Destabilization of NaBH4 by Transition Metal Fluorides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ball Milling Effects of the TMFs on NaBH4
2.2. Effect of TMFs on the Destabilization of NaBH4
2.2.1. Pure NaBH4 with Different Calorimetry Methods
2.2.2. Temperature-Programmed Desorption Results
2.2.3. Closed Crucible DSC-Setaram Discussion
2.2.4. DSC-Netzsch Discussion
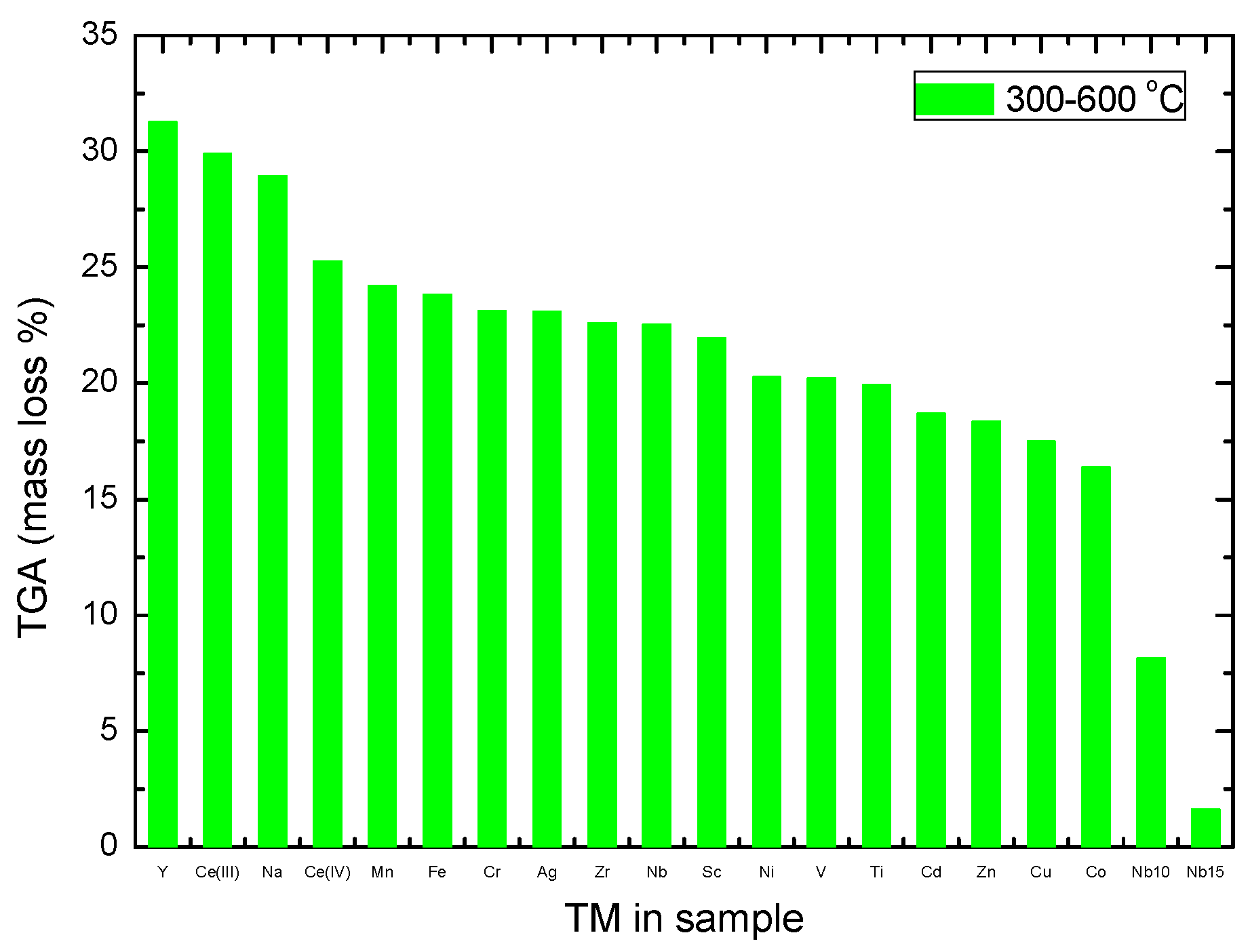
2.3. Thermogravimetric Analysis
3. Materials and Methods
4. Conclusions
- In particular, NbF5 and MnF3 were very good destabilizers of NaBH4, with a 30 C decrease of its melting temperature and a 50 to 57 C decrease of its decomposition temperature, while still giving high decomposition gas yields in the 300 and 600 C region of 24.2 and 22.5 wt%, for 2 mol% of MnF3 and NbF5, respectively, that might include evaporation of Na.
