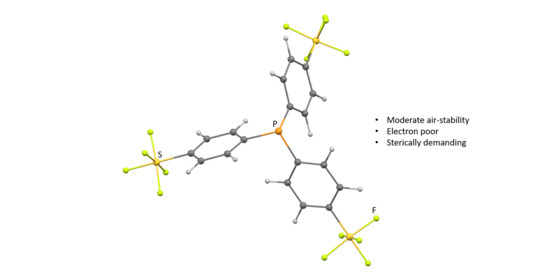
A SF5 Derivative of Triphenylphosphine as an Electron-Poor Ligand Precursor for Rh and Ir Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Air-Stability of P(p-C6H4SF5)3 (1)
2.2. Estimation of the Donor Properties
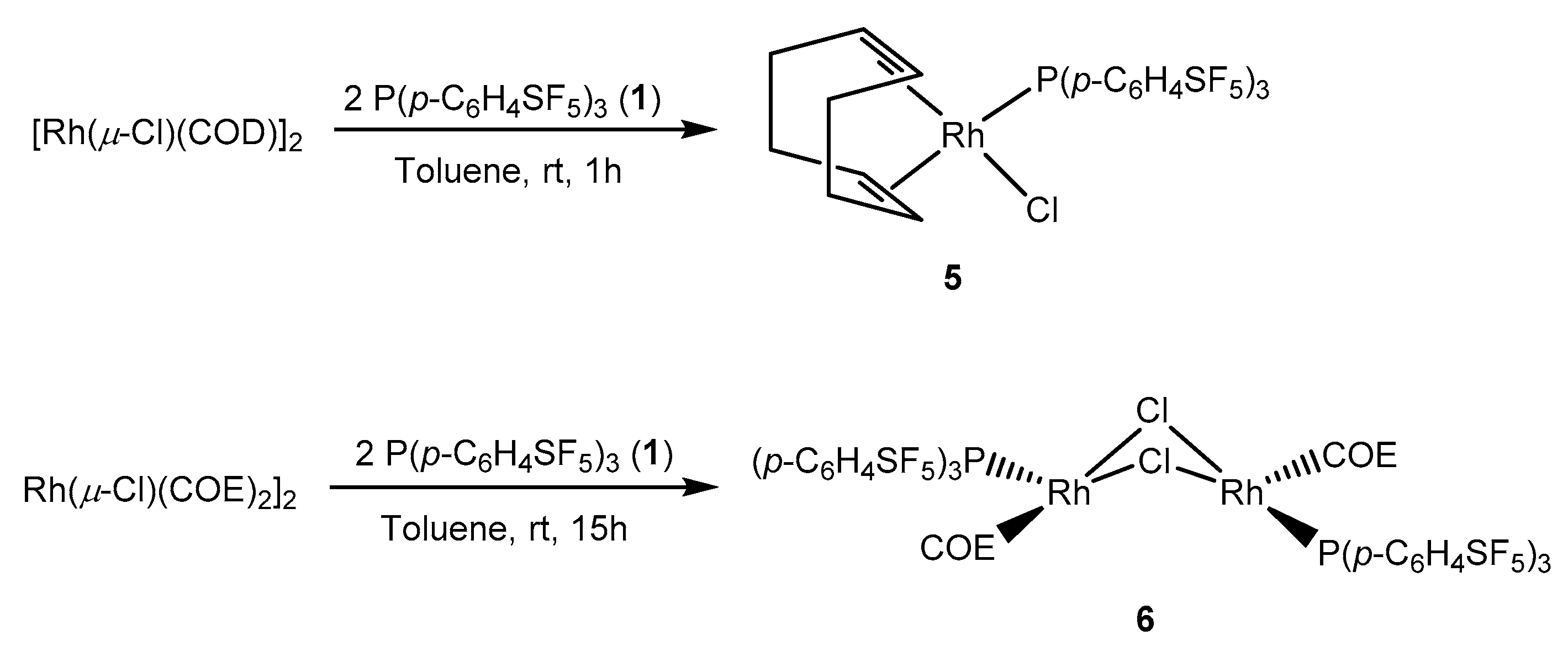
2.3. Synthesis of Iridium and Rhodium Complexes
2.4. Steric Properties of 1
2.5. Reactivity of Complexes 4 and 5 towards CO
3. Materials and Methods
3.1. General Procedures, Methods and Materials
3.2. Synthesis of Tris-(p-pentafluorosulfanylphenyl)phosphine (1)
3.3. Formation of Tris-(p-pentafluorosulfanylphenyl)phosphine oxide (2)
3.4. Synthesis of Tris-(p-pentafluorosulfanylphenyl)phosphine Selenide (3)
3.5. Synthesis of [IrCl(COD){P(p-C6H4SF5)3}] (4)
3.6. Synthesis of [RhCl(COD){P(p-C6H4SF5)3}] (5)
3.7. Synthesis of [Rh(µ-Cl)(COE){P(p-C6H4SF5)3}]2 (6)
3.8. Reaction of [IrCl(COD){P(p-C6H4SF5)3}] (4) with CO. Formation of [IrCl(CO)(COD){P(p-C6H4SF5)3}] (7) and [IrCl(CO)2{P(p-C6H4SF5)3}2] (8)
3.9. Independent Formation of [IrCl(CO)2{P(p-C6H4SF5)3}2] (8)
3.10. Formation of Trans,trans-[RhCl(CO)2{P(p-C6H4SF5)3}2] (9)
3.11. X-ray Diffraction Analysis
3.12. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 6th ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hartwig, J.F. Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar]
- McAuliffe, C.A.; Levason, W. Phosphine, Arsine and Stibine Complexes of the Transition Elements; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Pignolet, L.H. Homogeneous Catalysis with Metal Phosphine Complexes; Plenum Press: New York, NY, USA, 1983. [Google Scholar]
- Pollock, C.L.; Saunders, G.C.; Smyth, E.C.M.S.; Sorokin, V.I. Fluoroarylphosphines as ligands. J. Fluor. Chem. 2008, 129, 142–166. [Google Scholar] [CrossRef]
- Clarke, M.L.; Ellis, D.; Mason, K.L.; Orpen, A.G.; Pringle, P.G.; Wingad, R.L.; Zaher, D.A.; Baker, R.T. The electron-poor phosphines P{C6H3(CF3)2-3,5}3 and P(C6F5)3 do not mimic phosphites as ligands for hydroformylation. A comparison of the coordination chemistry of P{C6H3(CF3)2-3,5}3 and P(C6F5)3 and the unexpectedly low hydroformylation activity of their rhodium complexes. Dalton Trans. 2005, 7, 1294–1300. [Google Scholar]
- Fawcett, J.; Hope, E.G.; Kemmitt, R.D.; Paige, D.R.; Russell, D.R.; Stuart, A.M.; Stuart, M. Platinum group metal complexes of arylphosphine ligands containing perfluoroalkyl ponytails; crystal structures of [RhCl2(η5-C5Me5){P(C6H4C6F13-4)3}] and cis- and trans-[PtCl2{P(C6H4C6F13-4)3}2]. J. Chem. Soc. Dalton Trans. 1998, 22, 3751–3764. [Google Scholar] [CrossRef]
