Synthesis of Functionalized Cannabilactones
Abstract
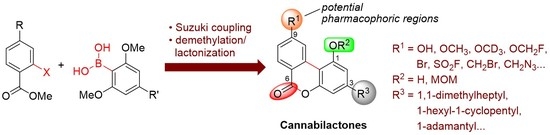
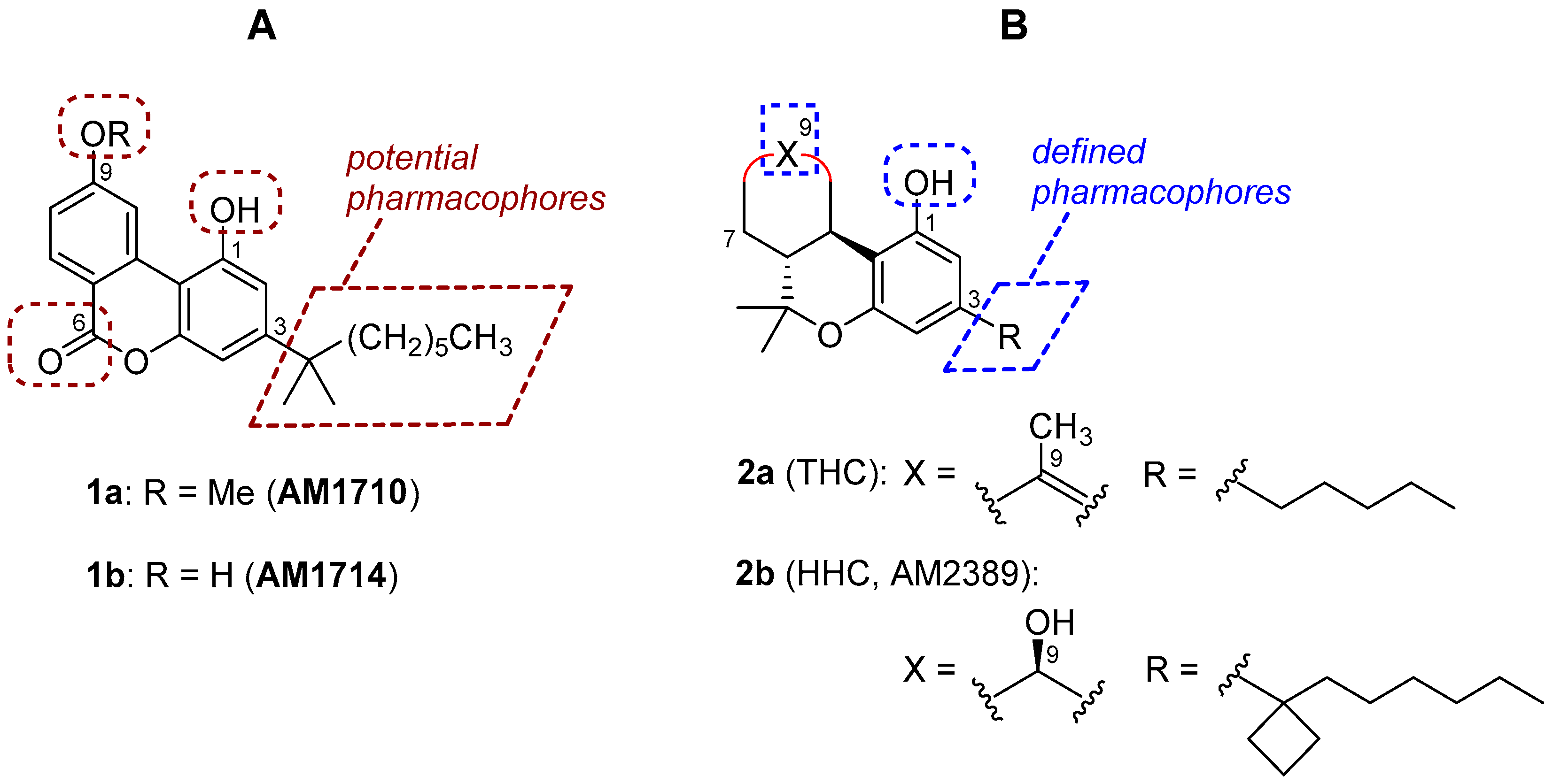
:1. Introduction
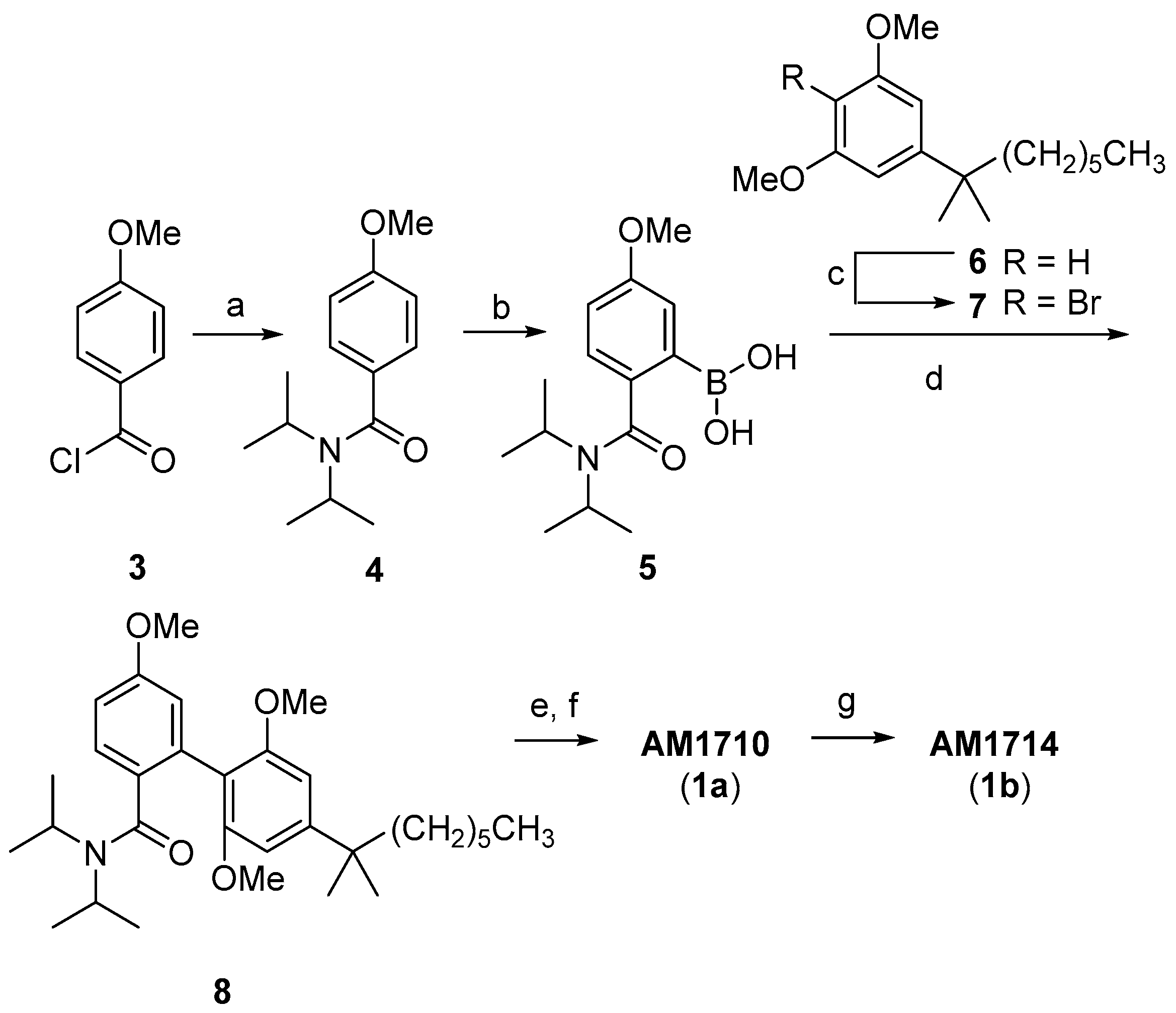
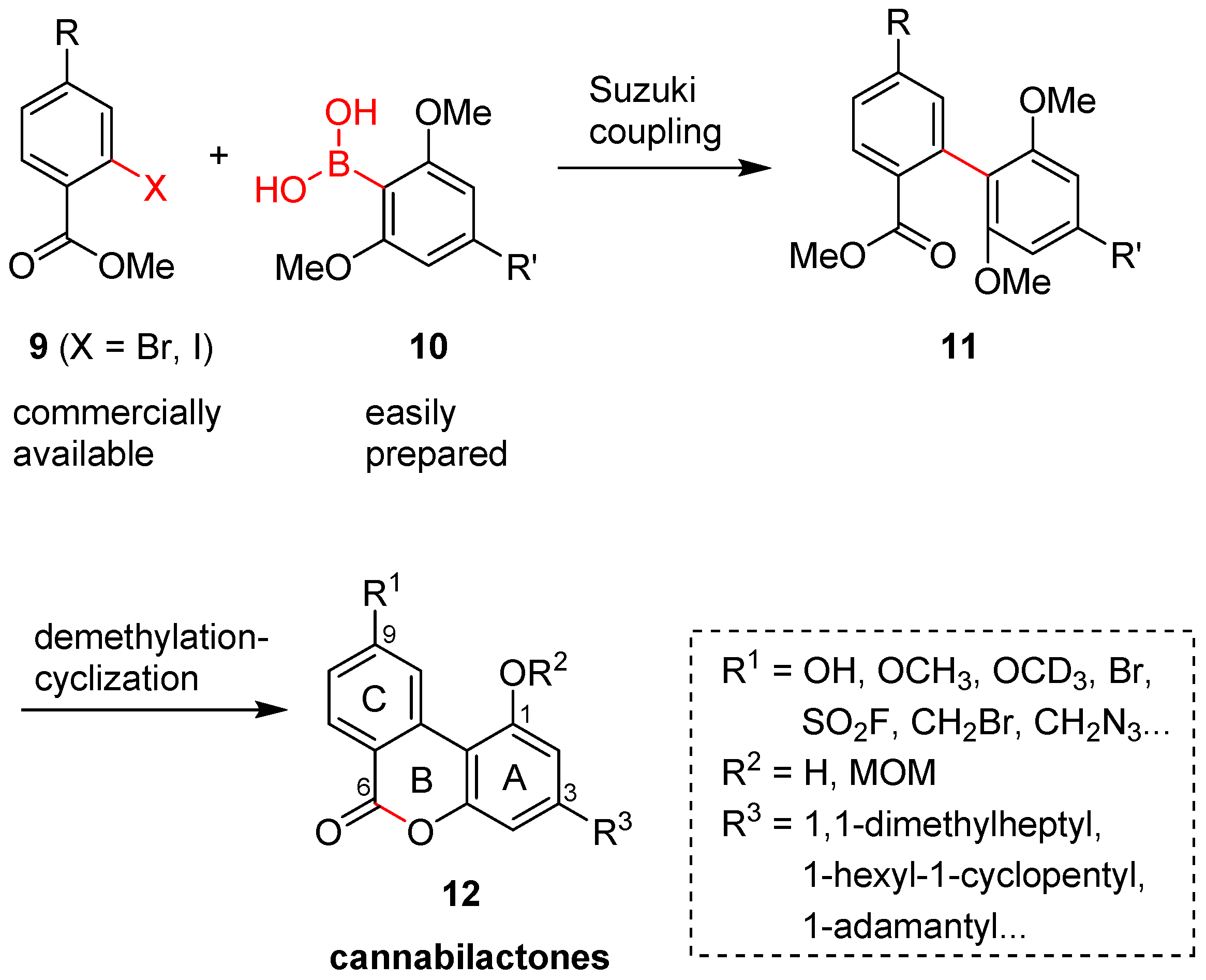
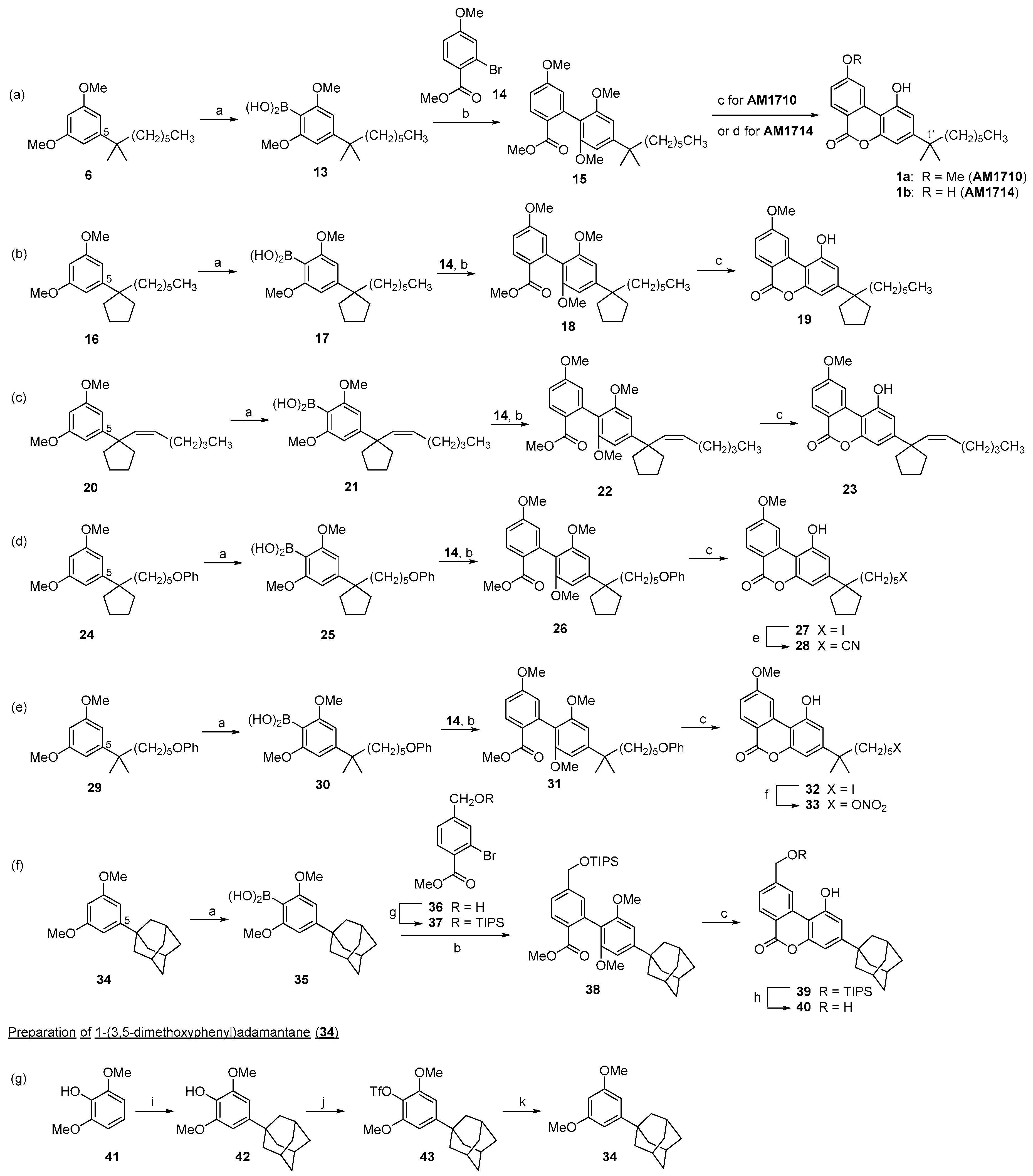
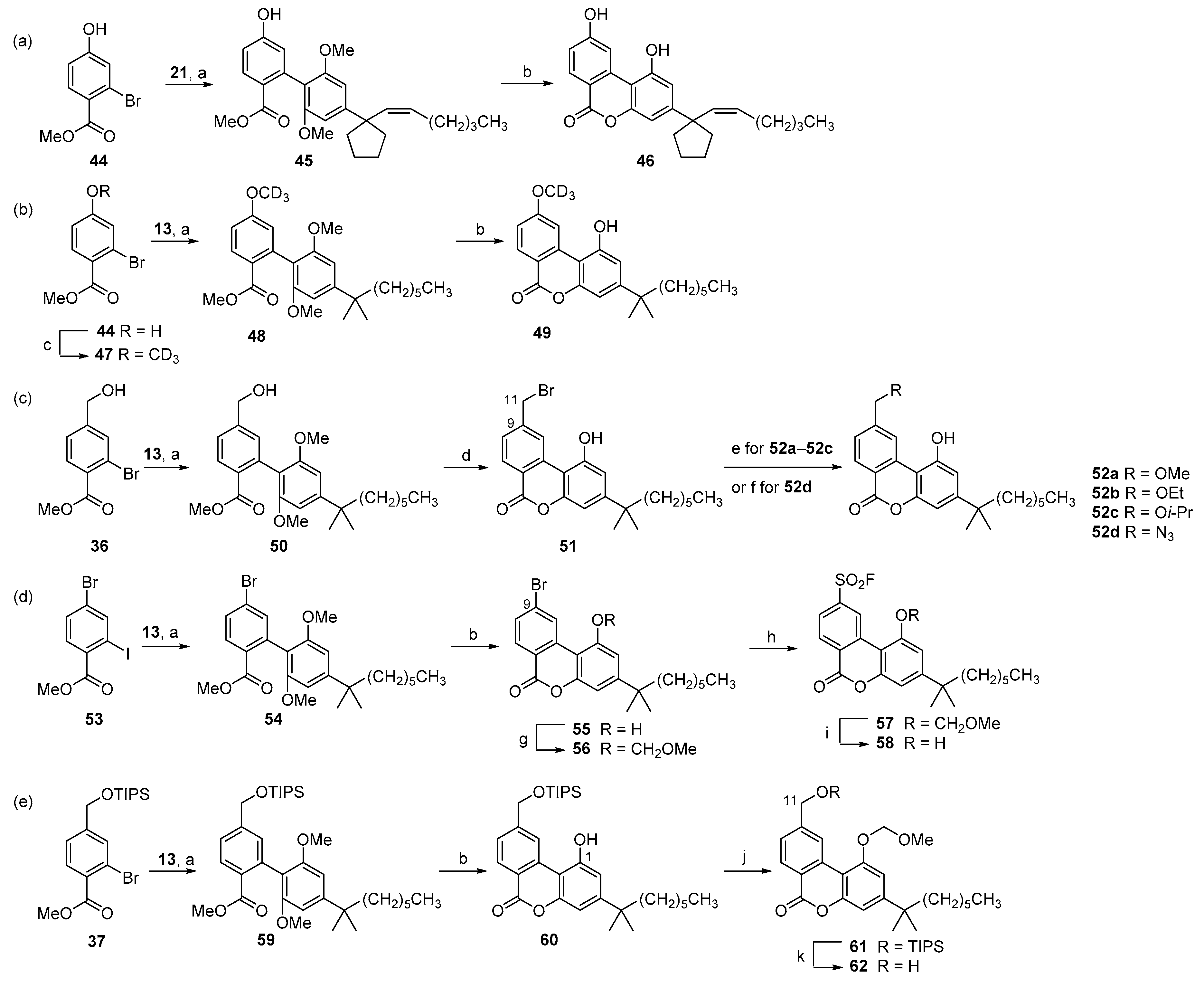
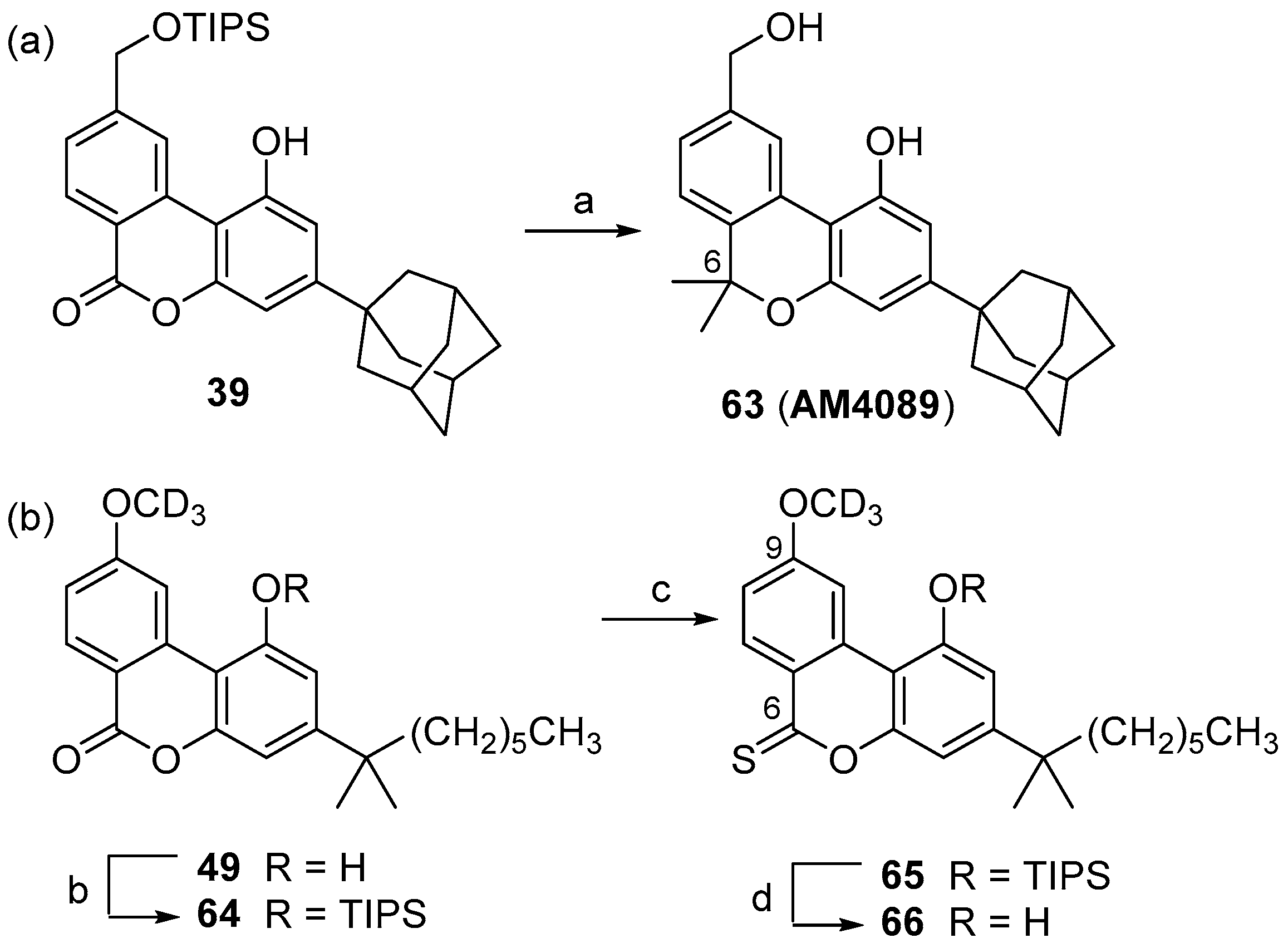
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makriyannis, A. 2012 Division of medicinal chemistry award address. Trekking the cannabinoid road: A personal perspective. J. Med. Chem. 2014, 57, 3891–3911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Han, S.; Thatte, J.; Buzard, D.J.; Jones, R.M. Therapeutic utility of cannabinoid receptor type 2 (CB2) selective agonists. J. Med. Chem. 2013, 56, 8224–8256. [Google Scholar] [CrossRef]
- Spinelli, F.; Capparelli, E.; Abate, C.; Colabufo, N.A.; Contino, M. Perspectives of cannabinoid type 2 receptor (CB2R) ligands in neurodegenerative disorders: Structure–affinity relationship (SAfiR) and structure–activity relationship (SAR) studies. J. Med. Chem. 2017, 60, 9913–9931. [Google Scholar] [CrossRef]
- Li, X.T.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.R.; Wu, L.J.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal structure of the human cannabinoid receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Johnston, C.A.; Nikas, S.P.; Song, F.; et al. Activation and signaling mechanism revealed by cannabinoid receptor-Gi complex structures. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Zhuang, Y.; Xu, T.-H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM structure of the human cannabinoid receptor CB2-Gi signaling complex. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.M.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.C.; Qu, L.; Han, G.W.; Wu, Y.R.; Zhao, S.W.; Shui, W.Q.; Li, S.S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef]
- Khanolkar, A.D.; Lu, D.; Ibrahim, M.; Duclos, R.I.; Thakur, G.A.; Malan, T.P.; Porreca, F.; Veerappan, V.; Tian, X.; George, C.; et al. Cannabilactones: A novel class of CB2 selective agonists with peripheral analgesic activity. J. Med. Chem. 2007, 50, 6493–6500. [Google Scholar] [CrossRef]
- Alapafuja, S.O.; Nikas, S.P.; Ho, T.C.; Tong, F.; Benchama, O.; Makriyannis, A. Chain substituted cannabilactones with selectivity for the CB2 cannabinoid receptor. Molecules 2019, 24, 3559. [Google Scholar] [CrossRef] [Green Version]
- Rahn, E.J.; Thakur, G.A.; Wood, J.A.T.; Zvonok, A.M.; Makriyannis, A.; Hohmann, A.G. Pharmacological characterization of AM1710, a putative cannabinoid CB2 agonist from the cannabilactone class: Antinociception without central nervous system side-effects. Pharmacol. Biochem. Behav. 