Revisiting the Radiosynthesis of [18F]FPEB and Preliminary PET Imaging in a Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
 | |||||
| Compound | Precursor (X) | Reported [18F]FPEB Radiochemical Yields (RCYs) | Radiolabeling Method | Validated for Human Use | Reference |
| (1) |  | 5% | Manual | ✓ | [9] |
| (2) | 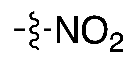 | 4–10% | Automated | ✓ | [8,10,18,19] |
| (3) | 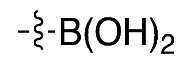 | 5% * | Automated | [11] | |
| (4) |  | 11% * | Manual | [12] | |
| (5) | 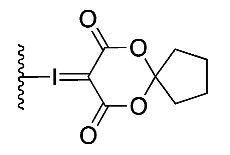 | 29% | Automated | ✓ | [13,15] |
| (6) | 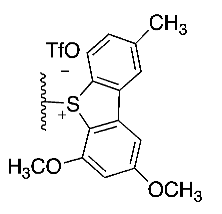 | 55% | Manual | [16] | |
2. Results and Discussion
2.1. Radiosyntheses of [18F]FPEB with Five Different Precursors
2.2. Small Animal PET/CT Imaging
3. Materials and Methods
3.1. Materials and General Methods
3.1.1. General Chemistry and Radiochemistry Methods
3.1.2. SCIDY-SPIAd Auxilary Synthesis and Characterization
3.1.3. General GE TRACERlab™ FX2 N Automated Synthesis Method
3.1.4. Preliminary Small Animal PET/CT Imaging Studies
3.2. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pillai, R.; Tipre, D. Metabotropic glutamate receptor 5—A promising target in drug development and neuroimaging. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1151–1170. [Google Scholar] [CrossRef] [PubMed]
- Terbeck, S.; Akkus, F.; Chesterman, L.P.; Hasler, G. The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: Combining preclinical evidence with human Positron Emission Tomography (PET) studies. Front. Mol. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.J.; Valenti, O.; Conn, P.J.; Conn, P.J. Glutamate Receptors and Parkinson’s Disease. Drugs Aging 2003, 20, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Alagille, D.; Dacosta, H.; Chen, Y.; Hemstapat, K.; Rodriguez, A.; Baldwin, R.M.; Conn, J.P.; Tamagnan, G.D. Potent mGluR5 antagonists: Pyridyl and thiazolyl-ethynyl-3,5-disubstituted-phenyl series. Bioorg. Med. Chem. Lett. 2011, 21, 3243–3247. [Google Scholar] [CrossRef] [Green Version]
- Rook, J.M.; Tantawy, M.N.; Ansari, M.S.; Felts, A.S.; Stauffer, S.R.; Emmitte, K.A.; Kessler, R.M.; Niswender, C.M.; Daniels, J.S.; Jones, C.K.; et al. Relationship between In Vivo Receptor Occupancy and Efficacy of Metabotropic Glutamate Receptor Subtype 5 Allosteric Modulators with Different In Vitro Binding Profiles. Neuropsychopharmacology 2014, 40, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Majo, V.J.; Prabhakaran, J.; Mann, J.J.; Kumar, J.S.D. PET and SPECT tracers for glutamate receptors. Drug Discov. Today 2013, 18, 173–184. [Google Scholar] [CrossRef]
- Wong, D.F.; Waterhouse, R.; Kuwabara, H.; Kim, J.; Chamroonrat, W.; Stabins, M.; Holt, D.P.; Dannals, R.F.; Hamill, T.G.; Mozley, P.D.; et al. 18F-FPEB, a PET Radiopharmaceutical for Quantifying Metabotropic Glutamate 5 Receptors: A First-in-Human Study of Radiochemical Safety, Biokinetics, and Radiation Dosimetry. J. Nucl. Med. 2013, 54, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.M.; Lim, K.; Labaree, D.; Lin, S.-F.; McCarthy, T.J.; Seibyl, J.P.; Tamagnan, G.; Huang, Y.; Carson, E.R.; Ding, Y.-S.; et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [18F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. Br. J. Pharmacol. 2012, 33, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Hamill, T.G.; Krause, S.; Ryan, C.; Bonnefous, C.; Govek, S.; Seiders, T.J.; Cosford, N.; Roppe, J.; Kamenecka, T.; Patel, S.; et al. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse 2005, 56, 205–216. [Google Scholar] [CrossRef]
- Liang, S.H.; Yokell, D.L.; Jackson, R.N.; Rice, P.A.; Callahan, R.; Johnson, K.A.; Alagille, D.; Tamagnan, G.; Collier, L.; Vasdev, N. Microfluidic continuous-flow radiosynthesis of [18F]FPEB suitable for human PET imaging. MedChemComm 2013, 5, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Mossine, A.V.; Brooks, A.F.; Makaravage, K.J.; Miller, J.M.; Ichiishi, N.; Sanford, M.S.; Scott, P.J.H. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett. 2015, 17, 5780–5783. [Google Scholar] [CrossRef] [PubMed]