- In addition, the strong reactivity of NbF5 meant that the yield of hydrogen from a mixture with NaBH4 decreased strongly with increasing fluoride amount (1.6 wt%, for 15 mol% of NbF5), since most of the hydrogen was lost during the ball milling process.
- Increasing the additive amount from 2 to 10 and 15 mol% led to the loss of the NaBH4 and therefore the loss of hydrogen yield during thermal decomposition.
- Higher oxidation states of the metal in the fluoride were more efficient in reducing the melting and decomposition temperatures of NaBH4. This was confirmed by the comparison between CeF3 and CeF4 (506 and 502 C, respectively), but also by the results showing NbF5, the TM fluoride with highest oxidation state, being one of the most efficient destabilizers.
- An increase of the oxidation state also seemed to lead to a decrease of the gas yield in the 300 and 600 C region, with 29.9 and 25.3 wt%, for CeF3 and CeF4, respectively).
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| PEMFCs | proton exchange membrane fuel cells |
| DBFCs | direct boron hydride fuel cells |
| TM | transition metals |
| PXD | powder X-ray diffraction |
| DSC | differential scanning calorimetry |
| TPD | temperature-programmed desorption |
| TMF | transition metal fluorides |
| TGA | thermogravimetric analysis |
| RT | room temperature |
| RGA | residual gas analyzer |
