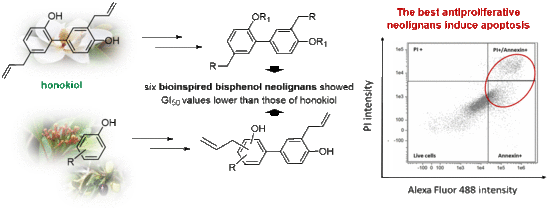
Synthesis of Bisphenol Neolignans Inspired by Honokiol as Antiproliferative Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Biochemical Assay
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compound 3
3.3. Synthesis of Compound 4
3.4. Synthesis of Compounds 5–7, 10a and b
3.5. Synthesis of Bromophenols 11a-c
3.6. Suzuki–Miyaura Cross-Coupling Reaction: Synthesis of Bisphenols 12a-c
3.7. Synthesis of O-Allyloxy Neolignans 8 and 13a-c
3.8. General Procedure for Claisen Rearrangement: Synthesis of Bisphenols 14a-c and 15a
3.9. Human Cell Cultures
3.10. Antiproliferative Assay
3.11. Apoptosis Analysis by Imaging Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Chen, J.J.; Liang, Z.Z.; Zhao, C.Q. New lignans and their biological activities. Chem. Biodivers. 2014, 11, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, H.; Richarz, R.; Gulder, T.A.M. The biocatalytic repertoire of natural biaryl formation. Ange. Chem. Int. Ed. 2014, 53, 8286–8293. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Patočka, J.; Jakl, J.; Strunecká, A. Expectations of biologically active compounds of the genus Magnolia in biomedicine. J. Appl. Biomed. 2006, 4, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Kelm, M.A.; Nair, M.G. A brief summary of biologically active compounds from Magnolia spp. Stud. Nat. Prod. Chem. 2000, 24, 845–873. [Google Scholar]
- Shen, J.L.; Man, K.M.; Huang, P.H.; Chen, W.C.; Chen, D.C.; Cheng, Y.W.; Liu, P.L.; Chou, M.C.; Chen, Y.H. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 2010, 15, 6452–6465. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant activity of magnolol and honokiol: Kinetic and mechanistic investigations of their reaction with peroxyl radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. 2007, 81, 1071–1078. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A neolignan from the Magnolia family for the prevention and treatment of cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.D.; Oh, J.; Park, H.J.; Bae, K.; Lee, S.K. Magnolol inhibits angiogenesis by regulating ROS–Mediated apoptosis and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived endothelial-like cells. Int. J. Oncol. 2013, 43, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, H.L.; Zhang, T.C.; Liu, H.; Yu, L.H.; Li, G.S.; Li, H.S.; Hu, M.C. Magnolol inhibits the growth of non-small cell lung cancer via inhibiting microtubule polymerization. Cell. Physiol. Biochem. 2017, 42, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, F.; Huang, R.M.; Yan, J.; Shen, B. Honokiol inhibits bladder cancer cell invasion through repressing SRC-3 expression and epithelial-mesenchymal transition. Oncol. Lett. 2017, 14, 4294–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, P.S.; Wang, W.; Chang, Y.A.; Lin, C.J.; Wang, J.J.; Chen, R.M. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016, 370, 66–77. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Bures, M.; Praestgaard, J.; Fesik, S.W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 2000, 43, 3443–3447. [Google Scholar] [CrossRef]
- Jada, S.; Doma, M.R.; Singh, P.P.; Kumar, S.; Malik, F.; Sharma, A.; Khan, I.A.; Qazi, G.N.; Kumar, H.M.S. Design and synthesis of novel magnolol derivatives as potential antimicrobial and antiproliferative compounds. Eur. J. Med. Chem. 2012, 51, 35–41. [Google Scholar] [CrossRef]