- Makaravage, K.J.; Brooks, A.F.; Mossine, A.V.; Sanford, M.S.; Scott, P.J.H. Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett. 2016, 18, 5440–5443. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.A.; Holland, J.P.; Kassenbrock, A.; Yokell, D.L.; Livni, E.; Liang, S.H.; Vasdev, N. Iodonium ylide-mediated radiofluorination of 18F-FPEB and validation for human use. J. Nucl. Med. 2015, 56, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotstein, B.; Stephenson, N.A.; Vasdev, N.; Liang, S.H. Spirocyclic hypervalent iodine(III)-mediated radiofluorination of non-activated and hindered aromatics. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.H.; Wang, L.; Stephenson, N.; Rotstein, B.; Vasdev, N. Facile 18F labeling of non-activated arenes via a spirocyclic iodonium(III) ylide method and its application in the synthesis of the mGluR5 PET radiopharmaceutical [18F]FPEB. Nat. Protoc. 2019, 14, 1530–1545. [Google Scholar] [CrossRef]
- Gendron, T.; Sander, K.; Cybulska, K.; Benhamou, L.; Sin, P.K.B.; Khan, A.; Wood, M.; Porter, M.; Årstad, E. Ring-Closing Synthesis of Dibenzothiophene Sulfonium Salts and Their Use as Leaving Groups for Aromatic 18F-Fluorination. J. Am. Chem. Soc. 2018, 140, 11125–11132. [Google Scholar] [CrossRef] [Green Version]
- Rotstein, B.; Wang, L.; Liu, R.Y.; Patteson, J.; Kwan, E.E.; Vasdev, N.; Liang, S.H. Mechanistic studies and radiofluorination of structurally diverse pharmaceuticals with spirocyclic iodonium(iii) ylides† †Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization of compounds, NMR spectra and computational studies. Chem. Sci. 2016, 7, 4407–4417. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Q.; Tueckmantel, W.; Zhu, A.; Pellegrino, D.; Brownell, A.L. Synthesis and preliminary biological evaluation of 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse 2007, 61, 951–961. [Google Scholar] [CrossRef]
- Lim, K.; Labaree, D.; Li, S.; Huang, Y. Preparation of the Metabotropic Glutamate Receptor 5 (mGluR5) PET Tracer [18F]FPEB for Human Use: An Automated Radiosynthesis and a Novel One-Pot Synthesis of its Radiolabeling Precursor. Appl. Radiat. Isot. 2014, 94, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.E.; Holland, J.P. Computational studies on hypervalent iodonium(III) compounds as activated precursors for 18F radiofluorination of electron-rich arenes. Comput. Theor. Chem. 2015, 1066, 34–46. [Google Scholar] [CrossRef]
- Casley, C.S.; Lakics, V.; Lee, H.-G.; Broad, L.M.; Day, T.A.; Cluett, T.; Smith, M.A.; O’Neill, M.J.; Kingston, A.E. Up-regulation of astrocyte metabotropic glutamate receptor 5 by amyloid-β peptide. Brain Res. 2009, 1260, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic Glutamate Receptor 5 Is a Coreceptor for Alzheimer Aβ Oligomer Bound to Cellular Prion Protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Dhull, D.K.; Mishra, P.S. Therapeutic potential of mGluR5 targeting in Alzheimer’s disease. Front. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Precursor (X=) | Base | Solvent | Temperature (°C) | Reaction Time (min) | RCY (%) | Am (GBq/µmol) |
|---|---|---|---|---|---|---|---|
| I | (1) | K2CO3/K222 | DMSO | 200 | 10 | N/A | N/A |
| II | (2) | K2CO3/K222 | DMSO | 150 | 5 | 4 | N/A |
| III | (5) | Et4NHCO3 | DMF | 80 | 5 | 23 | 22 |
| Et4NHCO3 | DMF | 80 | 10 | 19 | 54 | ||
| Et4NHCO3 | DMF | 100 | 5 | 25 ± 2 * | 37 ± 13 * | ||
| IV | 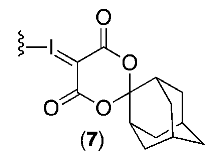 | Et4NHCO3 | DMF | 100 | 5 | 24 | 21 |
| V | (6) | Et4NHCO3 | CH3CN | 80 | 5 | 15 | 70 |
| Et4NHCO3 | CH3CN | 100 | 5 | 26 | 168 | ||
| KHCO3/K222 | CH3CN | 80 | 5 | 36 ± 6 * | 77 ± 35 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlow, C.; Murrell, E.; Holland, J.P.; Kassenbrock, A.; Shannon, W.; Liang, S.H.; Vasdev, N.; Stephenson, N.A. Revisiting the Radiosynthesis of [18F]FPEB and Preliminary PET Imaging in a Mouse Model of Alzheimer’s Disease. Molecules 2020, 25, 982. https://doi.org/10.3390/molecules25040982
Varlow C, Murrell E, Holland JP, Kassenbrock A, Shannon W, Liang SH, Vasdev N, Stephenson NA. Revisiting the Radiosynthesis of [18F]FPEB and Preliminary PET Imaging in a Mouse Model of Alzheimer’s Disease. Molecules. 2020; 25(4):982. https://doi.org/10.3390/molecules25040982
Chicago/Turabian StyleVarlow, Cassis, Emily Murrell, Jason P. Holland, Alina Kassenbrock, Whitney Shannon, Steven H. Liang, Neil Vasdev, and Nickeisha A. Stephenson. 2020. "Revisiting the Radiosynthesis of [18F]FPEB and Preliminary PET Imaging in a Mouse Model of Alzheimer’s Disease" Molecules 25, no. 4: 982. https://doi.org/10.3390/molecules25040982