Appendix A
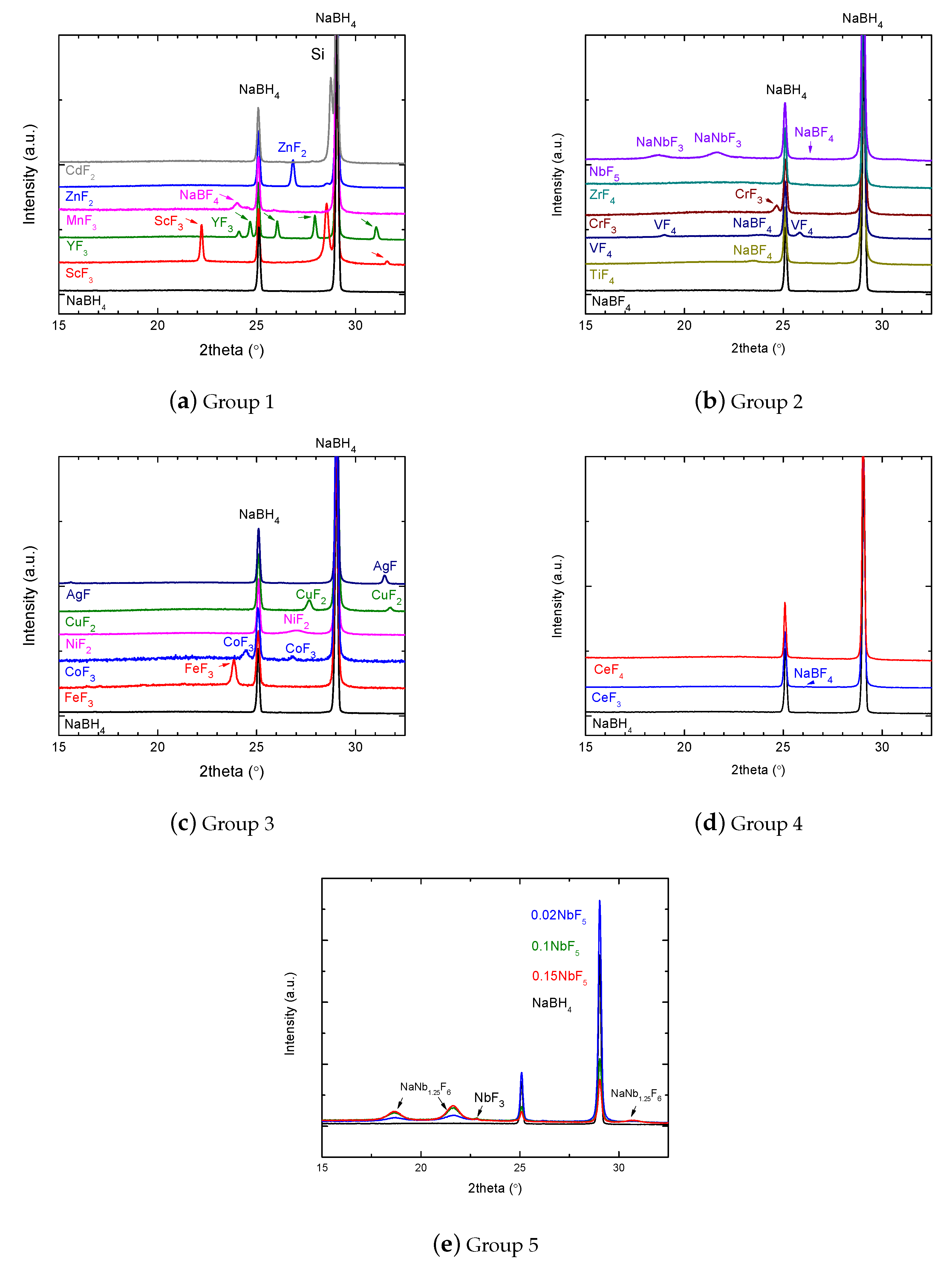
Appendix A.1. PXD Data in Plots, Separated into Groups
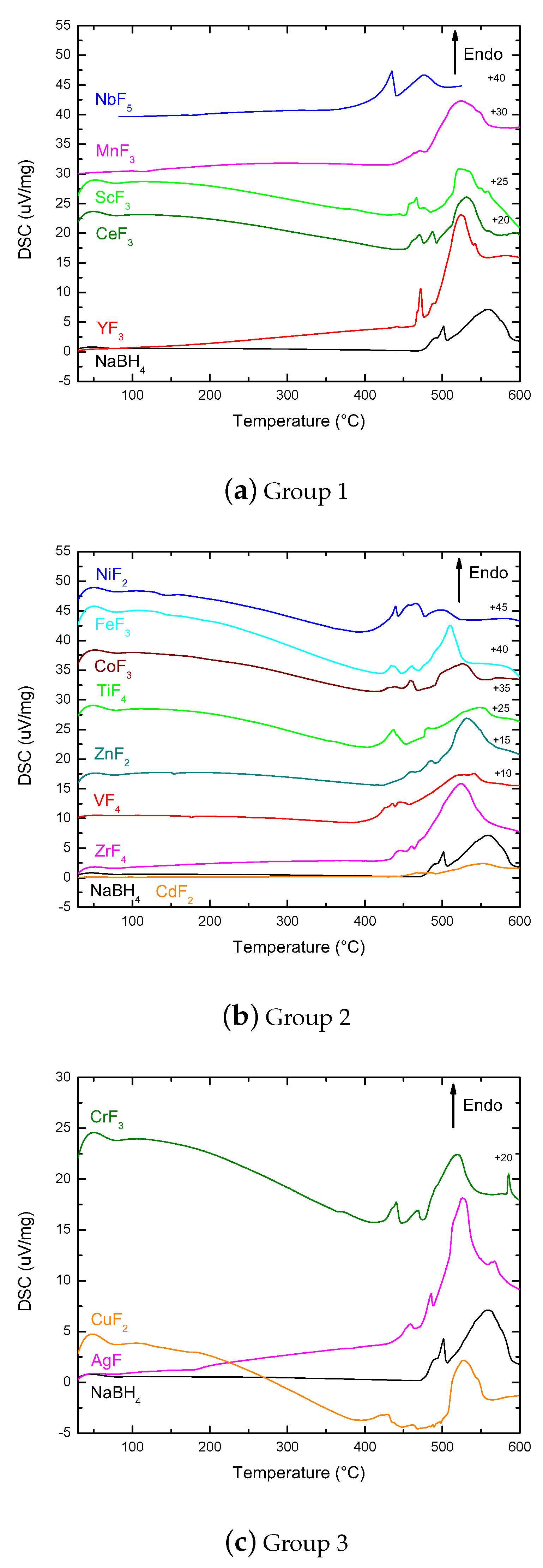
Appendix A.2. TGA Data in Plots, Separated into Groups

References
- Varin, R.A.; Bidabadi, A.S. Nanostructured, complex hydride systems for hydrogen generation. AIMS Energy 2015, 3, 121–143. [Google Scholar] [CrossRef]
- Xin, L.; Chuan, W.; Feng, W. Light metal complex hydride hydrogen storage systems. Prog. Chem. 2015, 27, 1167–1181. [Google Scholar]
- Callini, E.; Atakli, Z.Ö.K.; Hauback, B.C.; Orimo, s.I.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, R.; Orimo, S.I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2017, 2, 16091. [Google Scholar] [CrossRef]
- Frommen, C.; Sørby, M.H.; Heere, M.; Humphries, T.; Oslen, J.E.; Hauback, B.C. Rare earth borohydrides-crystal structures and thermal properties. Energies 2017, 10, 2115. [Google Scholar] [CrossRef] [Green Version]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef] [Green Version]
- Humphries, T.D.; Kalantzopoulos, G.N.; Llamas-Jansa, I.; Olsen, J.E.; Hauback, B.C. Reversible Hydrogenation Studies of NaBH4 Milled with Ni-Containing Additives. J. Phys. Chem. C 2013, 117, 6060–6065. [Google Scholar] [CrossRef] [Green Version]
- Llamas-Jansa, I.; Friedrichs, O.; Fichtner, M.; Bardaji, E.G.; Züttel, A.; Hauback, B.C. The Role of Ca(BH4)2 Polymorphs. J. Phys. Chem. C 2012, 116, 13472–13479. [Google Scholar] [CrossRef]