- Amblard, F.; Govindarajan, B.; Lefkove, B.; Rapp, K.L.; Detorio, M.; Arbiser, J.L.; Schinazi, R.F. Synthesis, cytotoxicity, and antiviral activities of new neolignans related to honokiol and magnolol. Bioorg. Med. Chem. Lett. 2007, 17, 4428–4431. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Chan, M.H.; Chen, C.P. An expedient synthesis of honokiol and its analogues as potential neuropreventive agents. Bioorg. Med. Chem. Lett. 2012, 22, 216–221. [Google Scholar] [CrossRef]
- Lee, S.H.; Fei, X.; Lee, C.; Do, H.T.T.; Rhee, I.; Seo, S.Y. Synthesis of either C2-or C4′-alkylated derivatives of honokiol and their biological evaluation for anti-inflammatory activity. Chem. Pharm. Bull. 2019, 67, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.M.; Gowda, A.S.P.; Sharma, A.K.; Amin, S. In vitro growth inhibition of human cancer cells by novel honokiol analogs. Bioorg. Med. Chem. 2012, 20, 3202–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maioli, M.; Basoli, V.; Carta, P.; Fabbri, D.; Dettori, M.A.; Cruciani, S.; Serra, P.A.; Delogu, G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS ONE 2018, 13, e0192178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Peris, M.; Murga, J.; Falomir, E.; Carda, M.; Marco, J.A. Synthesis of honokiol analogues and evaluation of their modulating action on VEGF protein secretion and telomerase-related gene expressions. Chem. Biol. Drug Des. 2017, 89, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Buchwald, S.L. Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Pulvirenti, L.; Muccilli, V.; Cardullo, N.; Spatafora, C.; Tringali, C. Chemoenzymatic synthesis and alpha-glucosidase inhibitory activity of dimeric neolignans inspired by magnolol. J. Nat. Prod. 2017, 80, 1648–1657. [Google Scholar] [CrossRef]
- Cardullo, N.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.; Tringali, C. Resveratrol-related polymethoxystilbene glycosides: Synthesis, antiproliferative activity, and glycosidase inhibition. J. Nat. Prod. 2015, 78, 2675–2683. [Google Scholar] [CrossRef]
- Cardullo, N.; Pulvirenti, L.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.F.; Tringalii, C. Dihydrobenzofuran neolignanamides: Laccase-mediated biomimetic synthesis and antiproliferative activity. J. Nat. Prod. 2016, 79, 2122–2134. [Google Scholar] [CrossRef]
- Chillemi, R.; Cardullo, N.; Greco, V.; Malfa, G.; Tomasello, B.; Sciuto, S. Synthesis of amphiphilic resveratrol lipoconjugates and evaluation of their anticancer activity towards neuroblastoma SH-SY5Y cell line. Eur. J. Med. Chem. 2015, 96, 467–481. [Google Scholar] [CrossRef]
- Capolupo, A.; Tosco, A.; Mozzicafreddo, M.; Tringali, C.; Cardullo, N.; Monti, M.C.; Casapullo, A. Proteasome as a new target for bio-inspired benzo[k,l]xanthene lignans. Chem. Eur. J. 2017, 23, 8371–8374. [Google Scholar] [CrossRef]
- Spatafora, C.; Barresi, V.; Bhusainahalli, V.M.; Di Micco, S.; Musso, N.; Riccio, R.; Bifulco, G.; Condorelli, D.; Tringali, C. Bio-inspired benzo[k,l]xanthene lignans: Synthesis, DNA-interaction and antiproliferative properties. Org. Biomol. Chem. 2014, 12, 2686–2701. [Google Scholar] [CrossRef]