- Hope, E.G.; Kemmitt, R.D.W.; Paige, D.R.; Stuart, A.M.; Wood, D.R.W. Synthesis and coordination chemistry of meta-perfluoroalkyl-derivatised triarylphosphines. Polyhedron 1999, 18, 2913–2917. [Google Scholar] [CrossRef]
- Corcoran, C.; Fawcett, J.; Friedrichs, S.; Holloway, J.H.; Hope, E.G.; Russell, D.R.; Saunders, G.C.; Stuart, A.M. Structural and electronic impact of fluorine in the ortho positions of triphenylphosphine and 1,2-bis(diphenylphosphino)ethane; a comparison of 2,6-difluorophenyl- with pentafluorophenyl-phosphines. J. Chem. Soc. Dalton Trans. 2000, 2, 161–172. [Google Scholar] [CrossRef]
- Croxtall, B.; Fawcett, J.; Hope, E.G.; Stuart, A.M. Synthesis and coordination chemistry of ortho-perfluoroalkyl-derivatised triarylphosphines. J. Chem. Soc. Dalton Trans. 2002, 4, 491–499. [Google Scholar] [CrossRef]
- Saunders, G.C. Structural and electronic properties of tris(4-trifluoromethyltetrafluorophenyl)phosphine. J. Fluor. Chem. 2015, 180, 15–20. [Google Scholar] [CrossRef]
- Uson, R.; Oro, L.A.; Fernandez, M.J. Preparation, reactions and catalytic activity of complexes of the type [Ir(COD){P(p-RC6H4)3}2]A (R = Cl, F, H, CH3 or CH3O.; A = ClO4− or B(C6H5)4−). J. Organomet. Chem. 1980, 193, 127–133. [Google Scholar] [CrossRef]
- Matsubara, K.; Fujii, T.; Hosokawa, R.; Inatomi, T.; Yamada, Y.; Koga, Y. Fluorine-Substituted Arylphosphine for an NHC-Ni(I) System, Air-Stable in a Solid State but Catalytically Active in Solution. Molecules 2019, 24, 3222. [Google Scholar] [CrossRef] [Green Version]
- Moser, W.R.; Papile, C.J.; Brannon, D.A.; Duwell, R.A.; Weininger, S.J. The mechanism of phosphine-modified rhodium-catalyzed hydroformylation studied by CIR-FTIR. J. Mol. Catal. 1987, 41, 271–292. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Shi, X.; Sun, W.; Zhao, J.; Zhu, Y.-P.; Liu, L.; Zhu, B. Palladium-Catalyzed C-2 and C-3 Dual C–H Functionalization of Indoles: Synthesis of Fluorinated Isocryptolepine Analogues. Org. Lett. 2020, 22, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.J.; Dunås, P.; Rahm, M.; Norrby, P.-O.; Kociok-Köhn, G.; Lewis, S.E.; Kann, N. Palladium Catalyzed Stereoselective Arylation of Biocatalytically Derived Cyclic 1,3-Dienes: Chirality Transfer via a Heck-Type Mechanism. Org. Lett. 2020, 22, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Jakab, A.; Dalicsek, Z.; Holczbauer, T.; Hamza, A.; Pápai, I.; Finta, Z.; Timári, G.; Soós, T. Superstable Palladium(0) Complex as an Air- and Thermostable Catalyst for Suzuki Coupling Reactions. Eur. J. Org. Chem. 2015, 2015, 60–66. [Google Scholar] [CrossRef]