- Albanese, E.; Kalantzopoulos, G.N.; Vitillo, J.G.; Pinatel, E.; Civalleri, B.; Deledda, S.; Bordiga, S.; Hauback, B.C.; Baricco, M. Theoretical and experimental study on Mg(BH4)2-Zn(BH4)2 mixed borohydrides. J. Alloy. Compd. 2013, 580, MH2012. [Google Scholar] [CrossRef] [Green Version]
- Kalantzopoulos, G.; Vitillo, J.; Albanese, E.; Pinatel, E.R.; Civalleri, B.; Deledda, S.; Bordiga, S.; Baricco, M.; Hauback, B.H. Hydrogen storage of Mg–Zn mixed metal borohydrides. J. Alloys Compd. 2014, 615 (Suppl. 1), S702–S705. [Google Scholar] [CrossRef] [Green Version]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives – synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrogen Energy 2016, 41, 14387–14403. [Google Scholar] [CrossRef] [Green Version]
- Pottmaier, D.; Baricco, M. Materials for hydrogen storage and the Na-Mg-B-H system. AIMS Energy 2015, 3, 75–100. [Google Scholar] [CrossRef]
- Urgnani, J.; Torres, F.J.; Palumbo, M.; Baricco, M. Hydrogen release from solid state NaBH4. Int. J. Hydrogen Energy 2008, 33, 3111–3115. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. Go/No-Go Recommendation for Sodium Borohydride for on-Board Vehicular Hydrogen Storage; Technical Report; Review Panel Recommendation Report; U.S. Department of Energy (DoE): Washington, DC, USA, 2007.
