Development of Taccalonolide AJ-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Treatment of Clear Cell Renal-Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Optimization, Preparation, and Characterization of AJ-HP-β-CD Inclusion Complexes
2.1.1. Optimization of Cyclodextrins for AJ-HP-β-CD Inclusion Complexes
2.1.2. Preparation of AJ-HP-β-CD Inclusion Complexes
2.1.3. Characterization of AJ-HP-β-CD Inclusion Complexes
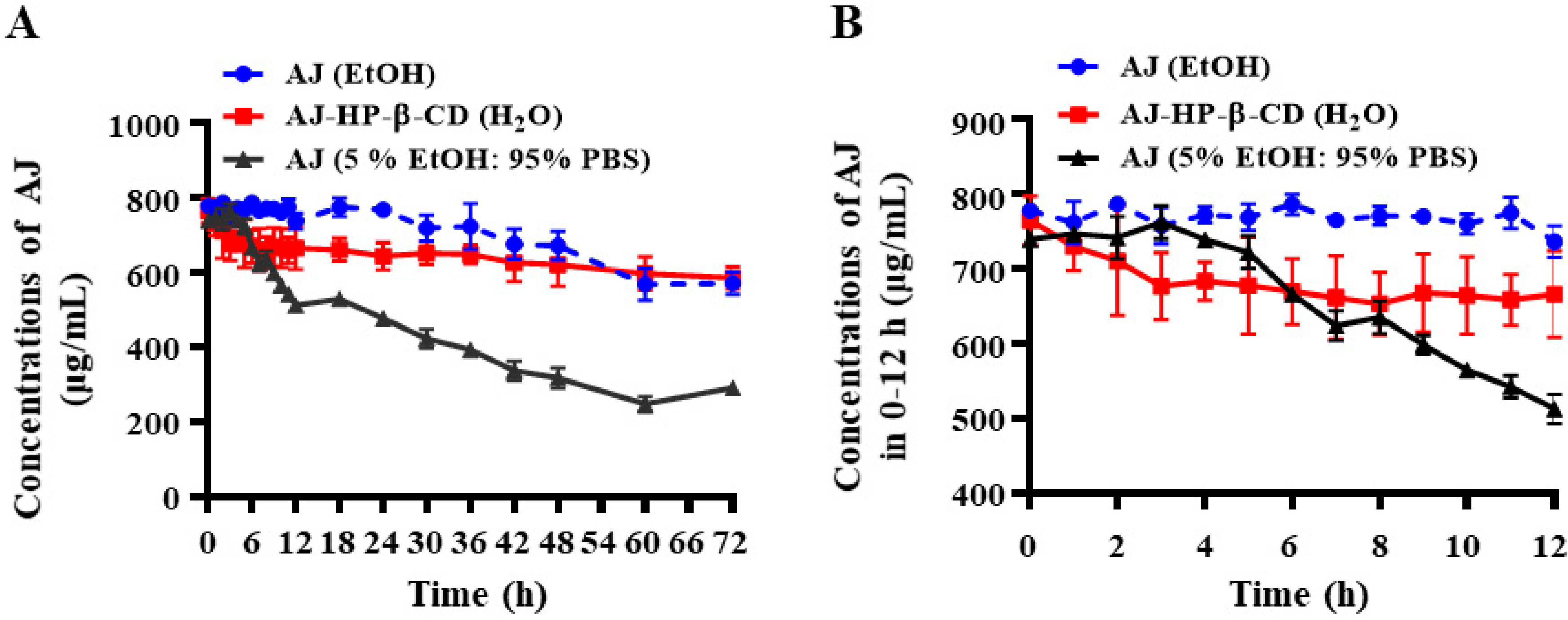
2.2. The Stability of AJ-HP-β-CD
2.3. In Vitro Cytotoxicity Study of AJ-HP-β-CD
2.4. AJ-HP-β-CD Inhibits 786-O Cell Invasion and Migration In Vitro
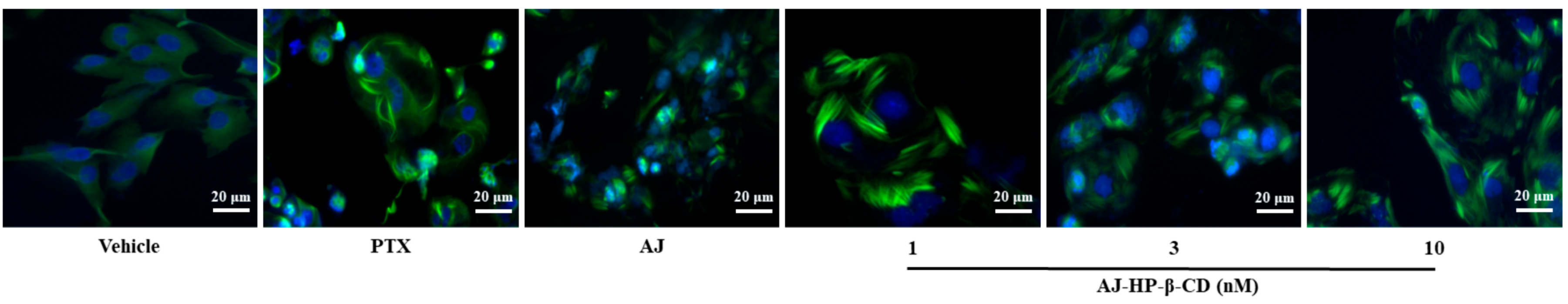
2.5. AJ-HP-β-CD Promotes Microtubule Accumulation in 786-O Cells
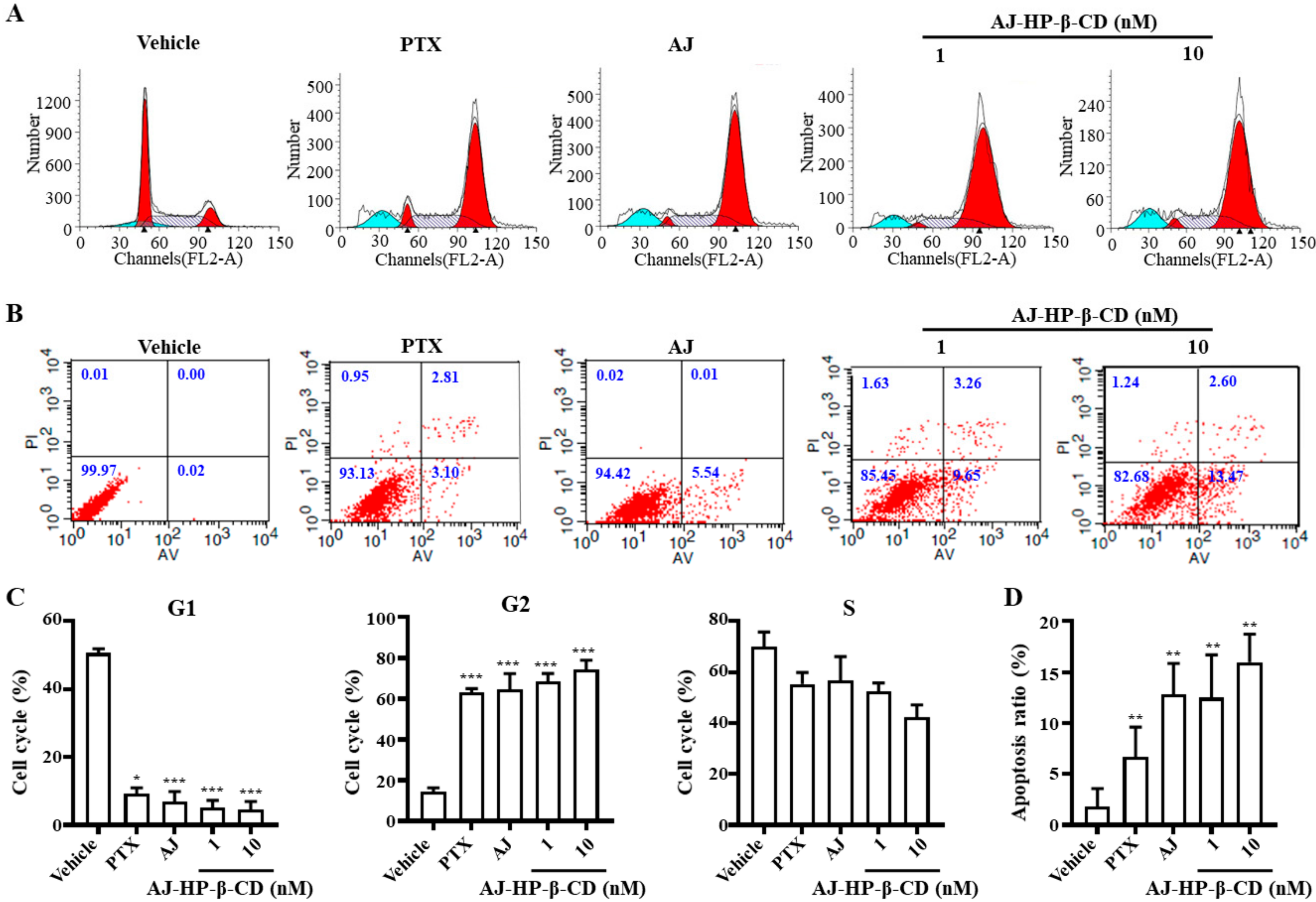
2.6. AJ-HP-β-CD Arrests 786-O Cells in G2/M Phase and Induces Cell Apoptosis
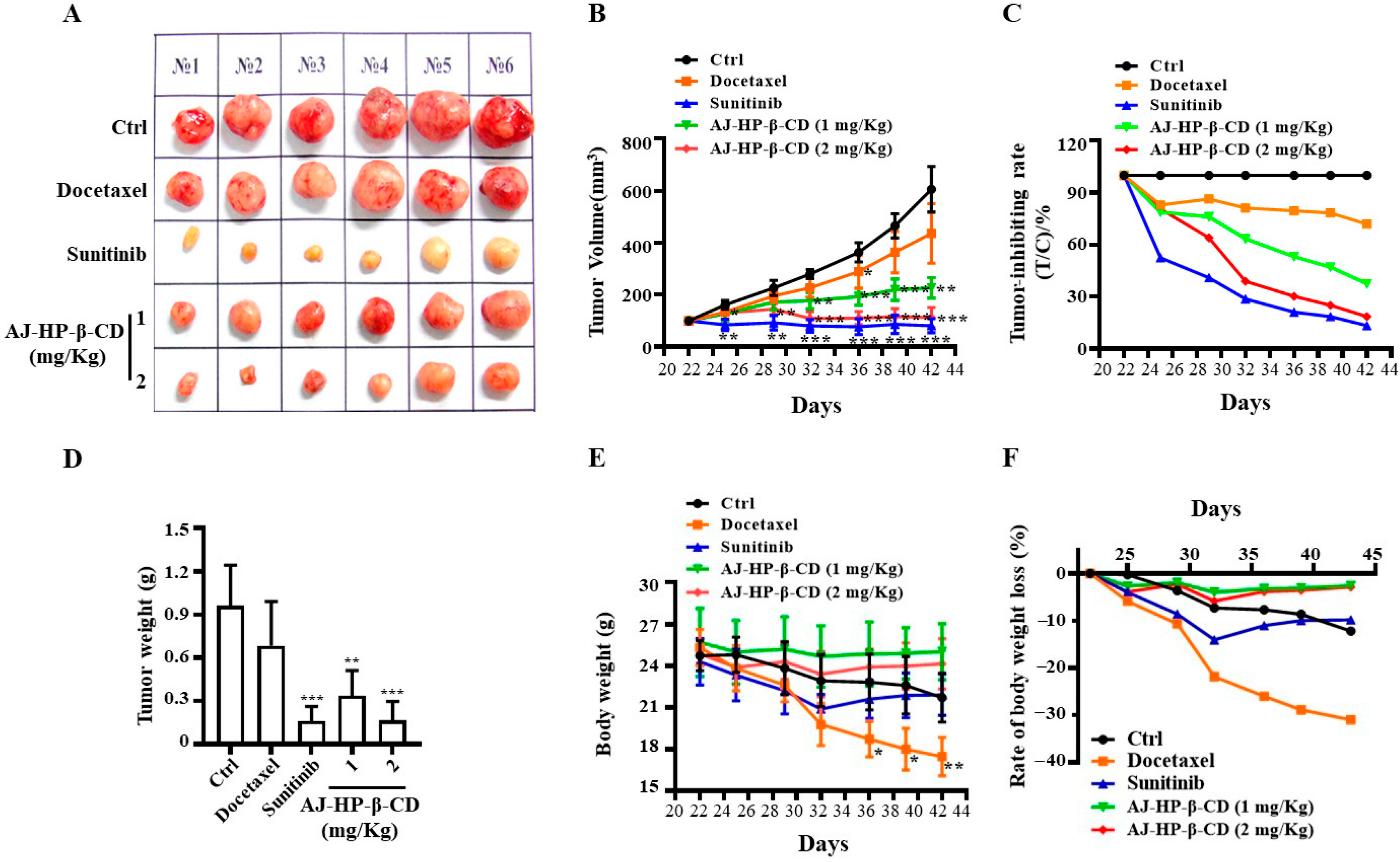
2.7. AJ-HP-β-CD Inhibits 786-O Xenograft Growth in Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Detection Method and Content Standard Curve of AJ
4.3. Phase Solubility and Inclusion Rate Study of the AJ-HP-β-CD Complex
4.4. Preparation of the AJ-HP-β-CD Complex
4.5. The Stability of AJ-HP-β-CD and AJ
4.6. Material Characterization of the AJ-HP-β-CD Complex
4.7. Cell Lines and Cell Culture
4.8. Cell Proliferation Assay
4.9. Cell Invasion and Migration Assays
4.10. Immunofluorescence Assays
4.11. Cell Cycle Distribution and Apoptosis Analysis
4.12. Xenografts
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Z.-L.; Wang, B.-D.; Chen, M.-Q. Steroidal bitter principles from tacca plantaginea structures of taccalonolide A and B. Tetrahedron Lett. 1987, 28, 1673–1675. [Google Scholar] [CrossRef]