- Cheng, L.; Li, M.-M.; Wang, B.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. Nickel-catalyzed hydroalkylation and hydroalkenylation of 1,3-dienes with hydrazones. Chem. Sci. 2019, 10, 10417–10421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, W.A. The Electrical Effect of the Sulfur Pentafluoride Group. J. Am. Chem. Soc. 1962, 84, 3072–3076. [Google Scholar] [CrossRef]
- Hansch, C.; Muir, R.M.; Fujita, T.; Maloney, P.P.; Geiger, F.; Streich, M. The Correlation of Biological Activity of Plant Growth Regulators and Chloromycetin Derivatives with Hammett Constants and Partition Coefficients. J. Am. Chem. Soc. 1963, 85, 2817–2824. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Savoie, P.R.; Welch, J.T. Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds. Chem. Rev. 2015, 115, 1130–1190. [Google Scholar] [CrossRef]
- Lentz, D.; Seppelt, K. The -SF5, -SeF5, and -TeF5 Groups in Organic Chemistry. In Chemistry of Hypervalent Compounds; Akiba, K.-Y., Ed.; Wiley-VCH: New York, NY, USA, 1999; pp. 295–325. [Google Scholar]
- Gard, G.L. Recent Milestones in SF5-Chemistry. Chim. Oggi 2009, 27, 10–13. [Google Scholar]
- Altomonte, S.; Zanda, M. Synthetic chemistry and biological activity of pentafluorosulphanyl (SF5) organic molecules. J. Fluor. Chem. 2012, 143, 57–93. [Google Scholar] [CrossRef] [Green Version]
- Kanishchev, O.S.; Dolbier, W.R. Chapter One—SF5-Substituted Aromatic Heterocycles. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 120, pp. 1–42. [Google Scholar]
- Chan, J.M.W. Pentafluorosulfanyl group: An emerging tool in optoelectronic materials. J. Mater. Chem. C 2019, 7, 12822–12834. [Google Scholar] [CrossRef]
- Beier, P. Synthesis and reactivity of novel sulfur pentafluorides—Effect of the SF5 group on reactivity of nitrobenzenes in nucleophilic substitution. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 212–215. [Google Scholar] [CrossRef]
- Beier, P. Pentafluorosulfanylation of Aromatics and Heteroaromatics. In Emerging Fluorinated Motifs; Ma, J.-A., Cahard, D., Eds.; Wiley-VCH: Weinheim, Germany, 2020; Volume 2, pp. 551–570. [Google Scholar]
- Haufe, G. Pentafluorosulfanylation of Aliphatic Substrates. In Emerging Fluorinated Motifs; Ma, J.-A., Cahard, D., Eds.; Wiley-VCH: Weinheim, Germany, 2020; Volume 2, pp. 571–609. [Google Scholar]
- Damerius, R.; Leopold, D.; Schulze, W.; Seppelt, K. Strukturen von SF5-substituierten Metallkomplexen. Z. Anorg. Allg. Chem. 1989, 578, 110–118. [Google Scholar] [CrossRef]