- Cardullo, N.; Catinella, C.; Floresta, G.; Muccilli, V.; Rosselli, S.; Rescifina, A.; Bruno, M.; Tringali, C. Synthesis of rosmarinic acid amides as antioxidative and hypoglycemic agents. J. Nat. Prod. 2019, 82, 573–582. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Poiségu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.; Pulvirenti, L.; Cardullo, N.; Muccilli, V.; Tempera, G.; Nicolosi, D.; Tringali, C. Bioinspired benzoxanthene lignans as a new class of antimycotic agents: Synthesis and Candida spp. growth inhibition. Nat. Prod. Res. 2018. publicated ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, S.; Spatafora, C.; Cardullo, N.; Riccio, R.; Fischer, K.; Pergola, C.; Koeberle, A.; Werz, O.; Chalal, M.; Vervandier-Fasseur, D.; et al. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem. 2016, 24, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Gerstmeier, J.; Kretzer, C.; Di Micco, S.; Miek, L.; Butschek, H.; Cantone, V.; Bilancia, R.; Rizza, R.; Troisi, F.; Cardullo, N.; et al. Novel benzoxanthene lignans that favorably modulate lipid mediator biosynthesis: A promising pharmacological strategy for anti-inflammatory therapy. Biochem. Pharmacol. 2019, 165, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, S.; Pulvirenti, L.; Bruno, I.; Terracciano, S.; Russo, A.; Vaccaro, M.C.; Ruggiero, D.; Muccilli, V.; Cardullo, N.; Tringali, C.; et al. Identification by inverse virtual screening of magnolol-based scaffold as new tankyrase-2 inhibitors. Bioorg. Med. Chem. 2018, 26, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef]
- Cardile, V.; Lombardo, L.; Spatafora, C.; Tringali, C. Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorg. Chem. 2005, 33, 22–33. [Google Scholar] [CrossRef]
- Ng, S.Y.; Cardullo, N.; Yeo, S.C.M.; Spatafora, C.; Tringali, C.; Ong, P.-S.; Lin, H.-S. Quantification of the resveratrol analogs trans-2,3-dimethoxy-stilbene and trans-3,4-dimethoxystilbene in rat plasma: Application to pre-clinical pharmacokinetic studies. Molecules 2014, 19, 9577–9590. [Google Scholar] [CrossRef] [Green Version]
- Schuhly, W.; Hufner, A.; Pferschy-Wenzig, E.M.; Prettner, E.; Adams, M.; Bodensieck, A.; Kunert, O.; Oluwemimo, A.; Haslinger, E.; Bauer, R. Design and synthesis of ten biphenyl-neolignan derivatives and their in vitro inhibitory potency against cyclooxygenase-1/2 activity and 5-lipoxygenase-mediated LTB4-formation. Bioorg. Med. Chem. 2009, 17, 4459–4465. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, Y.F.; Tu, X.; Wu, X.H.; Wang, G.X.; Ling, F. Isolation of anti-Saprolegnia lignans from Magnolia officinalis and SAR evaluation of honokiol/magnolol analogs. Bioorg. Med. Chem. Lett. 2019, 29, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sakurai, Y.; Okazaki, H.; Hasegawa, T.; Fukuyama, Y. Biphenyl Derivative Composition for Nerve Cell Degeneration Repairing or Protective Agent and Process for Preparing a Phenyl Derivative Contained in the Composition. U.S. Patent 5053548, 1 October 1991. [Google Scholar]
- Bovicelli, P.; Antonioletti, R.; Mancini, S.; Causio, S.; Borioni, G.; Annnendola, S.; Barontini, M. Expedient synthesis of hydroxytyrosol and its esters. Synt. Commun. 2007, 37, 4245–4252. [Google Scholar] [CrossRef]
- Ichikawa, T.; Netsu, M.; Mizuno, M.; Mizusaki, T.; Takagi, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a unique heterogeneous palladium catalyst for the Suzuki–Miyaura reaction using (hetero)aryl chlorides and chemoselective hydrogenation. Adv. Synth. Catal. 2017, 359, 2269–2279. [Google Scholar] [CrossRef]
- Brunel, J.M. Scope, limitations and mechanistic aspects in the selective homogeneous palladium-catalyzed reduction of alkenes under transfer hydrogen conditions. Tetrahedron 2007, 63, 3899–3906. [Google Scholar] [CrossRef]
- Freudenberg, K.; Renner, K.C. Biphenyls and diaryl ethers among the precursors of lignin. Chem. Ber. 1965, 98, 1879–1892. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, T.J.; Diao, H.P.; Li, M.P. Quantitative structure-activity relationship studies of phenol’s biological activity. Shanxi Daxue Xuebao 2011, 34, 468–474. [Google Scholar]