- Santos, D.; Sequeira, C. Sodium borohydride as a fuel for the future. Renew. Sustain. Energy Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Kim, T.; Kwon, S. Design and development of a fuel cell-powered small unmanned aircraft. Int. J. Hydrogen Energy 2012, 37, 615–622. [Google Scholar] [CrossRef]
- Mao, J.; Gregory, D.H. Recent Advances in the Use of Sodium Borohydride as a Solid State Hydrogen Store. Energies 2015, 8, 430–453. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, M.J.; Kang, S.; Kim, T. Development of a high-storage-density hydrogen generator using solid-state NaBH4 as a hydrogen source for unmanned aerial vehicles. Appl. Energy 2019, 251, 113331. [Google Scholar] [CrossRef]
- Llamas-Jansa, I.; Aliouane, N.; Deledda, S.; Fonneløp, J.E.; Frommen, C.; Humphries, T.; Lieutenant, K.; Sartori, S.; Sørby, M.H.; Hauback, B.C. Chloride substitution induced by mechano-chemical reactions between NaBH4 and transition metal chlorides. J. Alloys Compd. 2012, 530, 186–192. [Google Scholar] [CrossRef]
- Kalantzopoulos, G.N.; Guzik, M.N.; Deledda, S.; Heyn, R.H.; Muller, J.; Hauback, B.C. Destabilization effect of transition metal fluorides on sodium borohydride. Phys. Chem. Chem. Phys. 2014, 16, 20483–20491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Liu, B.H.; Li, Z.P. Destabilization of LiBH4 by (Ce, La)(Cl, F)3 for hydrogen storage. J. Alloys Compd. 2011, 509, 751–757. [Google Scholar] [CrossRef]
- Al–Kukhun, A.; Hwang, H.T.; Varma, A. NbF5 additive improves hydrogen release from magnesium borohydride. Int. J. Hydrogen Energy 2012, 37, 17671–17677. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, H.; Liu, J.W.; Zhu, M. Thermal decomposition behaviors of magnesium borohydride doped with metal fluoride additives. Thermochim. Acta 2013, 560, 82–88. [Google Scholar] [CrossRef]
- Minella, C.B.; Garroni, S.; Pistidda, C.; Baro, M.D.; Gutfleisch, O.; Klassen, T.; Dornheim, M. Sorption properties and reversibility of Ti(IV) and Nb(V)-fluoride doped-Ca(BH4)2–MgH2 system. J. Alloys Compd. 2015, 622, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhang, L.; Gao, S.; Liu, H.; Xu, L.; Wang, X.; Yan, M. Hydrogen storage properties of activated carbon confined LiBH4 doped with CeF3 as catalyst. Int. J. Hydrogen Energy 2017, 42, 23010–23017. [Google Scholar] [CrossRef]
- Richter, B.; Ravnsbaek, D.B.; Sharma, M.; Spyratou, A.; Hagemann, H.; Jensen, T.R. Fluoride substitution in LiBH4; destabilization and decomposition. Phys. Chem. Chem. Phys. 2017, 19, 30157–30165. [Google Scholar] [CrossRef]
- Rude, L.H.; Filsø, U.; D’Anna, V.; Spyratou, A.; Richter, B.; Hino, S.; Zavorotynska, O.; Baricco, M.; Sørby, M.H.; Hauback, B.C.; et al. Hydrogen–fluorine exchange in NaBH4–NaBF4. Phys. Chem. Chem. Phys. 2013, 15, 18185–18194. [Google Scholar] [CrossRef] [Green Version]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Effects of La fluoride and La hydride on the reversible hydrogen sorption behaviors of NaBH4: A comparative study. J. Mater. Chem. A 2014, 2, 8557–8570. [Google Scholar] [CrossRef]
