Hybrid Boron-Carbon Chemistry
Abstract
:1. Introduction
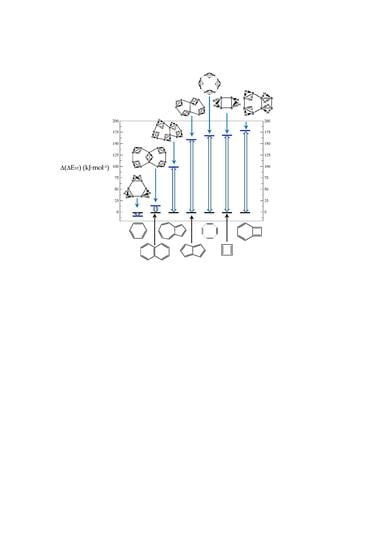
2. Results
3. Discussion
4. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. Z. Phys. 1931, 70, 204–286. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Heitler, W.; London, F. Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik. Z. Phys. 1927, 44, 455–472. [Google Scholar] [CrossRef]
- Hund, F. Zur Deutung einiger Erscheinungen in den Molekelspektren. Z. Phys. 1926, 36, 657. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic states and band spectrum structure in diatomic Molecules. I. Statement of the postulates. Interpretation of CuH, CH, and Co band-types. Phys. Rev. 1926, 28, 481. [Google Scholar] [CrossRef]
- Woodward, R.B.; Hoffmann, R. Stereochemistry of electrocyclic reactions. J. Am. Chem. Soc. 1965, 87, 395–397. [Google Scholar] [CrossRef]
- Kamtekar, K.T.; Monkman, A.P.; Bryce, M.R. Recent advances in white organic light-emitting materials and devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Shavitt, I. Geometry and singlet-triplet energy gap in methylene: A critical review of experimental and theoretical determinations. Tetrahedron 1985, 41, 1531–1542. [Google Scholar] [CrossRef]
- Chutjian, A.; Hall, R.I.; Trajmar, S. Electron-impact excitation of H2O and D2O at various scattering angles and impact energies in the energy-loss range 4.2–12 eV. J. Chem. Phys. 1975, 63, 892–898. [Google Scholar] [CrossRef]
- Stock, A. The Hydrides of Boron and Silicon; Cornell University Press: Ithaca, NY, USA, 1933. [Google Scholar]
- Brown, H.C. From little acorns to tall oaks from boranes through organoboranes. In Nobel Lecture, 8 December 1979; World Scientific Publishing Co.: Singapore, 1993. [Google Scholar]
- Marder, T.B.; Lin, Z. (Eds.) Contemporary Metal Boron Chemistry I.: Borylenes, Boryls, Borane Sigma-Complexes, and Borohydrides; Springer: Berlin, Germany, 2008. [Google Scholar]
- Lipscomb, W.N. Boron Hydrides; Dover Publications Inc.: Mineola, NY, USA, 2012. [Google Scholar]
- Štíbr, B. Carboranes other than C2B10H12. Chem. Rev. 1992, 92, 225–250. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Saxena, A.K.; Hosmane, N.S. Recent advances in the chemistry of carborane metal complexes incorporating d- and f-block elements. Chem. Rev. 1993, 93, 1081–1124. [Google Scholar] [CrossRef]