- Accardo, A.; Del Zoppo, L.; Morelli, G.; Condorelli, D.F.; Barresi, V.; Musso, N.; Spampinato, G.; Bellia, F.; Tabbi, G.; Rizzarelli, E. Liposome antibody-ionophore conjugate antiproliferative activity increases by cellular metallostasis alteration. Med. Chem. Comm. 2016, 7, 2364–2367. [Google Scholar] [CrossRef]
- Scherf, U.; Ross, D.T.; Waltham, M.; Smith, L.H.; Lee, J.K.; Tanabe, L.; Kohn, K.W.; Reinhold, W.C.; Myers, T.G.; Andrews, D.T.; et al. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3-9, 12a-c, 13a-c, 14a-c and 15a are available from the authors. |






| Entry | Brominating Agent | Catalyst | Solvent1 | % Yield (11a)2 |
|---|---|---|---|---|
| 1 | NBS | I2 | CH3CN | 10 |
| 2 | NBS | I2 | CHCl3 | 12 |
| 3 | NBS | AlCl3 | CH3CN | 15 |
| 4 | NBS | AlCl3 | CHCl3 | 25 |
| 5 | Br2 | AlCl3 | CH3CN | 18 |
| 6 | Br2 | / | CHCl3 | 47 |
| 73 | Br2 | / | CHCl3 | 63 |
| 84 | NaBr/oxone | / | acetone/water | 5 |
| Entry | Solvent | Temperature | % Yield (12a)1 |
|---|---|---|---|
| 1 | THF | 25 °C | 5 |
| 2 | THF | 70 °C | 10 |
| 3 | THF/H2O2 | 70 °C | 20 |
| 4 | THF/H2O3 | 70 °C | 67 |
| 5 | 1,4-dioxane | 70 °C | 6 |
| 6 | 1,4-dioxane | 180 °C | 8 |
| Compound | GI50 (µM) ± SD 1 | ||
|---|---|---|---|
| HCT-116 | HT-29 | PC3 | |
| 2 | 18.2 ± 2.1 | 40.6 ± 3.9 | 52.1 ± 7.1 |
| 3 | 22.5 ± 3.4 | > 100 | 51.3 ± 7.2 |
| 4 | 63.9 ± 5.9 | > 100 | > 100 |
| 5 | 21.2 ± 2.6 | 9.9 ± 2.1 | 10.5 ± 1.7 |
| 6 | 20.1 ± 2.9 | > 100 | 20.6 ± 3.2 |
| 7 | 64.7 ± 7.9 | 70.2 ± 8.7 | > 100 |
| 8 | 38.1 ± 0.6 | 40.7 ± 0.5 | > 100 |
| 9 | 11.1 ± 0.9 | 9.8 ± 1.4 | 19.8 ± 1.8 |
| 12a | 24.1 ± 3.0 | 65.9 ± 8.3 | > 100 |
| 12b | 14.7 ± 2.1 | 20.5 ± 3.1 | 47.9 ± 4.7 |
| 12c | > 100 | > 100 | > 100 |
| 13a | > 100 | > 100 | > 100 |
| 13b | 69.7 ± 6.9 | 42.3 ± 4.7 | > 100 |
| 13c | 84.5 ± 8.0 | 42.0 ± 4.1 | > 100 |
| 14a | 5.3 ± 1.5 | 13.0 ± 2.0 | 5.8 ± 1.9 |
| 14b | 8.2 ± 1.1 | 12.3 ± 1.6 | 17.2 ± 1.5 |
| 14c | 3.7 ± 0.7 | 11.3 ± 2.2 | 19.1 ± 2.6 |
| 15a | 3.6 ± 0.6 | 12.7 ± 2.1 | 8.9 ± 2.0 |
| 5-FU | 6.2 ± 0.8 | 7.3 ± 0.7 | 9.0 ± 0.9 |
| Entry | HTC-116 Cell Distribution (%) | PC3 Cell Distribution (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | 14a | 14c | 15a | 5-FU | Ctrl | 14a | 14c | 15a | 5-FU | |
| Live | 82.1 | 74.8 | 70.9 | 46.3 | 17.0 | 92.7 | 79.9 | 86.5 | 61.3 | 24.0 |
| Early apoptosis (Annexin+) | 1.5 | 9.5 | 6.0 | 17.4 | 55.0 | 0.9 | 12.4 | 5.3 | 26.0 | 50.0 |
| Necrotic cells (PI+) | 1.6 | 4.8 | 4.4 | 0.4 | 0.2 | 1.7 | 0.1 | 0.2 | 0.4 | 0.3 |
| Late apoptosis (Annexin+/PI+) | 14.9 | 10.9 | 18.7 | 35.9 | 27.8 | 4.7 | 7.7 | 8.0 | 12.4 | 25.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardullo, N.; Barresi, V.; Muccilli, V.; Spampinato, G.; D’Amico, M.; Condorelli, D.F.; Tringali, C. Synthesis of Bisphenol Neolignans Inspired by Honokiol as Antiproliferative Agents. Molecules 2020, 25, 733. https://doi.org/10.3390/molecules25030733
Cardullo N, Barresi V, Muccilli V, Spampinato G, D’Amico M, Condorelli DF, Tringali C. Synthesis of Bisphenol Neolignans Inspired by Honokiol as Antiproliferative Agents. Molecules. 2020; 25(3):733. https://doi.org/10.3390/molecules25030733
Chicago/Turabian StyleCardullo, Nunzio, Vincenza Barresi, Vera Muccilli, Giorgia Spampinato, Morgana D’Amico, Daniele Filippo Condorelli, and Corrado Tringali. 2020. "Synthesis of Bisphenol Neolignans Inspired by Honokiol as Antiproliferative Agents" Molecules 25, no. 3: 733. https://doi.org/10.3390/molecules25030733