2011, 98, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.T.; Guindon, J.; Cornett, B.L.; Makriyannis, A.; Mackie, K.; Hohmann, A.G. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol. Psychiatry 2015, 77, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Li, A.L.; Lin, X.; Dhopeshwarkar, A.S.; Thomaz, A.C.; Carey, L.M.; Liu, Y.; Nikas, S.P.; Makriyannis, A.; Mackie, K.; Hohmann, A.G. Cannabinoid CB2 agonist AM1710 differentially suppresses distinct pathological pain states and attenuates morphine tolerance and withdrawal. Mol. Pharmacol. 2019, 95, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Nikas, S.P.; Alapafuja, S.O.; Papanastasiou, I.; Paronis, C.A.; Shukla, V.G.; Papahatjis, D.P.; Bowman, A.L.; Halikhedkar, A.; Han, X.W.; Makriyannis, A. Novel 1′,1′-chain substituted hexahydrocannabinols: 9/beta-1-hydroxy-3-(1-hexyl-cyclobut-l-yl)hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J. Med. Chem. 2010, 53, 6996–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, G.A.; Nikas, S.P.; Li, C.; Makriyannis, A. Structural requirements for cannabinoid receptor probes. Handb. Exp. Pharmacol. 2005, 168, 209–246. [Google Scholar]
- Papahatjis, D.P.; Nahmias, V.R.; Nikas, S.P.; Andreou, T.; Alapafuja, S.O.; Tsotinis, A.; Guo, J.; Fan, P.; Makriyannis, A. C1′-cycloalkyl side chain pharmacophore in tetrahydrocannabinols. J. Med. Chem. 2007, 50, 4048–4060. [Google Scholar] [CrossRef] [PubMed]
