A Review: Halogenated Compounds from Marine Fungi
Abstract
:1. Introduction
2. Halogenated Compounds from Penicillium sp.
2.1. Sponges-Associated Penicillium sp.
2.2. Other Marine Animals-Associated Penicillium sp.
2.3. Marine Algae-Associated Penicillium sp.
2.4. Mangroves-Associated Penicillium sp.
2.5. Penicillium sp. from Marine Sediments
2.6. Penicillium sp. from Other Marine Sources
3. Halogenated Compounds from Aspergillus sp.
3.1. Sponges-Associated Aspergillus sp.
3.2. Other Marine Animals-Associated Aspergillus sp.
3.3. Marine Algae-Associated Aspergillus sp.
3.4. Aspergillus sp. from Marine Sediments
3.5. Aspergillus sp. from Other Marine Sources
4. Halogenated Compounds from Other Marine Fungi
4.1. Other Sponges-Associated Fungi
4.2. Other Marine Animals-Associated Fungi
4.3. Other marine Algae-Associated Fungi
4.4. Other Mangroves-Associated Fungi
4.5. Other Marine Plants-Associated Fungi
4.6. Other Marine Sediments-Associated Fungi
4.7. Other Marine Source-Associated Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, C.; Zhu, T.; Zhu, W. New marine natural products of microbial origin from 2010 to 2013. Chin. J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.; Sandy, M. Mechanistic considerations of halogenating enzymes. Nature 2009, 460, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Ballschmiter, K. Review: Pattern and sources of naturally produced organohalogens in the marine environment: Biogenic formation of organohalogens. Chemosphere 2003, 52, 313–324. [Google Scholar] [CrossRef]
- Gribble, G.-W. The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289–297. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hao, J.-D.; Ning, X.-Y.; Wu, J.-S.; Zhao, D.-L.; Kong, C.-J.; Shao, C.-L.; Wang, C.-Y. Penicilazaphilones D and E: Two new azaphilones from a sponge-derived strain of the fungus Penicillium sclerotiorum. RSC Adv. 2018, 8, 4348–4353. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.; Hartmann, R.; Plenker, M.; Mándi, A.; Kurtán, T.; Özkaya, F.-C.; Müller, W.-E.; Kassack, M.-U.; Hamacher, A.; Lin, W.; et al. Brominated azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14. 6a. J. Nat. Prod. 2019, 82, 2159–2166. [Google Scholar] [CrossRef]
- Kong, F.-D.; Ma, Q.-Y.; Huang, S.-Z.; Wang, P.; Wang, J.-F.; Zhou, L.-M.; Yuan, J.-Z.; Dai, H.-F.; Zhao, Y.-X. Chrodrimanins K–N and related meroterpenoids from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm, Sipunculus nudus. J. Nat. Prod. 2017, 80, 1039–1047. [Google Scholar] [CrossRef]
- Kong, F.-D.; Zhang, R.-S.; Ma, Q.-Y.; Xie, Q.-Y.; Wang, P.; Chen, P.-W.; Zhou, L.-M.; Dai, H.-F.; Luo, D.-Q.; Zhao, Y.-X. Chrodrimanins O–S from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm, Sipunculus nudus. Fitoterapia 2017, 122, 1–6. [Google Scholar] [CrossRef]
- Yang, G.; Yun, K.; Nenkep, V.-N.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. Induced production of halogenated diphenyl ethers from the marine-derived fungus Penicillium chrysogenum. Chem. Biodivers. 2010, 7, 2766–2770. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Feng, H.; Dai, J.; Li, J.; Che, Q.; Gu, Q.; Zhu, T.; Li, D. Penicisulfuranols A–F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 2017, 80, 71–75. [Google Scholar] [CrossRef]
- He, K.-Y.; Zhang, C.; Duan, Y.-R.; Huang, G.-L.; Yang, C.-Y.; Lu, X.-R.; Zheng, C.-J.; Chen, G.-Y. New chlorinated xanthone and anthraquinone produced by a mangrove-derived fungus Penicillium citrinum HL-5126. J. Antibiot. 2017, 70, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium Terrestre. J. Nat. Prod. 2008, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, L.; Zhu, T.; Kurtán, T.; Mándi, A.; Zhao, Z.; Li, J.; Gu, Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium Terrestre. Tetrahedron 2011, 67, 7913–7918. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Lin, A.; Gu, Q.; Zhu, T.; Li, D. Four new chloro-eremophilane sesquiterpenes from an Antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 2013, 11, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Cardoso-Martínez, F.; José, M.; Díaz-Marrero, A.-R.; Darias, J.; Cerella, C.; Diederich, M.; Cueto, M. Tanzawaic acids isolated from a marine-derived fungus of the genus Penicillium with cytotoxic activities. Org. Biomol. Chem. 2015, 13, 7248–7256. [Google Scholar] [CrossRef]
- Luo, M.; Cui, Z.; Huang, H.; Song, X.; Sun, A.; Dang, Y.; Lu, L.; Ju, J. Amino acid conjugated anthraquinones from the marine-derived fungus Penicillium sp. SCSIO sof101. J. Nat. Prod. 2017, 80, 1668–1673. [Google Scholar] [CrossRef]