- Poater, J.; Viñas, C.; Bennour, I.; Escayola, S.; Solà, M.; Teixidor, F. Too persistent to give up: Aromaticity in boron clusters survives radical structural changes. J. Am. Chem. Soc. 2020, 142, 9396–9407. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Fujita, T.; Cuong, N.T.; Tominaka, S.; Miyauchi, M.; Iimura, S.; Hirata, A.; Umezawa, N.; Okada, S.; Nishibori, E.; et al. Formation and characterization of hydrogen boride sheets derived from MgB2 by cation exchange. J. Am. Chem. Soc. 2017, 139, 13761–13769. [Google Scholar] [CrossRef] [PubMed]
- Wrackmeyer, B.; Thoma, P.; Kempe, R.; Glatz, G. 9-Borafluorenes—NMR spectroscopy and DFT calculations. Molecular structure of 1,2-(2,2′-diphenylylene)-1,2-diethyldiborane. Collect. Czech. Chem. Commun. 2010, 75, 743–756. [Google Scholar] [CrossRef]
- Das, A.; Hübner, A.; Weber, M.; Bolte, M.; Lerner, H.-W.; Wagner, M. 9-H-9-Borafluorene dimethyl sulfide adduct: A product of a unique ring-contraction reaction and a useful hydroboration reagent. Chem. Commun. 2011, 47, 11339–11341. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.; Qu, Z.-W.; Englert, U.; Bolte, M.; Lerner, H.-W.; Holthausen, M.C.; Wagner, M. Main-chain boron-containing oligophenylenes via ring-opening polymerization of 9-H-9-borafluorene. J. Am. Chem. Soc. 2011, 133, 4596–4609. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.; Diefenbach, M.; Bolte, M.; Lerner, H.-W.; Holthausen, M.C.; Wagner, M. Confirmation of an early postulate: B-C-B two-electron–three-center bonding in organo(hydro)boranes. Angew. Chem. Int. Ed. 2012, 51, 12514–12518. [Google Scholar] [CrossRef] [PubMed]
- Kaese, T.; Hübner, A.; Bolte, M.; Lerner, H.-W.; Wagner, M. Forming B−B Bonds by the Controlled Reduction of a Tetraaryldiborane(6). J. Am. Chem. Soc. 2016, 138, 6224–6233. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Enrich, J.M.; Kondo, T.; Alkorta, I.; Elguero, J.; Klein, D.J. Concatenation of diborane leads to new planar boron chemistry. Chem. Phys. Chem. 2020. [Google Scholar] [CrossRef]
- Rashid, Z.; van Lenthe, J.H. Generation of Kekulé valence structures and the corresponding valence bond wave function. J. Comput. Chem. 2010, 32, 696–708. [Google Scholar] [CrossRef]
- Cram, D.J.; Tanner, M.E.; Thoams, R. The taming of cyclobutadiene. Angew. Chem. Int. Ed. 1991, 30, 1024–1027. [Google Scholar] [CrossRef]
- Liu, Y.; Kilby, P.; Frankcombe, T.J.; Schmidt, T.W. The electronic structure of benzene from a tiling of the correlated 126-dimensional wavefunction. Nature 2020, 11, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Dougherty, D.A. The cation–π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Rozas, I.; Elguero, J. Interaction of anions with perfluoro aromatic compounds. J. Am. Chem. Soc. 2002, 124, 8593–8598. [Google Scholar] [CrossRef]
- Geuenich, D.; Hess, K.; Köhler, F.; Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 2005, 105, 3758–3772. [Google Scholar] [CrossRef]