- Tinley, T.L.; Randall-Hlubek, D.A.; Leal, R.M.; Jackson, E.M.; Cessac, J.W.; Quada, J.C.; Hemscheidt, T.K.; Mooberry, S.L. Taccalonolides E and A: Plant-derived steroids with microtubule-stabilizing activity. Cancer Res. 2003, 63, 3120–3211. [Google Scholar] [PubMed]
- Li, J.; Risinger, A.L.; Mooberry, S.L. Taccalonolide microtubule stabilizers. Bioorg. Med. Chem. 2014, 22, 5091–5096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Risinger, A.L.; Fest, G.A.; Jackson, E.M.; Helms, G.; Polin, L.A.; Mooberry, S.L. Identification and Biological Activities of New Taccalonolide Microtubule Stabilizers. J. Med. Chem. 2011, 54, 6117–6124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; Leboeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Ludueña, R.F.; Kruh, G.D.; Mooberry, S.L. The Taccalonolides: Microtubule Stabilizers That Circumvent Clinically Relevant Taxane Resistance Mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risinger, A.L.; Li, J.; Bennett, M.J.; Rohena, C.C.; Peng, J.; Schriemer, D.C.; Mooberry, S.L. Taccalonolide Binding to Tubulin Imparts Microtubule Stability and Potent In Vivo Activity. Cancer Res. 2013, 73, 6780–6792. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Risinger, A.L.; Peng, J.; Chen, Z.; Hu, L.; Mooberry, S.L. Potent Taccalonolides, AF and AJ, Inform Significant Structure–Activity Relationships and Tubulin as the Binding Site of These Microtubule Stabilizers. J. Am. Chem. Soc. 2011, 133, 19064–19067. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, Y.; Li, G.-B.; Li, S.-A.; Wu, C.; Gigant, B.; Qin, W.; Chen, H.; Wu, Y.; Chen, Q.; et al. Mechanism of microtubule stabilization by taccalonolide AJ. Nat. Commun. 2017, 8, 15787. [Google Scholar] [CrossRef]