- Chen, M.; Shen, N.-X.; Chen, Z.-Q.; Zhang, F.-M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.-Y.; Chen, Z.-Q.; Shen, N.-X.; Shen, L.; Zhang, F.-M.; Zhou, X.-J.; Wang, C.-Y. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374. [Google Scholar] [CrossRef]
- Vansteelandt, M.; Blanchet, E.; Egorov, M.; Petit, F.; Toupet, L.; Bondon, A.; Monteau, F.; Le Bizec, B.; Thomas, O.-P.; Pouchus, Y.-F. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium strain. J. Nat. Prod. 2013, 76, 297–301. [Google Scholar] [CrossRef]
- Blanchet, E.; Vansteelandt, M.; Le Bot, R.; Egorov, M.; Guitton, Y.; Pouchus, Y.-F.; Grovel, O. Synthesis and antiproliferative activity of ligerin and new fumagillin analogs against osteosarcoma. Eur. J. Med. Chem. 2014, 79, 244–250. [Google Scholar] [CrossRef]
- Bu, Y.-Y.; Yamazaki, H.; Ukai, K.; Namikoshi, M. Penicillimide, an open-chain hemisuccinimide from Okinawan marine-derived Penicillium copticola. J. Antibiot. 2015, 68, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Wang, M.; Zhao, H.-G.; Huang, Y.-H.; Lin, Y.-Y.; Tan, G.-H.; Chen, S.-L. Penicilazaphilone C, a new antineoplastic and antibacterial azaphilone from the marine fungus Penicillium sclerotiorum. Arch. Pharm. Res. 2016, 39, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Abrell, L.-M.; Borgeson, B.; Crews, P. Chloro polyketides from the cultured fungus (Aspergillus) separated from a marine sponge. Tetrahedron Lett. 1996, 37, 2331–2334. [Google Scholar] [CrossRef]
- Namikoshi, M.; Negishi, R.; Nagai, H.; Dmitrenok, A.; Kobayashi, H. Three new chlorine containing antibiotics from a marine-derived fungus Aspergillus ostianus collected in Pohnpei. J. Antibiot. 2003, 56, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureram, S.; Wiyakrutta, S.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Depsidones, Aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med. 2012, 78, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhao, C.; Hao, J.; Wang, C.; Wang, W.; Huang, X.; Zhu, W. New α-glucosidase inhibitors from a marine sponge-derived fungus, Aspergillus sp. OUCMDZ-1583. RSC Adv. 2015, 5, 68852–68863. [Google Scholar] [CrossRef]
- Gu, B.-B.; Jiao, F.-R.; Wu, W.; Liu, L.; Jiao, W.-H.; Sun, F.; Wang, S.-P.; Yang, F.; Lin, H.-W. Ochrasperfloroid, an ochratoxin–ergosteroid heterodimer with inhibition of IL-6 and NO production from Aspergillus flocculosus 16D-1. RSC Adv. 2019, 9, 7251–7256. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Greshock, T.-J.; Hirota, H.; Ohta, T.; Williams, R.-M. Isolation of antipodal (−)-versicolamide B and notoamides L–N from a marine-derived Aspergillus sp. Org. Lett. 2009, 11, 1297–1300. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Umaoka, H.; Yoshikawa, K.; Ikeda, T.; Hirota, H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P-R from a marine-derived fungus, Aspergillus sp. J. Nat. Prod. 2010, 73, 1438–1440. [Google Scholar] [CrossRef]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef]
- Ivanets, E.-V.; Yurchenko, A.-N.; Smetanina, O.-F.; Rasin, A.-B.; Zhuravleva, O.-I.; Pivkin, M.-V.; Popov, R.-S.; Von Amsberg, G.; Afiyatullov, S.-S.; Dyshlovoy, S.-A. Asperindoles A–D and a p-terphenyl derivative from the ascidian-derived fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuscher, F.; Lin, W.; Wray, V.; Edrada, R.; Padmakumar, K.; Proksch, P.; Ebel, R. Two new cyclopentanoids from the endophytic fungus Aspergillus sydowii associated with the marine alga Acanthophora spicifera. Nat. Prod. Commun. 2006, 1, 927–933. [Google Scholar] [CrossRef]
- Nguyen, H.-P.; Zhang, D.; Lee, U.; Kang, J.-S.; Choi, H.-D.; Son, B.-W. Dehydroxychlorofusarielin B, an antibacterial polyoxygenated decalin derivative from the marine-derived fungus Aspergillus sp. J. Nat. Prod. 2007, 70, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.; Feng, Z.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. New production of (R)-(–)-5-bromomellein, a dihydroisocoumarin derivative from the marine-derived fungus Aspergillus ochraceus. Chem. Nat. Compd. 2013, 49, 24–26. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Li, X.; Wang, B.-G. New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 2015, 12, 182–185. [Google Scholar] [CrossRef]
- Mandelare, P.-E.; Adpressa, D.-A.; Kaweesa, E.-N.; Zakharov, L.-N.; Loesgen, S. Coculture of two developmental stages of a marine-derived Aspergillus alliaceus results in the production of the cytotoxic bianthrone allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022. [Google Scholar] [CrossRef]