- Claus, K.H.; Krüger, C. Structure of cyclooctatetraene at 129 K. Acta Cryst. Sect. C 1988, 44, 1632–1634. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Pindelska, E.; Cyrański, M.K.; Häfelinger, G. Planarization of 1,3,5,7-cyclooctatetraene as a result of a partial rehybridization at carbon atoms: An MP2/6-31G* and B3LYP/6-311G** study. Chem. Phys. Lett. 2002, 359, 158–162. [Google Scholar] [CrossRef]
- Nishinaga, T.; Ohmae, T.; Iyoda, M. Recent studies on the aromaticity and antiaromaticity of planar cyclooctatetraene. Symmetry 2010, 2, 76. [Google Scholar] [CrossRef] [Green Version]
- Bally, T.; Chai, S.; Neuenschwander, M.; Zhu, Z. Pentalene: Formation, electronic, and vibrational structure. J. Am. Chem. Soc. 1997, 119, 1869–1875. [Google Scholar] [CrossRef]
- Hafner, K.; Süss, H.U. 1,3,5-Tri-tert-butylpentalene. A stabilized planar 8π electron system. Angew. Chem. Int. Ed. Engl. 1973, 12, 575–577. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry. Part A: Structure and Mechanisms, 2nd ed.; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- Mosher, O.A.; Flicker, W.M.; Kuppermann, A. Triplet states in 1,3-butadiene. Chem. Phys. Lett. 1973, 19, 332–333. [Google Scholar] [CrossRef]
- Gordon, M. The Azulenes. Chem. Rev. 1952, 50, 127–200. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, J.R.V.E. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]









| Cyclobutadiene C(4−2k)B(2k)H(4+2k) | ||||
| Formula (k) | C4H4 (0) | C2B2H6 (1) | B4H8 (2) | |
| Structure |  |  |  | |
| Energy | −154.734983 | −129.488436 | −104.182558 | |
| ΔEST,B3LYP (PGS) | 133.5 (D2h) | 272.9 (C2v) | 303.6 (D2h) | |
| ΔEST,B97D | 124.8 | 265.2 | 272.3 | |
| Benzene C(6−2k)B(2k)H(6+2k) | ||||
| C6H6 (0) | C4B2H8 (1) | C2B4H10 (2) | B6H12 (3) | |
 |  |  |  | |
 |  |  |  | |
 |  |  |  | |
| −232.333368 | −206.929239 | −181.596841 | −156.324833 | |
| 428.0 (D6h) | 325.9 (C2v) | 361.2 (C2v) | 419.8 (D3h) | |
| 414.4 | 312.9 | 353.1 | 373.2 | |
| Cyclooctatetraene C(8−2k)B(2k)H(8+2k) | ||||
| C8H8 (0) | C6B2H10 (1) | C4B4H12 (2) | C2B6H14 (3) | B8H16 (4) |
 |  |  |  |  |
| −309.697808 | −284.398420 | −259.096253 | −233.769903 | −208.442935 |
| 264.4 (D2d) | 312.2 (Cs) | (I) 377.1 (C2v) | 375.3 (Cs) | 433.1 (D2d) |
| 234.3 | 284.1 | 344.2 | 364.4 | 381.0 |
 | ||||
| −259.084995 | ||||
| (II) 355.7 (C2) | ||||
| 332.9 | ||||
| Pentalene C(8−2k)B(2k)H(6+2k) | ||||
|---|---|---|---|---|
| C8H6 (0) | C6B2H8 (1) | C4B4H10 (2) | C2B6H12 (3) | B8H14 (4) |
 |  |  |  |  |
| −308.476075 | −283.193095 | −257.898516 | −232.562207 | −207.215121 |