- Higashi, T. Cyclodextrin-Based Molecular Accessories for Drug Discovery and Drug Delivery. Chem. Pharm. Bull. 2019, 67, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Q.; Zhang, C.; Hao, J.; Xing, P.; Su, J.; Li, S.; Hao, A.; Liu, G. Self-Assembled Vesicles Prepared from Amphiphilic Cyclodextrins as Drug Carriers. Langmuir 2012, 28, 8625–8636. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, W.; Deleye, S.; Staelens, S.; De Smet, L.; Van Damme, N.; DeBergh, I.; Ceelen, W.P.; De Vos, F.; Remon, J.P.; Vervaet, C. Antitumour Efficacy of Two Paclitaxel Formulations for Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in an In Vivo Rat Model. Pharm. Res. 2011, 28, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Patrulea, V.; Borchard, G.; Bilensoy, E. Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model. Nanomaterials 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Shen, Y.; Jin, F.; Du, Y.-Z.; Ying, X.-Y. Paclitaxel/hydroxypropyl-β-cyclodextrin complex-loaded liposomes for overcoming multidrug resistance in cancer chemotherapy. J. Liposome Res. 2019, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.P.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef]
- Tan, M.-H.; Kanesvaran, R. Targeted therapy for renal cell carcinoma: The next lap. J. Carcinog. 2014, 13, 3. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuang, Q.; Cheng, K.; Ming, Y.; Zhao, Y.; Ye, Q.; Zhang, S. Long non-coding RNA TCL6 enhances preferential toxicity of paclitaxel to renal cell carcinoma cells. J. Cancer 2020, 11, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Scripture, C.D.; Figg, W.D.; Sparreboom, A. Paclitaxel chemotherapy: From empiricism to a mechanism-based formulation strategy. Ther. Clin. Risk Manag. 