- Huang, H.; Wang, F.; Luo, M.; Chen, Y.; Song, Y.; Zhang, W.; Zhang, S.; Ju, J. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012, 75, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, X.-Y.; Tu, Z.-C.; Xu, X.-Y.; Qi, S.-H. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 2013, 76, 983–987. [Google Scholar] [CrossRef]
- Liu, S.; Lu, C.; Huang, J.; Shen, Y. Three new compounds from the marine fungal strain Aspergillus sp. AF119. Rec. Nat. Prod. 2012, 6, 334–338. [Google Scholar]
- Uchida, R.; Nakajyo, K.; Kobayashi, K.; Ohshiro, T.; Terahara, T.; Imada, C.; Tomoda, H. 7-Chlorofolipastatin, an inhibitor of sterol O-acyltransferase, produced by marine-derived Aspergillus ungui NKH-007. J. Antibiot. 2016, 69, 647–651. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Varoglu, M.; Abrell, L.; Crews, P.; Lobkovsky, E.; Clardy, J. Chloriolins A-C, chlorinated sesquiterpenes produced by fungal cultures separated from a Jaspis marine sponge. J. Org. Chem. 1994, 59, 6344–6348. [Google Scholar] [CrossRef]
- Amagata, T.; Usami, Y.; Minoura, K.; Ito, T.; Numata, A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998, 51, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usami, Y.; Ikura, T.; Amagata, T.; Numata, A. First total syntheses and configurational assignments of cytotoxic trichodenones A–C. Tetrahedron: Asymmetry 2000, 11, 3711–3725. [Google Scholar] [CrossRef]
- Numata, A.; Amagata, T.; Minoura, K.; Ito, T. Gymnastatins, novel cytotoxic metabolites produced by a fungal strain from a sponge. Tetrahedron Lett. 1997, 38, 5675–5678. [Google Scholar] [CrossRef]
- Amagata, T.; Doi, M.; Ohta, T.; Minoura, K.; Ito, T.; Numata, A. Absolute stereostructures of novel cytotoxic metabolites, gymnastatins A-E, from a Gymnascella species separated from a Halichondria sponge. J. Chem. Soc. Perkin Trans. 1 1998, 21, 3585–3600. [Google Scholar] [CrossRef]
- Amagata, T.; Minoura, K.; Numata, A. Gymnastatins F-H, cytostatic metabolites from the sponge-derived fungus Gymnascella dankaliensis. J. Nat. Prod. 2006, 69, 1384–1388. [Google Scholar] [CrossRef]
- Amagata, T.; Takigawa, K.; Minoura, K. Gymnastatins I-K, Cancer cell growth inhibitors from a sponge-derived Gymnascella dankaliensis. Heterocycles 2010, 81, 897–907. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Minoura, K.; Numata, A. Gymnastatins and dankastatins, growth inhibitory metabolites of a Gymnascella species from a Halichondria Sponge. J. Nat. Prod. 2008, 71, 340–345. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Chen, Y.-P.; Minoura, K.; Numata, A. Additional cytotoxic substances isolated from the sponge-derived Gymnascella dankaliensis. Tetrahedron Lett. 2013, 54, 5960–5962. [Google Scholar] [CrossRef]
- Ogamino, T.; Ohnishi, S.; Ishikawa, Y.; Sugai, T.; Obata, R.; Nishiyama, S. Synthesis and biological assessment of hemiacetal spiro derivatives towards development of efficient chemotherapeutic agent. Sci. Technol. Adv. Mater. 2006, 7, 175. [Google Scholar] [CrossRef]
- Murayama, K.; Tanabe, T.; Ishikawa, Y.; Nakamura, K.; Nishiyama, S. A synthetic study on gymnastatins F and Q: The tandem Michael and aldol reaction approach. Tetrahedron Lett. 2009, 50, 3191–3194. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Kang, J.-S.; Choi, H.-D.; Son, B.-W. Chlorohydroaspyrones A and B, antibacterial aspyrone derivatives from the marine-derived fungus Exophiala sp. J. Nat. Prod. 2008, 71, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bao, B.; Dang, H.-T.; Hong, J.; Lee, H.-J.; Yoo, E.-S.; Bae, K.-S.; Jung, J.-H. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J. Nat. Prod. 2009, 72, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.-A.; Lambert, L.-K.; Pierens, G.-K.; Bernhardt, P.-V.; Garson, M.-J. Secondary metabolites of the sponge-derived fungus Acremonium persicinum. J. Nat. Prod. 2013, 76, 1432–1440. [Google Scholar]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Bruhn, T.; Bringmann, G.; Imhoff, J.-F. Helicusin E, isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides Strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef] [Green Version]
- Ngokpol, S.; Suwakulsiri, W.; Sureram, S.; Lirdprapamongkol, K.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Drimane sesquiterpene-conjugated amino acids from a marine isolate of the fungus Talaromyces minioluteus (Penicillium minioluteum). Mar. Drugs 2015, 13, 3567–3580. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2015, 68, 121–125. [Google Scholar] [CrossRef]
- Elsebai, M.-F.; Ghabbour, H.-A. Isocoumarin derivatives from the marine-derived fungus Phoma sp. 135. Tetrahedron Lett. 2016, 57, 354–356. [Google Scholar] [CrossRef]