| 100.1 (C2h) | (I) 176.7 (Cs) | (I) 232.3 (C2h) | (I) 292.9 (Cs) | 257.3 (C2h) |
 |  |  | ||
| −283.179450 | −257.881067 | −232.544645 | ||
| (II) 140.2 (Cs) | (II) 222.5 (Cs) | (II) 290.4 (Cs) | ||
 | ||||
| −257.869529 | ||||
| (III) 189.9 (Cs) | ||||
 | ||||
| −257.866865 | ||||
| (IV) 286.8 (C2h) | ||||
| (a) | ||||
| Benzocyclobutadiene Kekulé Structure K1: C(8−2k)B(2k)H(6+2k) | ||||
| C8H6 (0) | C6B2H8 (1) | C4B4H10 (2) | C2B6H12 (3) | B8H14 (4) |
 |  |  |  |  |
| −308.466884 | −283.206069 | −257.868229 | −232.534049 | −207.187765 |
| 195.3 (C2v) | (I) 303.0 (C2v) | (I) 225.8 (Cs) | (I) 361.7 (C2v) | 264.0 (C2v) |
 |  |  | ||
| −283.141948 | −257.847770 | −232.521784 | ||
| (II) 285.1 (C2v) | (II) 338.7 (C2v) | (II) 308.5 (Cs) | ||
 |  |  | ||
| −283.125618 | −257.820144 | −232.488989 | ||
| (III) 117.7 (Cs) | (III) 265.9 (Cs) | (III) 254.3 (C2v) | ||
 | ||||
| −257.791707 | ||||
| (IV) 108.4 (C2v) | ||||
| (b) | ||||
| Benzocyclobutadiene Kekulé Structure K2: C(8−2k)B(2k)H(6+2k) | ||||
| C6H6 (0) | C6B2H8 (1) | C4B4H10 (2) | C2B6H12 (3) | B6H14 (4) |
 |  |  |  |  |
| −308.466884 | −283.206069 | −257.880840 | −232.542344 | −207.194531 |
| 195.3 (C2v) | (I) C2v 302.9 = (I) in K1 | (I) C2v 367.5 | (I) Cs 342.3 | C2v 310.3 |
 |  |  | ||
| −283.162243 | −257.869238 | −232.529224 | ||
| (II) C2v 267.2 | (II) Cs 280.1 | (II) C2v 323.8 | ||
 |  |  | ||
| −283.161645 | −257.848002 | −232.517824 | ||
| (III) Cs 220.2 | (III) Cs 280.4 | (III) C2v 348.8 | ||
 | ||||
| −257.842989 | ||||
| (IV) C2v 328.5 | ||||
| (c) | ||||
| Benzocyclobutadiene Kekulé Structure K3: C(8−2k)B(2k)H(6+2k) | ||||
| C8H6 (0) | C6B2H8 (1) | C4B4H10 (2) | C2B6H12 (3) | B6H14 (4) |
 |  |  |  |  |
| −308.466884 | −283.151132 | −257.848644 | −232.527701 | −207.196234 |
| 195.3 (C2v) | (I) 137.7 (Cs) | (I) 229.1 (Cs) | (I) 210.9 (Cs) | 374.0 (C2v) |
 |  |  | ||
| −283.125618 | −257.841430 | −232.517894 | ||
| (II) 117.6 (Cs) | (II) 219.6 (C2v) | (II) 237.0 (Cs) | ||
 | ||||
| −257.838383 | ||||
| (III) 173.8 (Cs) | ||||
 | ||||
| −257.791707 | ||||
| (IV) 108.5 (C2v) | ||||
| (a) | |||||
| Naphthalene Kekulé Structure K1: C(10−2k)B(2k)H(8+2k) | |||||
| C10H8 (0) | C8B2H10 (1) | C6B4H12 (2) | C4B6H14 (3) | C2B8H16 (4) | B10H18 (5) |
 |  |  |  |  |  |
| −386.025545 | −360.701217 | −335.372607 | −310.030119 | −284.691550 | −259.335521 |
| 304.3 (D2h) | (I) 325.9 (Cs) | (I) 414.4 (C2v) | (I) 287.8 (Cs) | (I) 316.1 (D2h) | 313.6 (D2h) |
 |  |  |  | ||
| −360.673209 | −335.365370 | −310.015638 | −284.676167 | ||
| (II) 340.7 (D2h) | (II) 266.4 (C2h) | (II) 393.0 (C2v) | (II) 353.7 (Cs) | ||
