020. "High Carbon Dioxide Treatment Modulates Sugar Metabolism and Maintains the Quality of Fresh-Cut Pear Fruit" Molecules 25, no. 18: 4261. https://doi.org/10.3390/molecules25184261