- McDonald, L.-A.; Abbanat, D.-R.; Barbieri, L.-R.; Bernan, V.-S.; Discafani, C.-M.; Greenstein, M.; Janota, K.; Korshalla, J.-D.; Lassota, P.; Tischler, M.; et al. Spiroxins, DNA cleaving antitumor antibiotics from a marine-derived fungus. Tetrahedron Lett. 1999, 40, 2489–2492. [Google Scholar] [CrossRef]
- Ando, Y.; Tanaka, D.; Sasaki, R.; Ohmori, K.; Suzuki, K. Stereochemical dichotomy in two competing cascade processes: Total syntheses of both enantiomers of spiroxin A. Angew. Chem. 2019, 131, 12637–12643. [Google Scholar] [CrossRef]
- Shao, C.-L.; Wu, H.-X.; Wang, C.-Y.; Liu, Q.-A.; Xu, Y.; Wei, M.-Y.; Qian, P.-Y.; Gu, Y.-C.; Zheng, C.-J.; She, Z.-G.; et al. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J. Nat. Prod. 2011, 74, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mahankali, B.; Srihari, P.-A. Carbohydrate approach for the first total synthesis of cochliomycin C: Stereoselective total synthesis of paecilomycin E, paecilomycin F and 6′-epi-Cochliomycin, C. Eur. J. Org. Chem. 2015, 18, 3983–3993. [Google Scholar] [CrossRef]
- Li, H.-J.; Chen, T.; Xie, Y.-L.; Chen, W.-D.; Zhu, X.-F.; Lan, W.-J. Isolation and structural elucidation of chondrosterins F-H from the marine fungus Chondrostereum sp. Mar. Drugs 2013, 11, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.-Y.; Li, D.; Shao, C.-L.; Deng, D.-S.; Wang, C.-Y. (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Arredondo, V.; Roa, D.-E.; Yan, S.; Liu-Smith, F.; Van Vranken, D.-L. Total synthesis of (±)-pestalachloride C and (±)-pestalachloride D through a biomimetic knoevenagel/hetero-diels–alder cascade. Org. Lett. 2019, 21, 1755–1759. [Google Scholar] [CrossRef]
- Nielsen, J.; Nielsen, P.-H.; Frisvad, J.-C. Fungal depside, guisinol, from a marine derived strain of Emericella unguis. Phytochemistry 1999, 50, 263–265. [Google Scholar] [CrossRef]
- Afiyatullov, S.-S.; Kalinovsky, A.-I.; Antonov, A.-S. New virescenosides from the marine-derived fungus Acremonium striatisporum. Nat. Prod. Commun. 2011, 6, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Iritani, M.; Ohishi, H.; Tanaka, K.; Minoura, K.; Doi, M.; Numata, A. Pericosines, antitumour metabolites from the sea hare-derived fungus Periconia byssoides. Structures and biological activities. Org. Biomol. Chem. 2007, 5, 3979–3986. [Google Scholar] [CrossRef]
- Boyd, D.-R.; Sharma, N.-D.; Acaru, C.-A.; Malone, J.-F.; O’Dowd, C.-R.; Allen, C.-C.; Stevenson, P.-J. Chemoenzymatic synthesis of carbasugars (+)-pericosines A–C from diverse aromatic cis-dihydrodiol precursors. Org. Lett. 2010, 12, 2206–2209. [Google Scholar] [CrossRef]
- Mizuki, K.; Iwahashi, K.; Murata, N.; Ikeda, M.; Nakai, Y.; Yoneyama, H.; Harusawa, S.; Usami, Y. Synthesis of marine natural product (−)-pericosine E. Org. Lett. 2014, 16, 3760–3763. [Google Scholar] [CrossRef]
- Yamada, T.; Doi, M.; Shigeta, H.; Muroga, Y.; Hosoe, S.; Numata, A.; Tanaka, R. Absolute stereostructures of cytotoxic metabolites, chaetomugilins A-C, produced by a Chaetomium species separated from a marine fish. Tetrahedron Lett. 2008, 49, 4192–4195. [Google Scholar] [CrossRef]
- Yasuhide, M.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins, new selectively cytotoxic metabolites, produced by a marine fish-derived Chaetomium species. J. Antibiot. 2008, 61, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yasuhide, M.; Shigeta, H.; Numata, A.; Tanaka, R. Absolute stereostructures of chaetomugilins G and H produced by a marine-fish-derived Chaetomium species. J. Antibiot. 2009, 62, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muroga, Y.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins I-O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron 2009, 65, 7580–7586. [Google Scholar] [CrossRef]
- Yamada, T.; Muroga, Y.; Tanaka, R. New azaphilones, seco-chaetomugilins A and D, produced by a marine-fish-derived Chaetomium globosum. Mar. Drugs 2009, 7, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Muroga, Y.; Yamada, T.; Numata, A.; Tanaka, R. 11- and 4′-Epimers of chaetomugilin A, novel cytostatic metabolites from marine fish-derived fungus Chaetomium globosum. Helv. Chim. Acta 2010, 93, 542–549. [Google Scholar] [CrossRef]
- Yamada, T.; Muroga, Y.; Jinno, M.; Kajimoto, T.; Usami, Y.; Numata, A.; Tanaka, R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011, 19, 4106–4113. [Google Scholar] [CrossRef]
- Yamada, T.; Jinno, M.; Kikuchi, T.; Kajimoto, T.; Numata, A.; Tanaka, R. Three new azaphilones produced by a marine fish-derived chaetomium globosum. J. Antibiot. 2012, 65, 413–417. [Google Scholar] [CrossRef]