 |  | ||||
| −335.364766 | −310.013813 | ||||
| (III) 300.4 (C2v) | (III) 385.0 (C2h) | ||||
 |  | ||||
| −335.346472 | −310.011145 | ||||
| (IV) 322.7 (Cs) | (IV) 327.6 (C2v) | ||||
| (b) | |||||
| Naphthalene Kekulé Structure K2: C(10−2k)B(2k)H(8+2k) | |||||
| C8B2H10 (1) | C6B4H12 (2) | C4B6H14 (3) | C2B8H16 (4) | B10H18 (5) | |
 |  |  |  |  | |
| −360.701217 | −335.372607 | −310.028387 | −284.687476 | −259.338265 | |
| (I) 325.9 (Cs) = (I) in K1 | (I) 414.4 (C2v) = (I) in K1 | (I) 297.5 (C2v) | (I) 331.2 (Cs) | 307.0 (C2v) | |
 |  |  |  | ||
| −360.678565 | −335.362684 | −310.025977 | −284.679641 | ||
| (II) 223.1 (Cs) | (II) Cs 303.3 | (II) 301.6 (Cs) | (II) C2v 351.0 | ||
 |  |  |  | ||
| −360.675945 | −335.356631 | −310.014490 | −284.675123 | ||
| (III) C2v 187.8 | (III) 256.1 (Cs) | (III) Cs 251.0 | (III) 313.2 (Cs) | ||
 |  | ||||
| −335.352046 | −310.014211 | ||||
| (IV) 244.0 (Cs) | (IV) 333.7 (Cs) | ||||
 |  | ||||
| −335.335292 | −309.998099 | ||||
| (V) C2v 252.3 | (V) C2v 234.9 | ||||
| Azulene C(10−2k)B(2k)H(8+2k) | |||
| C10H8 (0) | C8B2H10 (1) | C6B4H12 (2) | C4B6H14 (3) |
 |  |  |  |
| −385.971486 | −360.664485 | −335.342898 | −310.017400 |
| 196.3 (C2v) | (I) 224.4 (Cs) | (I) 260.7 (Cs) | (I) 329.9 (Cs) |
 |  |  | |
| −360.653691 | −335.338375 | −310.010310 | |
| (II) 180.5 (Cs) | (II) 235.9 (Cs) | (II) 252.5 (Cs) | |
 |  |  | |
| −360.644455 | −335.335924 | −310.009032 | |
| (III) 203.1 (Cs) | (III) 207.6 (Cs) | (III) 335.1 (Cs) | |
 |  |  | |
| −360.643019 | −335.333474 | −310.006879 | |
| (IV) 183.3 (Cs) | (IV) 239.7 (Cs) | (IV) 248.1 (Cs) | |
 |  |  | |
| −360.637439 | −335.332218 | −310.005650 | |
| (V) 179.7 (Cs) | (V) 212.6 (Cs) | (V) 250.4 (Cs) | |
| Azulene C(10−2k)B(2k)H(8+2k) | |||
| C6B4H12 (2) | C4B6H14 (3) | ||
 |  | ||
| −335.324248 | −309.999674 | ||
| (VI) 219.3 (Cs) | (VI) 327.6 (Cs) | ||
 |  | ||
| −335.321141 | −309.999405 | ||
| (VII) 272.0 (Cs) | (VII) 319.5 (Cs) | ||
 |  | ||
| −335.320601 | −309.996626 | ||
| (VIII) 217.0 (Cs) | (VIII) 249.8 (Cs) | ||
 |  | ||
| −335.319266 | −309.992937 | ||
| (IX) 208.9 (Cs) | (IX) 210.9 (Cs) | ||
 |  | ||
| −335.313370 | −309.989768 | ||
| (X) 163.1 (Cs) | (X) 262.6 (Cs) | ||
| Azulene C(10−2k)B(2k)H(8+2k) | |||
| C2B8H16 (4) | B10H18 (5) | ||
 |  | ||
| −284.679284 | −259.332171 | ||
| (I) 315.4 (Cs) | (I) 293.3 (Cs) | ||
 | |||
| −284.675773 | |||
| (II) 311.6 (Cs) | |||
 | |||
| −284.667945 | |||
| (III) 320.1 (Cs) | |||
 | |||
| −284.666066 | |||
| (IV) 317.1 (Cs) | |||
 | |||
| −284.666006 | |||
| (V) 313.2 (Cs) | |||
| d(C-C) | d(C=C) | d(C-H) | d(B-B) | d(B(Hb)2B) | d(B-H) | d(Hb-Hb) | |