2005, 1, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Westerdijk, K.; Krens, S.D.; Van Der Graaf, W.T.; Mulder, S.F.; Van Herpen, C.M.; Smilde, T.; Van Erp, N.P.; Desar, I.M. The relationship between sunitinib exposure and both efficacy and toxicity in real-world patients with renal cell carcinoma and gastrointestinal stromal tumour. Br. J. Clin. Pharmacol. 2020, 1–10. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.; Wang, M.; Luo, J.-M.; Wang, S.-Y.; Zhang, Y.-P.; Zhang, Y.-J. Optimization of Early Response Monitoring and Prediction of Cancer Antiangiogenesis Therapy via Noninvasive PET Molecular Imaging Strategies of Multifactorial Bioparameters. Theranostics 2016, 6, 2084–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Yin, P.; Li, L.; Kang, G.; Ning, G.; Cao, Y.; Gao, F.; Su, Y.; Wu, Y.; Zhang, X. Knockdown of Matrix Metallopeptidase 9 Inhibits Metastasis of Oral Squamous Cell Carcinoma Cells in a Zebrafish Xenograft Model. BioMed Res. Int. 2020, 2020, 4350783. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qin, W.; Lu, S.; Wang, X.; Zhang, J.; Sun, T.; Hu, X.; Li, Y.; Chen, Q.; Wang, Y.; et al. Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2′-O-methylation via NOP58 recruitment in colorectal cancer. Mol. Cancer 2020, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wobser, M.; Weber, A.; Glunz, A.; Tauch, S.; Seitz, K.; Butelmann, T.; Hesbacher, S.; Goebeler, M.; Bartz, R.; Kohlhof, H.; et al. Elucidating the mechanism of action of domatinostat (4SC-202) in cutaneous T cell lymphoma cells. J. Hematol. Oncol. 2019, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.