- Papahatjis, D.P.; Nikas, S.P.; Kourouli, T.; Chari, R.; Xu, W.; Pertwee, R.G.; Makriyannis, A. Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1′. J. Med. Chem. 2003, 46, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Alonso, E.; Ramón, D.J.; Yus, M. Naphthalene-catalysed lithiation of N,N-diisopropylbenzamide and its methoxy derivatives. Tetrahedron 1998, 54, 13629–13638. [Google Scholar] [CrossRef]
- Makriyannis, A.; Nikas, S.P.; Alapafuja, S.O.; Shukla, V.G. Monoacylglycerol Lipase Inhibitors for Modulation of Cannabinoid Activity. Patent WO2009052319A1, 23 April 2009. [Google Scholar]
- Busch-Petersen, J.; Hill, W.A.; Fan, P.S.; Khanolkar, A.; Xie, X.Q.; Tius, M.A.; Makriyannis, A. Unsaturated side chain beta-11-hydroxyhexahydrocannabinol analogs. J. Med. Chem. 1996, 39, 3790–3796. [Google Scholar] [CrossRef]
- Lu, D.; Meng, Z.; Thakur, G.A.; Fan, P.; Steed, J.; Tartal, C.L.; Hurst, D.P.; Reggio, P.H.; Deschamps, J.R.; Parrish, D.A.; et al. Adamantyl cannabinoids: a novel class of cannabinergic ligands. J. Med. Chem. 2005, 48, 4576–4585. [Google Scholar] [CrossRef]