- Watts, K.-R.; Loveridge, S.-T.; Tenney, K.; Media, J.; Valeriote, F.-A.; Crews, P. Utilizing DART Mass Spectrometry to pinpoint halogenated metabolites from a marine invertebrate-derived fungus. J. Org. Chem. 2011, 76, 6201–6208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shao, C.-L.; Chen, M.; Liu, Q.-A.; Wang, C.-Y. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 2014, 55, 4888–4891. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.-M.; Jensen, P.-R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-D.; Zakarian, A. Total synthesis of (±)-Trichodermamide B and of a putative biosynthetic precursor to Aspergillazine A using an oxaza-cope rearrangement. Angew. Chem. Int. Ed. 2008, 47, 6829–6831. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.-L.; Williams, D.-E.; Ioca, L.-P.; Morais-Urano, R.-P.; Santos, M.-F.; Patrick, B.-O.; Elias, L.-M.; Lira, S.-P.; Ferreira, A.-G.; Passarini, M.-R.; et al. Structure and biogenesis of roussoellatide, a dichlorinated polyketide from the marine-derived fungus Roussoella sp. DLM33. Org. Lett. 2015, 17, 5152–5155. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.-F.; Lan, W.-J.; Wang, K.-T.; Huang, L.; Jiang, C.-W.; Li, H.-J. Two chlorinated benzofuran derivatives from the marine fungus Pseudallescheria boydii. Nat. Prod. Commun. 2015, 10, 621–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cueto, M.; Jensen, P.-R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Slavov, N.; Cvengroš, J.; Neudörfl, J.-M.; Schmalz, H.-G. Total synthesis of the marine antibiotic pestalone and its surprisingly facile conversion into pestalalactone and pestalachloride A. Angew. Chem. Int. Ed. 2010, 49, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, S.-K.; Kang, J.-S.; Choi, H.-D.; Son, B.-W. Polyketide and sesquiterpenediol metabolites from a marine-derived fungus. Bull. Korean Chem. Soc. 2004, 25, 607–608. [Google Scholar] [CrossRef]
- Lira, S.-P.; Vita-Marques, A.-M.; Seleghim, M.-H.-R.; Bugni, T.-S.; LaBarbera, D.-V.; Sette, L.-D.; Sponchiado, S.-R.; Ireland, C.-M.; Berlinck, R.-G. New destruxins from the marine-derived fungus Beauveria felina. J. Antibiot. 2006, 59, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, D.; Lee, U.; Li, X.; Cheng, J.; Zhu, W.; Jung, J.-H.; Choi, H.-D.; Son, B.-W. Bromomyrothenone B and botrytinone, cyclopentenone derivatives from a marine isolate of the fungus Botrytis. J. Nat. Prod. 2007, 70, 307–309. [Google Scholar] [CrossRef]
- Pontius, A.; Mohamed, I.; Krick, A.; Kehraus, S.; König, G.-M. Aromatic polyketides from marine algicolous fungi. J. Nat. Prod. 2008, 71, 272–274. [Google Scholar] [CrossRef]
- Pontius, A.; Krick, A.; Kehraus, S.; Brun, R.; König, G.-M. Antiprotozoal activities of heterocyclic- substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 2008, 71, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.-J.; Eguereva, E.; Kehraus, S.; König, G.-M. Ten-membered lactones from the marine-derived fungus Curvularia sp. J. Nat. Prod. 2008, 71, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Nenkep, V.; Yun, K.; Zhang, D.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. Induced production of bromomethylchlamydosporols A and B from the marine-derived fungus Fusarium tricinctum. J. Nat. Prod. 2010, 73, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Nenkep, V.-N.; Yun, K.; Li, Y.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. New production of haloquinones, bromochlorogentisylquinones A and B, by a halide salt from a marine isolate of the fungus Phoma herbarum. J. Antibiot. 2010, 63, 199–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, H.; Takahashi, O.; Murakami, K.; Namikoshi, M. Induced production of a new unprecedented epitrithiodiketopiperazine, chlorotrithiobrevamide, by a culture of the marine-derived Trichoderma cf. brevicompactum with dimethyl sulfoxide. Tetrahedron Lett. 2015, 56, 6262–6265. [Google Scholar] [CrossRef]
- Song, Y.-P.; Miao, F.-P.; Fang, S.-T.; Yin, X.-L.; Ji, N.-Y. Halogenated and nonhalogenated metabolites from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Mar. Drugs 2018, 16, 266. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Guo, Z.-Y.; Li, Q.; Zhang, D.; She, Z.; Vrijmoed, L.-L. A new griseofulvin derivative from the mangrove endophytic fungus Sporothrix sp. Chem. Nat. Compd. 2010, 46, 363–365. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, S.; Zhu, T.; Lin, Z.; Gu, J.; Li, D.; Gu, Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry 2011, 72, 1436–1442. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Tadpetch, K.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Sakayaroj, J. Chlorinated chromone and diphenyl ether derivatives from the mangrove-derived fungus Pestalotiopsis sp. PSU-MA69. Tetrahedron 2012, 68, 2299–2305. [Google Scholar] [CrossRef]