- Henkel, T.; Klauck, A.; Seppelt, K. Pentafluoro-λ6-sulfanylacetylene complexes of cobalt. J. Organomet. Chem. 1995, 501, 1–6. [Google Scholar] [CrossRef]
- Preugschat, D.; Thrasher, J.S. Pentacarbonylchrom-Komplexe SF5-substituierter Isocyanide. Z. Anorg. Allg. Chem. 1996, 622, 1411–1414. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Xie, G.; Varghese, S.; Cordes, D.B.; Slawin, A.M.Z.; Momblona, C.; Ortí, E.; Bolink, H.J.; Samuel, I.D.W.; Zysman-Colman, E. Green Phosphorescence and Electroluminescence of Sulfur Pentafluoride-Functionalized Cationic Iridium(III) Complexes. Inorg. Chem. 2015, 54, 5907–5914. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-F.; Luo, X.-F.; Yan, Z.-P.; Wu, Z.-G.; Zhao, Y.; Zheng, Y.-X.; Zuo, J.-L. Syntheses, Crystal Structures, and Photoluminescence of a Series of Iridium(III) Complexes Containing the Pentafluorosulfanyl Group. Organometallics 2019, 38, 3553–3559. [Google Scholar] [CrossRef]
- Pal, A.K.; Henwood, A.F.; Cordes, D.B.; Slawin, A.M.Z.; Samuel, I.D.W.; Zysman-Colman, E. Blue-to-Green Emitting Neutral Ir(III) Complexes Bearing Pentafluorosulfanyl Groups: A Combined Experimental and Theoretical Study. Inorg. Chem. 2017, 56, 7533–7544. [Google Scholar] [CrossRef]
- Groves, L.M.; Schotten, C.; Beames, J.; Platts, J.A.; Coles, S.J.; Horton, P.N.; Browne, D.L.; Pope, S.J.A. From Ligand to Phosphor: Rapid, Machine-Assisted Synthesis of Substituted Iridium(III) Pyrazolate Complexes with Tuneable Luminescence. Chem. A Eur. J. 2017, 23, 9407–9418. [Google Scholar] [CrossRef] [Green Version]
- Henwood, A.F.; Webster, J.; Cordes, D.; Slawin, A.M.Z.; Jacquemin, D.; Zysman-Colman, E. Phosphorescent platinum(ii) complexes bearing pentafluorosulfanyl substituted cyclometalating ligands. RSC Adv. 2017, 7, 25566–25574. [Google Scholar] [CrossRef] [Green Version]
- Berg, C.; Braun, T.; Laubenstein, R.; Braun, B. Palladium-mediated borylation of pentafluorosulfanyl functionalized compounds: The crucial role of metal fluorido complexes. Chem. Commun. 2016, 52, 3931–3934. [Google Scholar] [CrossRef] [PubMed]
- Golf, H.R.A.; Reissig, H.-U.; Wiehe, A. Synthesis of SF5-Substituted Tetrapyrroles, Metalloporphyrins, BODIPYs, and Their Dipyrrane Precursors. J. Org. Chem. 2015, 80, 5133–5143. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.D.; De Marco, R.A. Reaction of pentafluoro[(trifluoromethyl)acetylenyl]sulfur with nickel tetracarbonyl. Inorg. Chem. 1982, 21, 457–458. [Google Scholar] [CrossRef]