- Huang, T.; Zou, J.; Zhao, N.; Zeng, X.; Ding, W. Reversible hydrogen storage system of 3NaBH4-0.5ScF3-0.5YF3: The synergistic effect of ScF3 and YF3. J. Alloys Compd. 2019, 791, 1270–1276. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, J.; Zeng, X.; Ding, W. Mechanisms of partial hydrogen sorption reversibility in a 3NaBH4/ScF3 composite. RSC Adv. 2018, 8, 9211–9217. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Zou, J.; Meng, F.; Wang, J.; Liu, H.; Ding, W. Reversible hydrogen sorption behaviors of the 3NaBH4-(x)YF3-(1 - x)GdF3 system: The effect of double rare earth metal cations. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts 2018, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Guo, Z.; Nevirkovets, I.; Liu, H.; Dou, S. Hydrogen De-/Absorption Improvement of NaBH4 Catalyzed by Titanium-Based Additives. J. Phys. Chem. C 2011, 116, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Lee, C.H.; Matsui, K.; Kousaka, K.; Isobe, S.; Hashimoto, N.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Doping effect of Nb species on hydrogen desorption properties of AlH3. J. Alloys Compd. 2018, 734, 55–59. [Google Scholar] [CrossRef]
- Malka, I.E.; Bystrzycki, J. The effect of storage time on the thermal behavior of nanocrystalline magnesium hydride with metal halide additives. Int. J. Hydrogen Energy 2014, 39, 3352–3359. [Google Scholar] [CrossRef]
- Malka, I.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball-milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Jin, S.A.; Shim, J.H.; Cho, Y.W.; Yi, K.W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sources 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Ma, L.P.; Cheng, H.M. Hydrogen sorption kinetics of MgH2 catalyzed with NbF5. J. Alloys Compd. 2008, 453, 138–142. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Zhang, T.; Kitamura, M.; Isobe, S.; Hino, S.; Hashimoto, N.; Ohnuki, S. A Systematic Study of the Effects of Metal Chloride Additives on H2 Desorption Properties of Ammonia Borane. J. Chem. Eng. Data 2016, 61, 1924–1929. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Roine, A. Peep Database, HSC Outkumpu Chemistry for Windows; Vs. 5.1; 02103-ORC-T; Schlumberger Limited: Honston, TX, USA, 2002; ISBN 952-9507-08-9. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. They were destroyed due to age. |





| Sample | NaBH4 (wt%) | TMF (wt%) | NaBF4 (wt%) | TM (wt%) | Other (wt%) | NaBH4 (wt%) | TMF (wt%) |
|---|---|---|---|---|---|---|---|
| ScF3 | 95.5 | 4.5 | 94.9 | 5.1 | |||
| TiF4 | 82.0 | 18.0 | 93.9 | 6.2 | |||
| VF4 | 77.9 | 3.6 | 18.5 | 93.7 | 6.3 | ||
| CrF3 | 95.7 | 4.0 | 0.4 | 94.6 | 5.5 | ||
| MnF3 | 55.3 | 44.7 | 94.4 | 5.6 | |||
| FeF3 | 94.9 | 5.1 | 94.4 | 5.6 | |||
| CoF3 | 95.8 | 3.2 | 1.0 | 94.2 | 5.8 | ||
| NiF2 | 94.5 | 5.5 | 95.1 | 4.9 | |||
| CuF2 | 88.8 | 9.8 | 1.4 | 94.9 | 5.1 | ||
| ZnF2 | 95.1 | 4.9 | 94.8 | 5.2 | |||
| YF3 | 92.5 | 7.5 | 92.8 | 7.2 | |||
| ZrF4 | 100 | 91.9 | 8.1 | ||||
| NbF5 (2 mol%) | 88.1 | 4.6 | 2.8 | 91.0 | 9.0 | ||
| NbF5 (10 mol%) | 70.2 | 8.0 | 2.4 | 66.8 | 33.2 | ||