- Hammerschmidt, L.; Debbab, A.; Ngoc, T.-D.; Wray, V.; Hemphil, C.-P.; Lin, W.; Broetz-Oesterhelt, H.; Kassack, M.-U.; Proksch, P.; Aly, A.-H. Polyketides from the mangrove-derived endophytic fungus Acremonium strictum. Tetrahedron Lett. 2014, 55, 3463–3468. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Rachtawee, P.; Srichomthong, K.; Srikitikulchai, P.; Kongsaeree, P.; Prabpai, S. Cytotoxic hydroanthraquinones from the mangrove-derived fungus Paradictyoarthrinium diffractum BCC 8704. J. Antibiot. 2015, 68, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Williams, K.; Proksch, P.; Ji, N.-Y.; Wang, B.-G. Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Heering, C.; Janiak, C.; Müller, W.-E.; Akoné, S.-H.; Liu, Z.; Proksch, P. Sesquiterpenoids from the endophytic fungus Rhinocladiella similis. J. Nat. Prod. 2019, 82, 1055–1062. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Kannai, S.; Klaiklay, S.; Phongpaichit, S.; Sakayaroj, J. Rare 2-phenylpyran-4-ones from the seagrass-derived fungi Polyporales PSU-ES44 and PSU-ES83. Tetrahedron 2013, 69, 6981–6986. [Google Scholar] [CrossRef]
- Uchida, R.; Tomoda, H.; Arai, M.; Omura, S. Chlorogentisylquinone, a new neutral sphingo-myelinase inhibitor, produced by a marine fungus. J. Antibiot. 2001, 54, 882–889. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A-O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef]
- Fredimoses, M.; Zhou, X.; Lin, X.; Tian, X.; Ai, W.; Wang, J.; Liao, S.; Liu, J.; Yang, B.; Yang, X.; et al. New prenylxanthones from the deep-sea derived fungus Emericella sp. SCSIO 05240. Mar. Drugs 2014, 2, 3190–3202. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, X.; Liu, C.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342. RSC Adv. 2016, 6, 76498–79504. [Google Scholar] [CrossRef]
- Wang, W.; Park, C.; Oh, E.; Sung, Y.; Lee, J.; Park, K.-H.; Kang, H. Benzophenone Compounds, from a Marine-Derived Strain of the Fungus Pestalotiopsis neglecta, Inhibit Proliferation of Pancreatic Cancer Cells by Targeting the MEK/ERK Pathway. J. Nat. Prod. 2019, 82, 3357–3365. [Google Scholar] [CrossRef]
- Sun, C.; Ge, X.; Mudassir, S.; Zhou, L.; Yu, G.; Che, Q.; Zhang, G.; Peng, J.; Gu, Q.; Zhu, T.; et al. New glutamine-containing azaphilone alkaloids from deep-sea-derived fungus Chaetomium globosum HDN151398. Mar. Drugs 2019, 17, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, M.-K.; Jensen, P.-R.; Fenical, W. Neomangicols: Structures and absolute stereochemistries of unprecedented halogenated sesterterpenes from a marine fungus of the genus Fusarium. J. Org. Chem. 1998, 63, 8346–8354. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]

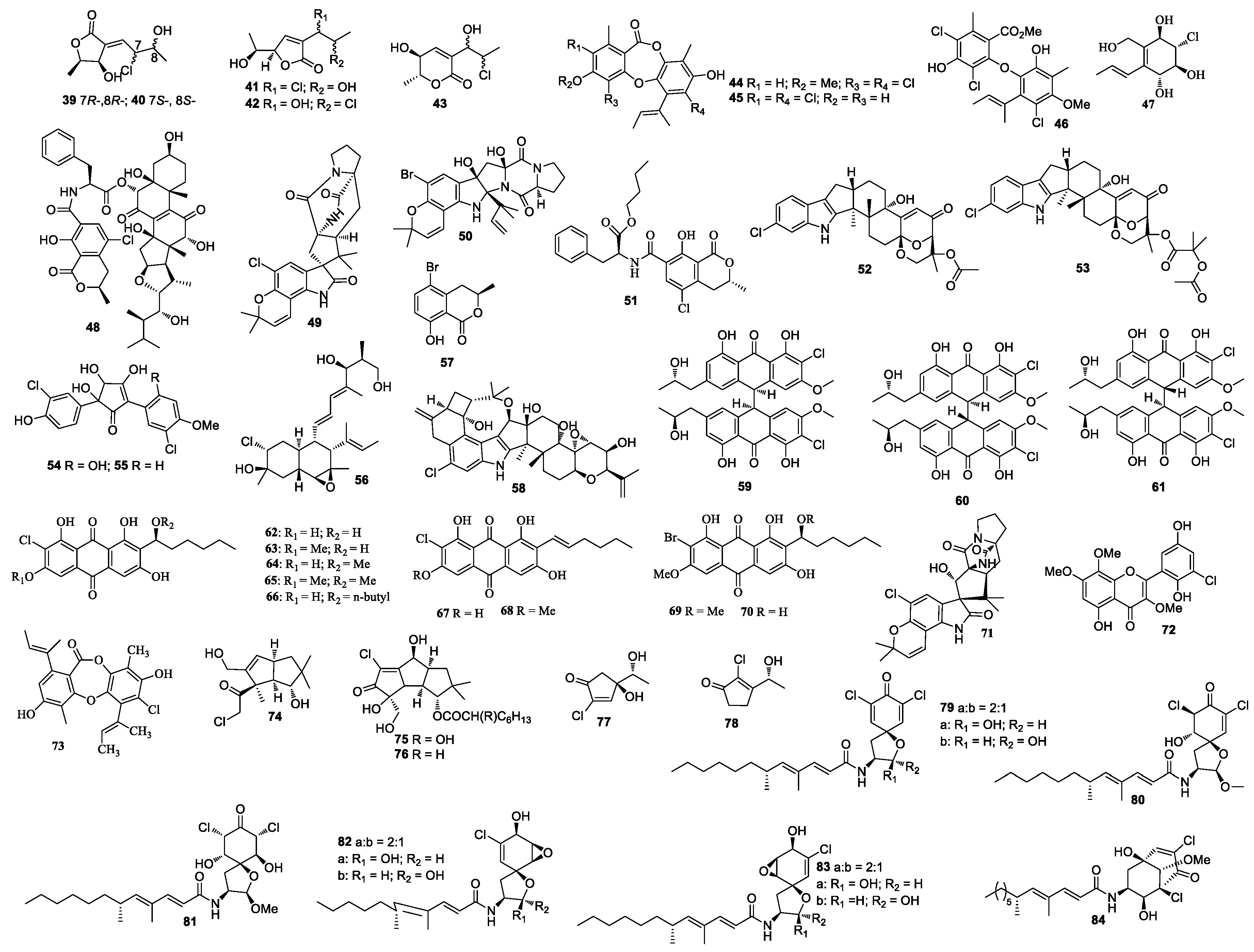
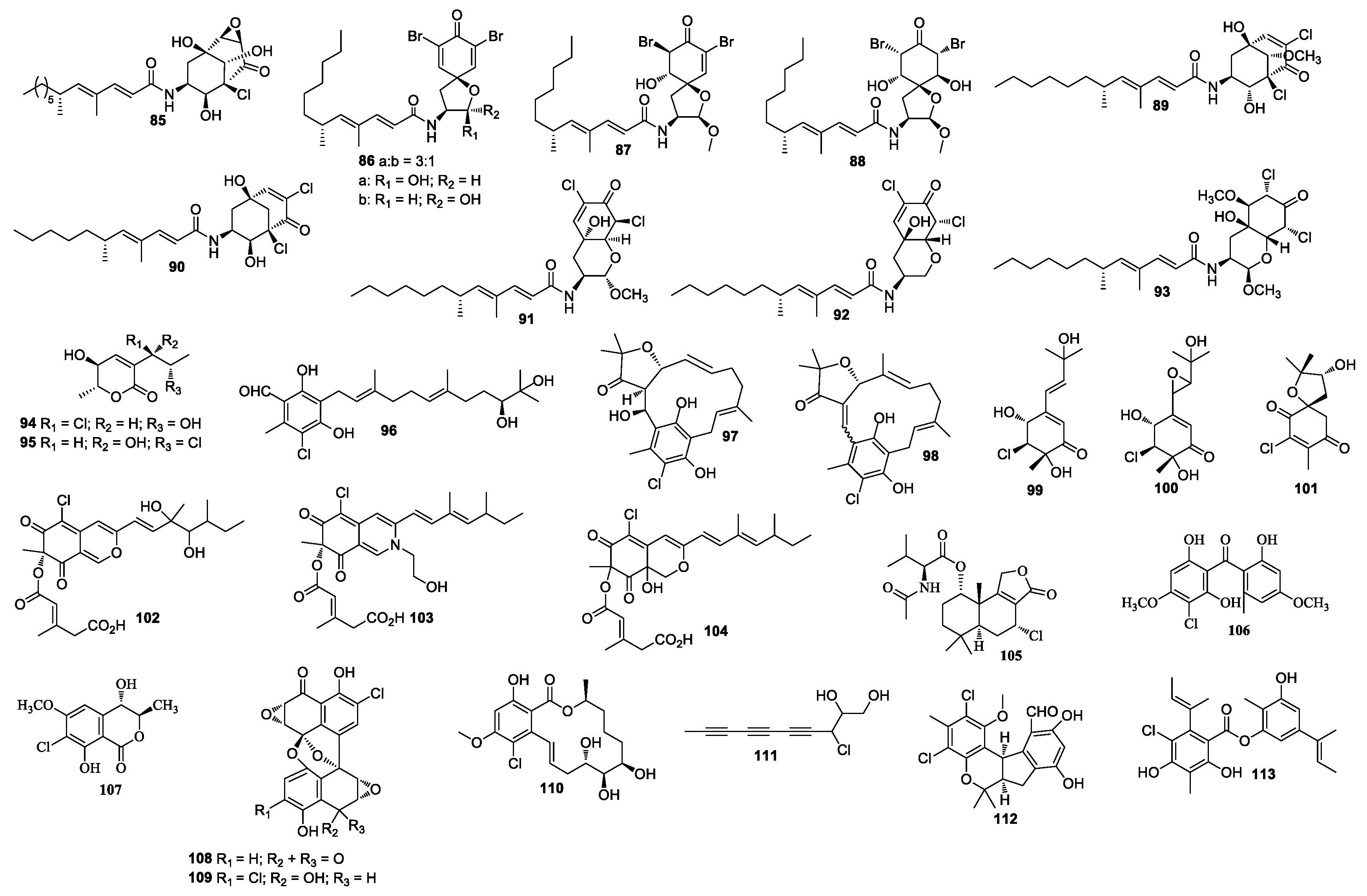
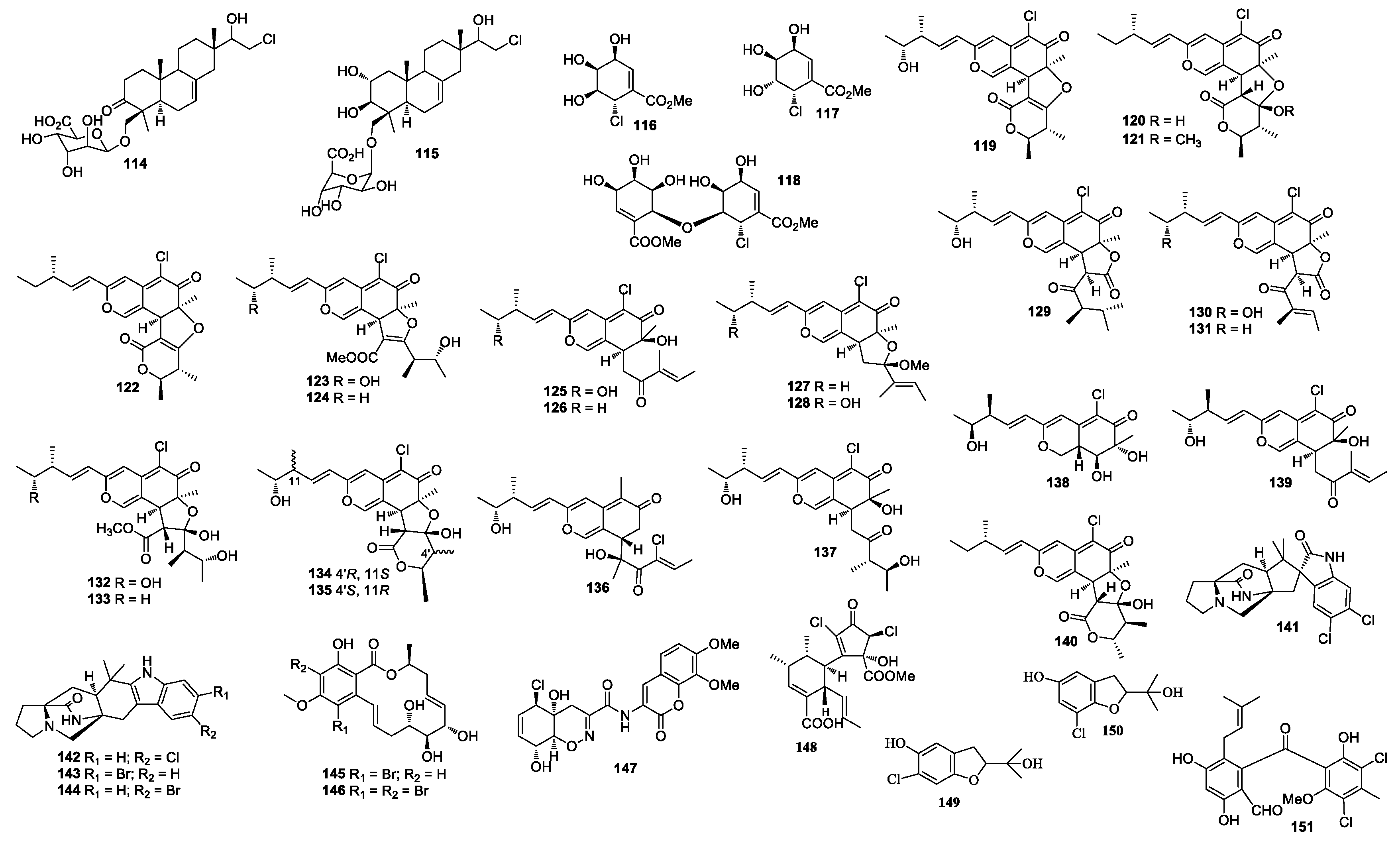

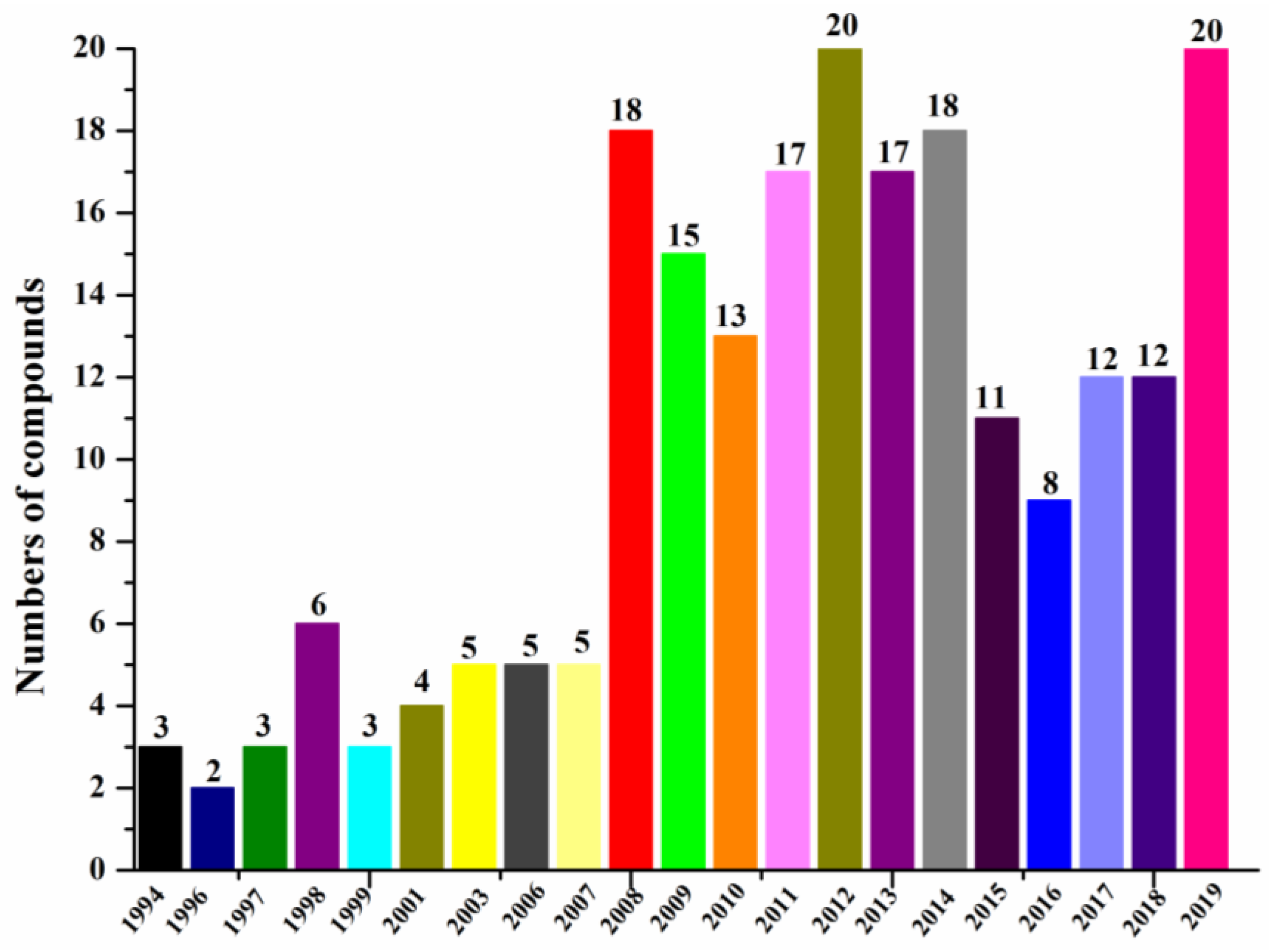
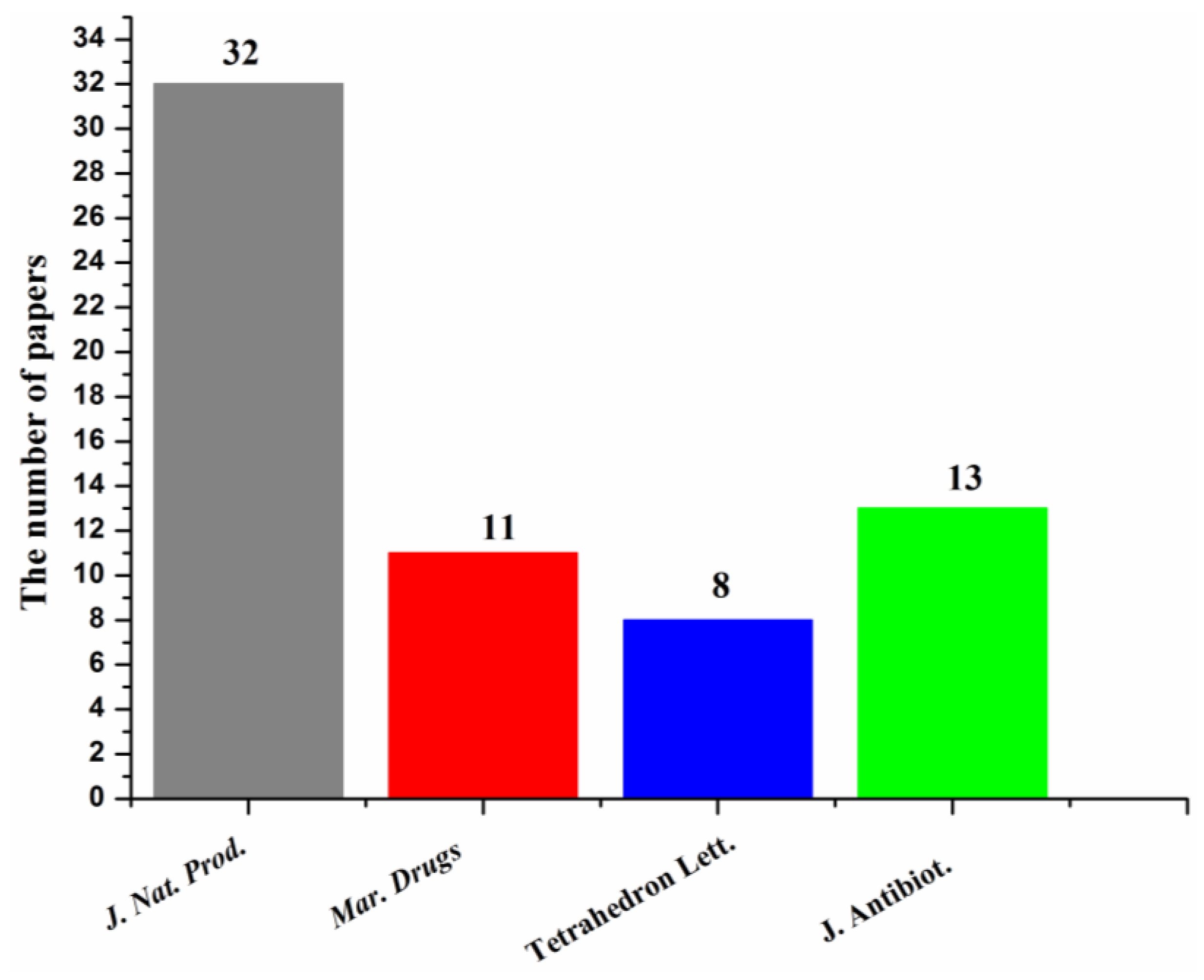
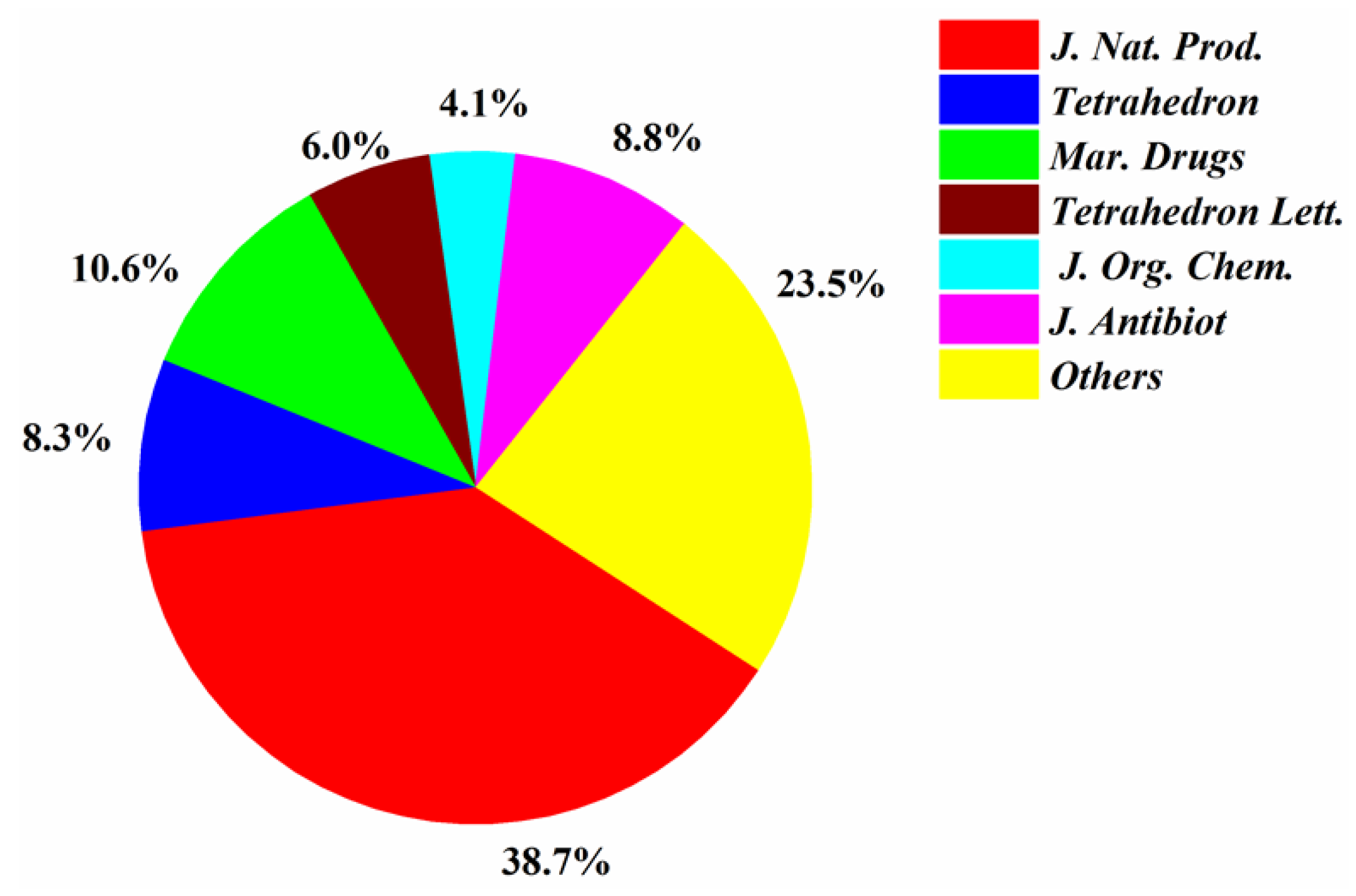

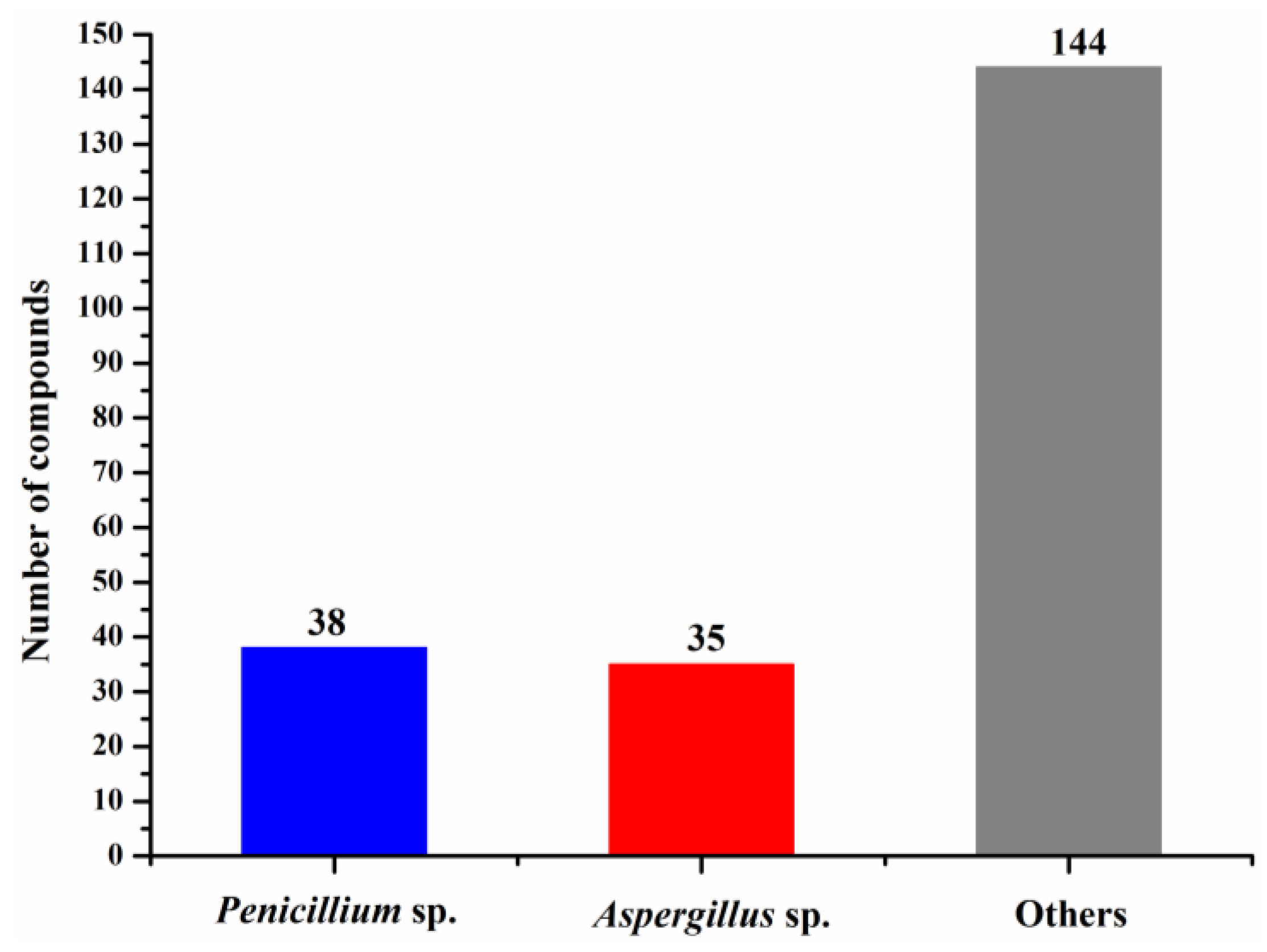
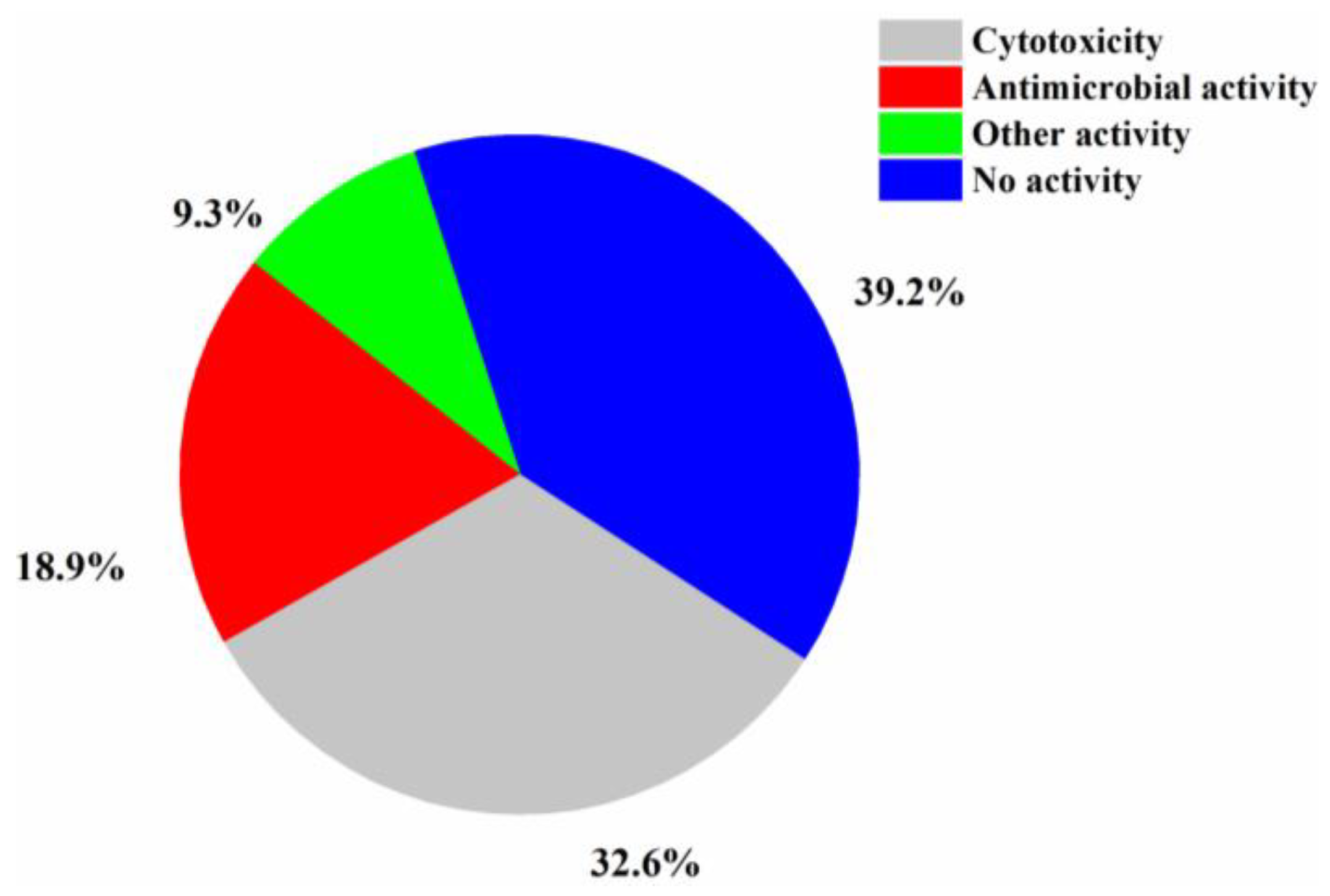

| First Producing Strain | Environment Source | Compound | Time |
|---|---|---|---|
| Penicillium Terrestre | Sediment, Jiaozhou Bay, China | Terrestrols B, D, F–G, a monomer (19) | 2008 |
| Aspergillus cf. ochraceus 941026 | Jaspis of Coriacea, Indian-Pacific Ocean | Chlorocarolides A–B (39 and 40) | 1996 |
| Unidentified fungus | Indo-Pacific sponge Jaspis aff. johnstoni | Chloriolins A–C (74–76) | 1994 |
| Compound | Producing Strain | Environment Source | Bioactivity | Ref. |
|---|---|---|---|---|
| 1–2 | Penicillium sclertiorum GDST-2013-0415 | Unidentified sponge GDST-2013-04, the coral reef at a depth of 10 m in the sea area of Shantou, Guangdong, China | - | [5] |
| 3–5 | Penicillium canescens 4.14. 6a. | The inner tissues of the marine sponge Agelas oroides, the coast of Sığacı̧kİzmir, Turkey | - | [6] |
| 6–8 | Penicillium sp. SCS-KFD09 | A marine worm, Sipunculus nudus (HK10404), Haikou Bay, China | 6: Anti-H1N1 activity; 8: Protein tyrosine phosphatase 1B inhibitory activity | [7,8] |
| 9–10 | Penicillium chrysogenum | Hypnea complex, South Gyeongsang, Korea | DPPH activity | [9] |
| 11–12 | Penicillium janthinellum HDN13-309 | Sonneratia caseolari, Hainan, China | cytotoxicity | [10] |
| 13–14 | Penicillium citrinum HL-5126 | Bruguiera sexangula var. rhynchopetala, the South China Sea | 14: Antibacterial activity | [11] |