- Sergeeva, T.A.; Dolbier, W.R. A New Synthesis of Pentafluorosulfanylbenzene. Org. Lett. 2004, 6, 2417–2419. [Google Scholar] [CrossRef]
- Dunne, B.J.; Orpen, A.G. Triphenylphosphine: A redetermination. Acta Cryst. 1991, C47, 345–347. [Google Scholar] [CrossRef]
- Eapen, K.C.; Tamborski, C. The synthesis of tris-(trifluoromethylphenyl)phosphines and phosphine oxides. J. Fluor. Chem. 1980, 15, 239–243. [Google Scholar] [CrossRef]
- See, R.F.; Dutoi, A.D.; Fettinger, J.C.; Nicastro, P.J.; Ziller, J.W. The crystal structures of (p-ClPh)3PO and (p-OMePh)3PO, including an analysis of the P-O bond in triarylphosphine oxides. J. Chem. Crystallogr. 1998, 28, 893–898. [Google Scholar] [CrossRef]
- Stewart, B.; Harriman, A.; Higham, L.J. Predicting the Air Stability of Phosphines. Organometallics 2011, 30, 5338–5343. [Google Scholar] [CrossRef]
- Dunne, B.J.; Morris, R.B.; Orpen, A.G. Structural systematics. Part 3. Geometry deformations in triphenylphosphine fragments: A test of bonding theories in phosphine complexes. J. Chem. Soc. Dalton Trans. 1991, 653–661. [Google Scholar] [CrossRef]
- Palau, C.; Berchadsky, Y.; Chalier, F.; Finet, J.-P.; Gronchi, G.; Tordo, P. Tris(monochlorophenyl)- and Tris(dichlorophenyl)phosphines: Molecular Geometry, Anodic Behavior, and ESR Studies. J. Phys. Chem. 1995, 99, 158–163. [Google Scholar] [CrossRef]
- Chevykalova, M.N.; Manzhukova, L.F.; Artemova, N.V.; Luzikov, Y.N.; Nifant’ev, I.E.; Nifant’ev, E.E. Electron-donating ability of triarylphosphines and related compounds studied by 31P NMR spectroscopy. Russ. Chem. Bull. 2003, 52, 78–84. [Google Scholar] [CrossRef]
- Howell, J.S.; Lovatt, J.; Yates, P.; Gottlieb, H.; Hursthouse, M.; Light, M. Effect of fluorine and trifluoromethyl substitution on the donor properties and stereodynamical behaviour of triarylphosphines. J. Chem. Soc. Dalton Trans. 1999, 17, 3015–3028. [Google Scholar] [CrossRef]
- Allen, D.W.; Taylor, B.F. The chemistry of heteroarylphosphorus compounds. Part 15. Phosphorus-31 nuclear magnetic resonance studies of the donor properties of heteroarylphosphines towards selenium and platinum(II). J. Chem. Soc. Dalton Trans. 1982, 1, 51–54. [Google Scholar] [CrossRef]
- Tolman, C.A. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 1977, 77, 313–348. [Google Scholar] [CrossRef]
- Gusev, D.G. Donor Properties of a Series of Two-Electron Ligands. Organometallics 2009, 28, 763–770. [Google Scholar] [CrossRef]
- Perrin, L.; Clot, E.; Eisenstein, O.; Loch, J.; Crabtree, R.H. Computed Ligand Electronic Parameters from Quantum Chemistry and Their Relation to Tolman Parameters, Lever Parameters, and Hammett Constants. Inorg. Chem. 2001, 40, 5806–5811. [Google Scholar] [CrossRef]