| NbF5 (15 mol%) | 59.0 | 10.6 | 2.6 | 57.3 | 42.7 | ||
| AgF | 93.7 | 0.8 | 2.9 | 1.0 | 1.5 | 93.7 | 6.3 |
| CdF2 | 90.5 | 6.6 | 2.9 | 92.6 | 7.4 | ||
| CeF3 | 97.6 | 2.4 | 90.6 | 9.4 | |||
| CeF4 | 100 | 89.8 | 10.3 |
| Sample | Decomposition | Difference | Melting | Difference |
|---|---|---|---|---|
| C | C | C | C | |
| NaBH4 | 535 | 0 | 503 | 0 |
| TiF4 | 522 | 13 | 452 | 51 |
| YF3 | 513 | 22 | 462 | 41 |
| CdF2 | 512 | 23 | 461 | 42 |
| CeF3 | 506 | 29 | 487 | 16 |
| ZnF2 | 504 | 31 | 460 | 43 |
| ZrF4 | 503 | 32 | 460 | 43 |
| CeF4 | 502 | 33 | 457 | 46 |
| CoF3 | 501 | 34 | 452 | 51 |
| ScF3 | 501 | 34 | 455 | 48 |
| CrF3 | 500 | 35 | 464 | 39 |
| VF4 | 499 | 36 | 452 | 51 |
| FeF3 | 498 | 37 | 460 | 43 |
| AgF | 498 | 37 | 466 | 37 |
| MnF3 | 483 | 52 | 453 | 50 |
| CuF2 | 476 | 59 | 425 | 78 |
| NiF2 | 452 | 83 | 446 | 57 |
| NbF5 (2 mol%) | 442 | 93 | 190 | 313 |
| NbF5 (10 mol%) | 379 | 156 | 315 | 188 |
| NbF5 (15 mol%) | 379 | 156 | 315 | 188 |
| Sample | TG Mass Loss % | TG Mass Loss % |
|---|---|---|
| 100–300 C | 300–600 C | |
| NaBH4 | 14.0 | |
| YF3 | 0.7 | 31.3 |
| CeF3 | 0.3 | 29.9 |
| CeF4 | 1.4 | 25.3 |
| MnF3 | 0.2 | 24.2 |
| FeF3 | 0.4 | 24.0 |
| CrF3 | 0.1 | 23.1 |
| AgF | 0.3 | 23.1 |
| ZrF4 | 0.4 | 22.6 |
| NbF5 (2 mol%) | 0.3 | 22.5 |
| ScF3 | 0.3 | 22.0 |
| NiF2 | 0.6 | 20.3 |
| VF4 | 0.1 | 20.2 |
| TiF4 | 0.3 | 20.0 |
| CdF2 | 0.9 | 19.0 |
| ZnF2 | 0.2 | 18.3 |
| CuF2 | 0.5 | 17.5 |
| CoF3 | 0.4 | 16.4 |
| NbF5 (10 mol%) | 3.5 | 8.2 |
| NbF5 (15 mol%) | 3.6 | 1.6 |
| 1st period | ScF3 | TiF4 | VF4 | CrF3 | MnF3 | FeF3 | CoF3 | NiF2 | CuF2 | ZnF2 |
|---|---|---|---|---|---|---|---|---|---|---|
| electronic | Ar | Ar | Ar3d | Ar3d | Ar3d | Ar3d | Ar3d | Ar3d | Ar3d | Ar3d |
| melting point/C | 1552 | 377 | 325 | 1100 | 600 | 1200 | 927 | 1474 | 836 | 1500 |
| enthalpy of decomposition | −385.2 | −394.2 | −335.4 | −277.2 | −256.0 | −236.7 | −188.9 | −157.1 | −128.8 | −182.7 |
| H/kcal mol | ||||||||||
| 2nd period | YF3 | ZrF4 | NbF5 | AgF | CdF2 | |||||
| electronic | Kr | Kr | Kr | Kr4d5s | Kr4d | |||||
| melting point/C | 1387 | 910 | 90 | 435 | 1110 | |||||
| enthalpy of decomposition | −410.7 | −456.8 | −433.5 | −48.5 | −167.4 | |||||
| H/kcal mol | ||||||||||
| other | CeF3 | CeF4 | ||||||||
| electronic | Xe4p | Xe | ||||||||
| melting point/C | 817 | 650 | ||||||||
| enthalpy of decomposition | −403.7 | −442.0 | ||||||||
| H/kcal mol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas Jansa, I.; Kalantzopoulos, G.N.; Nordholm, K.; Hauback, B.C. Destabilization of NaBH4 by Transition Metal Fluorides. Molecules 2020, 25, 780. https://doi.org/10.3390/molecules25040780
Llamas Jansa I, Kalantzopoulos GN, Nordholm K, Hauback BC. Destabilization of NaBH4 by Transition Metal Fluorides. Molecules. 2020; 25(4):780. https://doi.org/10.3390/molecules25040780
Chicago/Turabian StyleLlamas Jansa, Isabel, Georgios N. Kalantzopoulos, Kari Nordholm, and Bjørn C. Hauback. 2020. "Destabilization of NaBH4 by Transition Metal Fluorides" Molecules 25, no. 4: 780. https://doi.org/10.3390/molecules25040780