| 15–19 | Penicillium Terrestre | Sediment, Jiaozhou Bay, China | cytotoxicity; 15: DPPH activity | [12] |
| 20–23 | Penicillium Terrestre | Sediment, Jiaozhou Bay, China | 20–21: Cytotoxicity | [13] |
| 24–27 | Penicillium sp. PR19N-1 | Sediment (−1000 m), Prydz Bay, Antarctica | 24: Cytotoxicity | [14] |
| 28 | Penicillium CF07370 | Sediment (~100 m), Bahia de Los Angeles (Gulf of California, Mexico) | cytotoxicity | [15] |
| 29–31 | Penicillium sp. SCSIO sof101 | Sediment (2448 m), the South China Sea (112°124′ E, 18°0.541′ N) | 29: Cytotoxicity | [16] |
| 32–35 | Penicillium janthinellum HK1-6 | The mangrove rhizosphere soil, Dongzhaigang mangrove natural reserve, Hainan Island | antibacterial activity | [17,18] |
| 36 | Penicillium canescentia MMS351 | Seawater, French Atlantic coast | cytotoxicity | [19,20] |
| 37 | Penicillium copticola TPU1270 | Marine foam, Iriomote Island, Okinawa Prefecture, Japan | - | [21] |
| 38 | Penicillium sclerotiorum M-22 | The rotten leaf sample, on the west coast of Haikou, Hainan, China | cytotoxicity, antibacterial activity | [22] |
| 39–40 | Aspergillus cf. ochraceus 941026 | Jaspis of Coriacea, Indian-Pacific Ocean | - | [23] |
| 41–43 | A. ostianus TUF 01F313 | Unidentified sponge, Pohnpei, Micronesia | antibacterial activity | [24] |
| 44–46 | Aspergillus unguis CRI282-03 | Unidentified sponge CRI282, Thailand | 44–46: Aromatase inhibitor 45: DPPH | [25] |
| 47 | Aspergillus sp. OUCMDZ-1583 | An unidentified marine sponge XD10410, Xisha Islands, China | α-glucosidase inhibitor | [26] |
| 48 | Aspergillus flocculosus 16D-1 | The sponge Phakellia fusca, Yongxing Island, China | inhibitory activity towards THP-1 and NO production in LPS-activated RAW264.7 | [27] |
| 49–50 | Aspergillus sp. MF297-2 | Mytilus edulis, Japan | - | [28,29] |
| 51 | Aspergillus sp. SCSGAF0093 | Melitodes squamata collected from the South China Sea | - | [30] |
| 52–53 | Aspergillus sp. KMM 4676 | Unidentified colonial ascidian, Shikotan Island, Pacific Ocean | 52: Cytotoxicity | [31] |
| 54–55 | Aspergillus sydowii | Acanthophora spicifera, Bay of Bengal India | - | [32] |
| 56 | Aspergillus sp. MFB024 | Sargassum horneri, Korea | antibacterial activity | [33] |
| 57 | Aspergillus ochraceus | Marine red alga Chondria crassicualis, Yokji Island, Kyeongnam, Korea | DPPH activity | [34] |
| 58 | Aspergillus nidulans EN-330 | Marine red alga P. scopulorum var. villum, Yantai, China | cytotoxicity, antibacterial activity | [35] |
| 59–61 | Aspergillus alliaceus | Marine alga by Bioviotica GmbH | cytotoxicity | [36] |
| 62–70 | Aspergillus sp. SCSIO F063 | A marine sediment sample, the South China Sea | 63, 64, 70: Cytotoxicity | [37] |
| 71 | A. westerdijkiae DFFSCS013 | A marine sediment sample, the South China Sea | cytotoxicity | [38] |
| 72 | Aspergillus sp. AF119 | Sediment, Xiamen beach, China | - | [39] |
| 73 | Aspergillus ungui NKH-007 | Soil (331 m), Suruga Bay, Japan (138°18.1207’ E, 34°22.4813’ N) | inhibitor of sterol O-acyltransferase | [40] |
| 74–76 | unidentified fungus | Indo-Pacific sponge Jaspis aff. johnstoni | 74: Cytotoxicity | [41] |
| 77–78 | Trichoderma harzianum OUPS-N115 | Halichondria okadai, Japan | cytotoxicity | [42,43] |
| 79–93 | Gymnascella dankaliensis | Halichondria japonica, Japan | cytotoxicity | [44,45,46,47,48,49,50,51] |
| 94–95 | Exophiala sp. | Sponge Halichondria panicea, Bogil Island, Jeonnam Province, Korea | antibacterial activity | [52] |
| 96–98 | Acremonium sp. J05B-1-F-3 | Sponge Stelletta sp. (J05B-1), the coast of Jeju Island, Korea | - | [53] |
| 99–101 | Acremonium persicinum | Sponge Anomoianthella rubrawere, the gneering reef offshore from Mooloolaba | - | [54] |
| 102–104 | Bartalinia robillardoides LF550 | Marine sponge Tethya aurantium, the Limsky kanal (Canal di Lemme or Limsky channel, Croatia) | 104: Antibacterial activity 103–104: Inhibitory activity towards PDE4 | [55] |
| 105 | Talaromyces minioluteus | Unidentified marine sponge, Pilae Bay, Phi Phi Island, Krabi Province, Thailand | cytotoxicity | [56] |
| 106 | Stachybotry sp. HH1 ZSDS1F1-2 | Sponge, Xisha Island, China | anti-virus activity | [57] |
| 107 | Phoma sp. 135 | Sponge Ectyplasia perox, Dominica | - | [58] |
| 108–109 | fungus LL-37H248 | Orange coral, Dixon Bay, Vancouver Island, Canada | 108: Cytotoxicity | [59,60] |
| 110 | Cochliobolus lunatus | Gorgonian Dichotella gemmacea, the South China Sea | - | [61,62] |
| 111 | Chondrostereum sp. nov. SF002 | Sarcophyton tortuosum, Sanya, Hainan | antibacterial activity | [63] |