-A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Risinger, A.L.; Li, J.; Du, L.; Benavides, R.; Robles, A.J.; Cichewicz, R.H.; Kuhn, J.G.; Mooberry, S.L. Pharmacokinetic Analysis and in Vivo Antitumor Efficacy of Taccalonolides AF and AJ. J. Nat. Prod. 2017, 80, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Shi, W.; Tang, T.; Wang, Y.; Yin, X.; Chen, Y.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. miR-29a contributes to breast cancer cells epithelial–mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis. 2019, 10, 176. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Augello, F.R.; Giusti, I.; Dolo, V.; Leocata, P.; Cifone, M.G.; Cinque, B. Biological effects of selective COX-2 inhibitor NS398 on human glioblastoma cell lines. Cancer Cell Int. 2020, 20, 167. [Google Scholar] [CrossRef]


| Cell Line | Origin | IC50 (nM) | |||
|---|---|---|---|---|---|
| Taccalonolide B | Taccalonolide AJ | PTX | AJ-HP-β-CD | ||
| A2780 | Epithelial ovarian cancer | >30,000 | 6.18 ± 0.446 | 1.87 ± 0.37 | 2.06 ± 0.13 |
| 786-O | Primary clear renal cell carcinoma | 26,100.27 | 16.01 ± 1.54 | 4.85 ± 0.46 | 3.51 ± 0.79 |
| ACHN | Metastatic papillary renal cell carcinoma | >30,000 | 35.96 ± 1.08 | 5.57 ± 0.28 | 9.82 ± 1.02 |
| HepG2 | Hepatocellular carcinoma | >30,000 | 9.49 ± 0.64 | 2.94 ± 0.25 | 1.90 ± 0.23 |
| Hep-3B | Hepatocellular carcinoma | >30,000 | 8.36 ± 0.28 | 4.50 ± 0.57 | 1.90 ± 0.06 |
| Huh-7 | Hepatocellular carcinoma | >30,000 | 9.96 ± 0.21 | 5.18 ± 0.03 | 3.09 ± 0.20 |
| HCT116 | Human colorectal carcinoma | >30,000 | 14.62 ± 1.13 | 3.83 ± 0.35 | 4.24 ± 0.51 |
| MCF-7 | Human breast carcinoma | >30,000 | 23.87 ± 1.71 | 3.41 ± 0.69 | 16.54 ± 0.49 |
| Group | Ctrl | Docetaxel, 10, QW | Sunitinib, | AJ-HP-β-CD | AJ-HP-β-CD |