- Thakur, G.A.; Bajaj, S.; Paronis, C.; Peng, Y.; Bowman, A.L.; Barak, L.S.; Caron, M.G.; Parrish, D.; Deschamps, J.R.; Makriyannis, A. Novel adamantyl cannabinoids as CB1 receptor probes. J. Med. Chem. 2013, 56, 3904–3921. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, G.; Tius, M.A.; Zhou, H.; Nikas, S.P.; Halikhedkar, A.; Mallipeddi, S.; Makriyannis, A. 3′-functionalized adamantyl cannabinoid receptor probes. J. Med. Chem. 2015, 58, 3104–3116. [Google Scholar] [CrossRef] [Green Version]
- Nikas, S.P.; Sharma, R.; Paronis, C.A.; Kulkarni, S.; Thakur, G.A.; Hurst, D.; Wood, J.T.; Gifford, R.S.; Rajarshi, G.; Liu, Y.; et al. Probing the carboxyester side chain in controlled deactivation (-)-delta(8)-tetrahydrocannabinols. J. Med. Chem. 2015, 58, 665–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makriyannis, A.; D’Souza, M.R.; Bajaj, S.; Nikas, S.P.; Thakur, G.A. 2-Cycloalkyl Resorcinol Cannabinergic Ligands. Patent WO2014062965A1, 24 April 2014. [Google Scholar]
- Kulkarni, S.; Nikas, S.P.; Sharma, R.; Jiang, S.; Paronis, C.A.; Leonard, M.Z.; Zhang, B.; Honrao, C.; Mallipeddi, S.; Raghav, J.G.; et al. Novel C-ring-hydroxy-substituted controlled deactivation cannabinergic analogues. J. Med. Chem. 2016, 59, 6903–6919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gant, T.G. Using deuterium in drug discovery: Leaving the label in the drug. J. Med. Chem. 2014, 57, 3595–3611. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Jones, L.H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Peng, L. Photoactivatable lipid probes for studying biomembranes by photoaffinity labeling. Chem. Rev. 2013, 113, 7880–7929. [Google Scholar] [CrossRef]