| 112 | Pestalotiopsis sp. ZJ-2009-7-6 | Sarcophyton sp., Yongxing Island | antibacterial activity | [64,65] |
| 113 | Emericella unguis M87-2 | Stomolopus meliagris, Paria Bay, Venezuela | antibacterial activity | [66] |
| 114–115 | Acremonium striatisporum KMM 4401 | Eupentacta fraudatrix, Japan | - | [67] |
| 116–118 | Periconia byssaides OUPS-N133 | Sea hare Aplysia kurodai, Japan | - | [68,69,70] |
| 119–140 | Chaetomium globosum OUPS-T106B-6 | Marine fish Mugil cephalus, Japan | 119–128, 130–131, 133: Cytotoxicity | [71,72,73,74,75,76,77,78] |
| 141–144 | Malbranchea graminicola 086937A | Unidentified invertebrate, Kona, Hawaii | - | [79] |
| 145–146 | Cochliobolus lunatus TA26-46 | Palythoa haddoni, Weizhou Island | - | [80] |
| 147 | Trichoderma virens CNL910 | Didemnum mole, Papua New Guinea | cytotoxicity, antimicrobial activity | [81,82] |
| 148 | Roussoella sp. DLM33 | the ascidian Didemnum ligulum, the north coast of São Paulo state, Brazil | - | [83] |
| 149–150 | Pseudallescheria boydii | Acanthaster planci, Hainan Sanya National Coral Reef Reserve, Hainan | - | [84] |
| 151 | Pestalotia sp. CNL-365 | Rosenvingea sp. Bahamas | cytotoxicity, antimicrobial activity | [85,86] |
| 152–153 | unidentified fungus | Gracillaria verrucose, Korea | - | [87] |
| 154 | Beauveria felina | Caulerpa sp., São Paulo | - | [88] |
| 155 | Enteromorpha compressa, Busan, Korea | Botrytis sp. | - | [89] |
| 156 | Acremonium sp. | Plocamium sp., Heligoland | - | [90] |
| 157 | Chaetomium sp. | The algal species (taxonomy not determined), Kamari on the island Santorini, Greece | antiprotozoal activities | [91] |
| 158 | Curvularia sp. 768 | Acanthophora spicifera, The Territory of Guam | - | [92] |
| 159–160 | Fusarium tricinctum | Sargassum ringgoldium, Yeosu, Korea | antibacterial activity | [93] |
| 161–162 | Phoma herbarum | Gloiopeitis tenax, Korea | DPPH activity | [94] |
| 163 | Trichoderma sp. (cf. T. brevicompactum) TPU199 | A red alga, Palau | - | [95] |
| 164 | Trichoderma asperellum cf44-2 | Marine brown alga Sargassum sp., Zhoushan Islands | antibacterial activity | [96] |
| 165 | Sporothrix sp. 4335 | The bark of an estuarine mangrove, the South China Sea | - | [97] |
| 166–167 | Emericella sp. HK-ZJ | A. corniculatu, Haikou, China | antiviral activity | [98] |
| 168–173 | Pestalotiopsis sp. PSU-MA69 | R. apiculate, Thailand | - | [99] |
| 174 | Acremonium strictum | The mangrove tree Rhizophora apiculate Blume | - | [100] |
| 175 | Paradictyoarthrinium diffractum BCC 8704 | A mangrove wood in Laem Son National Park, Ranong Province, Thailand | cytotoxicity | [101] |
| 176–177 | Lasiodiplodia theobromae ZJ-HQ1 | The marine mangrove A. ilicifolius, China | cytotoxicity, antibacterial activity | [102] |
| 178–179 | Mucor irregularis QEN-189 | Mangrove plant Rhizophora stylosa, Hainan Island, China | cytotoxicity | [103] |
| 180–183 | Rhinocladiella similis | Acrostichums aureum (Pteridaceae), Douala, Cameroon | 180: Cytotoxicity | [104] |
| 184 | Polyporales sp. PSU-ES44 | Thalassia hemprichii | - | [105] |
| 185 | marine-derived fungus FOM-8108 | Marine sand, Katase Enoshima Beach, Kanagawa, Japan | nSMase activity | [106] |
| 186–199 | Spiromastix sp. MCCC3A00308 | Deep-sea sediment (2869 m), the South Atlantic Ocean (GPS 13.7501 W, 15.1668 S) | antibacterial activity | [107] |
| 200 | Emericella sp. SCSIO 05240 | A sediment sample (3258 m), the South China Sea | antibacterial activity | [108] |
| 201 | Cladosporium cladosporioides HDN14-342 | Sediment sample, Indian Ocean | cytotoxicity | [109] |
| 202–208 | Pestalotiopsis neglecta | Marine sediment (−10 m), Gageo, Korea | 205–208: Cytotoxicity | [110] |
| 209–211 | Chaetomium globosum HDN151398 | The sediment sample, South China Sea | 211: Cytotoxicity | [111] |
| 212–213 | F. heterosporum CNC-477 | A driftwood sample, Sweetings Cay, Bahamas | 212: Cytotoxicity; 213: Antibacterial activity | [112] |
| 214–217 | Chaetomium sp. NA-S01-R1 | A seawater sample, the West Pacific Ocean | antimicrobial activity, cytotoxicity | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Lu, H.; Lan, J.; Zaman, K.A.; Cao, S. A Review: Halogenated Compounds from Marine Fungi. Molecules 2021, 26, 458. https://doi.org/10.3390/molecules26020458
Wang C, Lu H, Lan J, Zaman KA, Cao S. A Review: Halogenated Compounds from Marine Fungi. Molecules. 2021; 26(2):458. https://doi.org/10.3390/molecules26020458
Chicago/Turabian StyleWang, Cong, Huanyun Lu, Jianzhou Lan, KH Ahammad Zaman, and Shugeng Cao. 2021. "A Review: Halogenated Compounds from Marine Fungi" Molecules 26, no. 2: 458. https://doi.org/10.3390/molecules26020458