|---|---|---|---|---|---|
| 80→40, QD | (1 mg/Kg, QW) | (2 mg/Kg, QW) | |||
| Day22 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Day25 | 160.76 ± 15.35 | 133.20 ± 13.34 | 84.30 ± 21.76 ** | 126.70 ± 29.78 | 129.69 ± 24.15 |
| Day29 | 226.117 ± 26.82 | 195.30 ± 22.50 | 92.96 ± 27.57 ** | 171.30 ± 30.29 ** | 144.59 ± 20.81 |
| Day32 | 279.26 ± 16.41 | 226.60 ± 36.36 | 80.60 ± 24.80 *** | 177.90 ± 31.69 *** | 108.59 ± 25.76 ** |
| Day36 | 363.38 ± 34.04 | 288.90 ± 64.19 * | 76.40 ± 30.65 *** | 192.31 ± 32.03 *** | 109.99 ± 25.49 *** |
| Day39 | 464.99 ± 42.54 | 364.20 ± 80.19 | 86.19 ± 35.07 *** | 218.52 ± 41.42 *** | 116.39 ± 30.53 *** |
| Day42 | 606.04 ± 80.43 | 435.60 ± 114.31 | 80.18 ± 26.90 *** | 226.30 ± 39.39 *** | 112.39 ± 38.61 *** |
Sample Availability: Samples of the compounds of AJ, TA, TB are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Zhang, S.; Niu, J.; Zhang, C.; Dai, W.; Wu, Y.; Hu, L. Development of Taccalonolide AJ-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Treatment of Clear Cell Renal-Cell Carcinoma. Molecules 2020, 25, 5586. https://doi.org/10.3390/molecules25235586
Han J, Zhang S, Niu J, Zhang C, Dai W, Wu Y, Hu L. Development of Taccalonolide AJ-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Treatment of Clear Cell Renal-Cell Carcinoma. Molecules. 2020; 25(23):5586. https://doi.org/10.3390/molecules25235586
Chicago/Turabian StyleHan, Jing, Siwang Zhang, Junxin Niu, Chunli Zhang, Weichen Dai, Yuanyuan Wu, and Lihong Hu. 2020. "Development of Taccalonolide AJ-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Treatment of Clear Cell Renal-Cell Carcinoma" Molecules 25, no. 23: 5586. https://doi.org/10.3390/molecules25235586