- Rodríguez, G.; Albrecht, M.; Schoenmaker, J.; Ford, A.; Lutz, M.; Spek, A.L.; van Koten, G. Bifunctional pincer-type organometallics as substrates for organic transformations and as novel building blocks for polymetallic materials. J. Am. Chem. Soc. 2002, 124, 5127–5138. [Google Scholar] [CrossRef] [Green Version]
- Joshi, G.; Adimurthy, S. New method for the synthesis of benzyl alkyl ethers mediated by FeSO4. Synth. Commun. 2011, 41, 720–728. [Google Scholar] [CrossRef]
- Emmett, E.J.; Hayter, B.R.; Willis, M.C. Palladium-catalyzed synthesis of ammonium sulfinates from aryl halides and a sulfur dioxide surrogate: A gas- and reductant-free process. Angew. Chem. Int. Ed. 2014, 53, 10204–10208. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.T.; Curto, J.M.; Bagley, S.W.; Willis, M.C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 2017, 8, 1233–1237. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.C.; Shimada, N.; Tius, M.A.; Nikas, S.P.; Zhang, W.; Makriyannis, A. C1′-azacycloalkyl hexahydrocannabinols. J. Org. Chem. 2017, 82, 7839–7849. [Google Scholar] [CrossRef]
- Crocker, P.J.; Mahadevan, A.; Wiley, J.L.; Martin, B.R.; Razdan, R.K. The role of fluorine substitution in the structure-activity relationships (SAR) of classical cannabinoids. Bioorg. Med. Chem. Lett. 2007, 17, 1504–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareau, Y.; Dufresne, C.; Gallant, M.; Rochette, C.; Sawyer, N.; Slipetz, D.M.; Tremblay, N.; Weech, P.K.; Metters, K.M.; Labelle, M. Structure activity relationships of tetrahydrocannabinol analogues on human cannabinoid receptors. Bioorg. Med. Chem. Lett. 1996, 6, 189–194. [Google Scholar] [CrossRef]
- Huffman, J.W.; Hepburn, S.A.; Lyutenko, N.; Thompson, A.L.S.; Wiley, J.L.; Selley, D.E.; Martin, B.R. 1-Bromo-3-(1′,1′-dimethylalkyl)-1-deoxy-Δ(8)-tetrahydrocannabinols: New selective ligands for the cannabinoid CB(2) receptor. Bioorg. Med. Chem. 2010, 18, 7809–7815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffman, J.W.; Yu, S.; Showalter, V.; Abood, M.E.; Wiley, J.L.; Compton, D.R.; Martin, B.R.; Bramblett, R.D.; Reggio, P.H. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. J. Med. Chem. 1996, 39, 3875–3877. [Google Scholar] [CrossRef]
- Burdick, D.; DeOrazio, R.; Guzzo, P.; Habershaw, A.; Helle, M.; Paul, B.; Wolf, M. Synthesis and structure–activity relationship of substitutions at the C-1 position of Δ9-tetrahydrocannabinol. Bioorg. Med. Chem. Lett. 2010, 20, 1424–1426. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yanagita, R.C.; Hamada, N.; Murakami, A.; Takahashi, H.; Saito, N.; Nagai, H.; Irie, K. A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: Development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 2009, 131, 7573–7579. [Google Scholar] [CrossRef]
- Bunnelle, W.H.; Mckinnis, B.R.; Narayanan, B.A. Difluorination of esters—Preparation of α,α-difluoro ethers. J. Org. Chem. 1990, 55, 768–770. [Google Scholar] [CrossRef]
- Cava, M.P.; Levinson, M.I. Thionation reactions of Lawesson reagents. Tetrahedron 1985, 41, 5061–5087. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ho, T.C.; Baradwan, M.; Pascual Lopez-Alberca, M.; Iliopoulos-Tsoutsouvas, C.; Nikas, S.P.; Makriyannis, A. Synthesis of Functionalized Cannabilactones. Molecules 2020, 25, 684. https://doi.org/10.3390/molecules25030684
Liu Y, Ho TC, Baradwan M, Pascual Lopez-Alberca M, Iliopoulos-Tsoutsouvas C, Nikas SP, Makriyannis A. Synthesis of Functionalized Cannabilactones. Molecules. 2020; 25(3):684. https://doi.org/10.3390/molecules25030684
Chicago/Turabian StyleLiu, Yingpeng, Thanh C. Ho, Mohammed Baradwan, Maria Pascual Lopez-Alberca, Christos Iliopoulos-Tsoutsouvas, Spyros P. Nikas, and Alexandros Makriyannis. 2020. "Synthesis of Functionalized Cannabilactones" Molecules 25, no. 3: 684. https://doi.org/10.3390/molecules25030684