- Kawaguchi, S.-I.; Minamida, Y.; Okuda, T.; Sato, Y.; Saeki, T.; Yoshimura, A.; Nomoto, A.; Ogawa, A. Photoinduced Synthesis of P-Perfluoroalkylated Phosphines from Triarylphosphines and Their Application in the Copper-Free Cross-Coupling of Acid Chlorides and Terminal Alkynes. Adv. Synth. Catal. 2015, 357, 2509–2519. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Wang, K.; Zhang, L.; Li, X.-J.; Li, R.-X. Selective Hydrogenation of Avermectin Catalyzed by Iridium-Phosphine Complexes. Chin. J. Chem. 2007, 25, 1503–1507. [Google Scholar] [CrossRef]
- Tiburcio, J.; Bernès, S.; Torrens, H. Electronic and steric effects of triarylphosphines on the synthesis, structure and spectroscopical properties of mononuclear rhodium(I)–chloride complexes. Polyhedron 2006, 25, 1549–1554. [Google Scholar] [CrossRef]
- Naaktgeboren, A.J.; Nolte, R.J.M.; Drenth, W. Phosphorus-31 nuclear magnetic resonance studies of polymer-anchored rhodium(I) complexes. J. Am. Chem. Soc. 1980, 102, 3350–3354. [Google Scholar] [CrossRef]
- Canepa, G.; Brandt, C.D.; Werner, H. Mono- and Dinuclear Rhodium(I) and Rhodium(III) Complexes with the Bulky Phosphine 2,6-Me2C6H3CH2CH2PtBu2, Including the First Structurally Characterized Cis-Configurated Dicarbonyl Compound, cis-[RhCl(CO)2(PR3)]. Organometallics 2004, 23, 1140–1152. [Google Scholar] [CrossRef]
- Müller, T.E.; Mingos, D.M.P. Determination of the Tolman cone angle from crystallographic parameters and a statistical analysis using the crystallographic data base. Transit. Met. Chem. 1995, 20, 533–539. [Google Scholar] [CrossRef]
- Reyna-Madrigal, A.; Ortiz-Pastrana, N.; Paz-Sandoval, M.A. Cyclooctadiene iridium complexes with phosphine and pentadienyl ligands. J. Organomet. Chem. 2019, 886, 13–26. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Joerg, S.; Drago, R.S.; Sales, J. Reactivity of Phosphorus Donors. Organometallics 1998, 17, 589–599. [Google Scholar] [CrossRef]
- Ortega-Moreno, L.; Fernández-Espada, M.; Moreno, J.J.; Navarro-Gilabert, C.; Campos, J.; Conejero, S.; López-Serrano, J.; Maya, C.; Peloso, R.; Carmona, E. Synthesis, properties, and some rhodium, iridium, and platinum complexes of a series of bulky m-terphenylphosphine ligands. Polyhedron 2016, 116, 170–181. [Google Scholar] [CrossRef]
- Von Hahmann, C.N.; Talavera, M.; Xu, C.; Braun, T. Reactivity of 3,3,3-Trifluoropropyne at Rhodium Complexes: Development of Hydroboration Reactions. Chem. A Eur. J. 2018, 24, 11131–11138. [Google Scholar] [CrossRef]