| Ethylene | --- | 1.324 | 1.083 | --- | --- | --- | --- |
| 1.332 | 1.089 | ||||||
| Diborane(6) | --- | --- | --- | 1.758 | 1.185 | 1.948 | |
| 1.777 | 1.195 | 1.973 | |||||
| Cyclobutadiene (k) | d(C-C) | d(C=C) | d(C-H) | d(B-B) | d(B(Hb)2B) | d(B-H) | d(Hb-Hb) |
| C4H4 (0) | 1.575 | 1.329 | 1.079 | --- | --- | --- | --- |
| 1.582 | 1.337 | 1.085 | |||||
| C2B2H6 (1) | --- | 1.338 | 1.083 | --- | 1.741 | 1.185 | 1.858 |
| 1.347 | 1.09 | 1.749 | 1.194 | 1.886 | |||
| B4H8 (2) | --- | --- | --- | 1.728 | 1.76 | 1.189 | 1.856 |
| 1.739 | 1.771 | 1.199 | 1.883 | ||||
| Benzene (k) | d(C-C) | d(C=C) | d(C-H) | d(B-B) | d(B(Hb)2B) | d(B-H) | d(Hb-Hb) |
| C6H6 (0) | 1.391 | 1.391 | 1.082 | --- | --- | --- | --- |
| 1.399 | 1.399 | 1.087 | |||||
| C4B2H8 (1) | 1.439 | 1.359 | 1.085 | --- | 1.799 | 1.186 | 1.926 |
| 1.442 | 1.369 | 1.09 | 1.823 | 1.196 | 1.951 | ||
| C2B4H10 (2) | --- | 1.35 | 1.088 | 1.695 | 1.789 | 1.188 | 1.926 |
| 1.359 | 1.094 | 1.708 | 1.812 | 1.198 | 1.952 | ||
| B6H12 (3) | --- | --- | --- | 1.713 | 1.793 | 1.19 | 1.923 |
| 1.724 | 1.812 | 1.2 | 1.948 | ||||
| Cyclooctatetraene (k) | d(C-C) | d(C=C) | d(C-H) | d(B-B) | d(B(Hb)2B) | d(B-H) | d(Hb-Hb) |
| C8H8 (0) | 1.469 | 1.335 | 1.087 | --- | --- | --- | --- |
| 1.471 | 1.343 | 1.092 | |||||
| C6B2H10 (1) | 1.47 | 1.338 | 1.088 | --- | 1.77 | 1.191 | 1.944 |
| 1.471 | 1.347 | 1.093 | 1.788 | 1.202 | 1.972 | ||
| C4B4H12 (2) (I) | --- | 1.341 | 1.09 | --- | 1.785 | 1.19 | 1.94 |
| 1.349 | 1.096 | 1.804 | 1.202 | 1.966 | |||
| C4B4H12 (2) (II) | 1.475 | 1.34 | 1.089 | 1.694 | 1.776 | 1.191 | 1.941 |
| 1.476 | 1.348 | 1.095 | 1.706 | 1.791 | 1.202 | 1.969 | |
| C2B6H14 (3) | --- | 1.343 | 1.091 | 1.696 | 1.777 | 1.191 | 1.938 |
| 1.351 | 1.096 | 1.706 | 1.796 | 1.202 | 1.964 | ||
| B8H16 (4) | --- | --- | --- | 1.698 | 1.786 | 1.191 | 1.934 |
| 1.708 | 1.8 | 1.202 | 1.96 |
| Cyclobutadiene | NICS(0) | NICS(1) | Benzene | NICS(0) | NICS(1) |
|---|---|---|---|---|---|
 | 26.8 | 17.4 |  | −8.2 | −10.4 |
 | 10.2 | 6.3 |  | −3.6 | −5.9 |
 | 4.7 | 2.4 |  | 0.5 | −1.4 |
 | 2.2 | 1.0 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva-Enrich, J.M.; Alkorta, I.; Elguero, J. Hybrid Boron-Carbon Chemistry. Molecules 2020, 25, 5026. https://doi.org/10.3390/molecules25215026
Oliva-Enrich JM, Alkorta I, Elguero J. Hybrid Boron-Carbon Chemistry. Molecules. 2020; 25(21):5026. https://doi.org/10.3390/molecules25215026
Chicago/Turabian StyleOliva-Enrich, Josep M., Ibon Alkorta, and José Elguero. 2020. "Hybrid Boron-Carbon Chemistry" Molecules 25, no. 21: 5026. https://doi.org/10.3390/molecules25215026