- Vaska, L. Reversible Combination of Carbon Monoxide with a Synthetic Oxygen Carrier Complex. Science 1966, 152, 769–771. [Google Scholar] [CrossRef]
- Sanger, A.R. Five-coordinate dicarbonyl complexes of rhodium(I): [RhX(CO)2(PPh3)2] (X = Cl, Br, I). Can. J. Chem. 1985, 63, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Butterworth/Heinemann: London/Oxford, UK, 1988. [Google Scholar]
- Herde, J.-L.; Lambert, J.C.; Senoff, C.V. Cyclooctene and 1,5-Cyclooctadiene Complexes of Iridium. Inorg. Synth. 1974, 15, 18–20. [Google Scholar]
- Van der Ent, A.; Onderdelinden, A.L. Chlorobis(cyclooctene)rhodium(I) and -iridium(I) Complexes. Inorg. Synth. 1973, 14, 92–95. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data, May 2014; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-2014, Program for the Solution of Crystal Structures from X-ray Data; University of Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Sheldrick, G.M. SHELXL-2018, Program for the Refinement of Crystal Structures from X-ray Data; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Ar Group | SOMO | Ar Group | SOMO |
|---|---|---|---|
| p-C6H4SF5 | −12.22 | 3,4,5-C6H2F3 | –11.93 |
| p-C6H4CF3 | −11.82 | p-C6H4Me | –10.62 |
| m-C6H3(CF3)2 | –12.36 | p-C6H4OMe | –10.01 |
| p-C6H4F | –11.21 | C6H5 | −11.11 |
| Ar Group | HOMO (eV) | TEP (cm−1) [a] | 1JSe-P (Hz) |
|---|---|---|---|
| p-C6H4SF5 | −8.69 | 2072.6 | 792 |
| p-C6H4CF3 | −8.17 | 2069.3 | 765 [b] |
| m-C6H3(CF3)2 | −8.78 | 2075.2 | 802 [b] |
| p-C6H4F | −7.58 | n.d. | 741 [b] |
| 3,4,5-C6H2F3 | −8.36 | n.d. | 792 [c] |
| p-C6H4Me | −7.10 | 2061.7 (2166.7) | 715 [b] |
| p-C6H4OMe | −6.78 | n.d. | 710 [b] |
| C6H5 | −7.34 | 2063.5 (2068.9) | 733 [b] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talavera, M.; Hinze, S.; Braun, T.; Laubenstein, R.; Herrmann, R. A SF5 Derivative of Triphenylphosphine as an Electron-Poor Ligand Precursor for Rh and Ir Complexes. Molecules 2020, 25, 3977. https://doi.org/10.3390/molecules25173977
Talavera M, Hinze S, Braun T, Laubenstein R, Herrmann R. A SF5 Derivative of Triphenylphosphine as an Electron-Poor Ligand Precursor for Rh and Ir Complexes. Molecules. 2020; 25(17):3977. https://doi.org/10.3390/molecules25173977
Chicago/Turabian StyleTalavera, Maria, Silke Hinze, Thomas Braun, Reik Laubenstein, and Roy Herrmann. 2020. "A SF5 Derivative of Triphenylphosphine as an Electron-Poor Ligand Precursor for Rh and Ir Complexes" Molecules 25, no. 17: 3977. https://doi.org/10.3390/molecules25173977