Silver Nanoparticles Stimulates Spermatogenesis Impairments and Hematological Alterations in Testis and Epididymis of Male Rats
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Ag-NP
2.2. Body Weight
2.3. Relative Organ Weight
2.4. Hematological Parameters
2.5. Hormonal Concentrations
2.6. Effect of the Ag-NP on Sperm Parameters
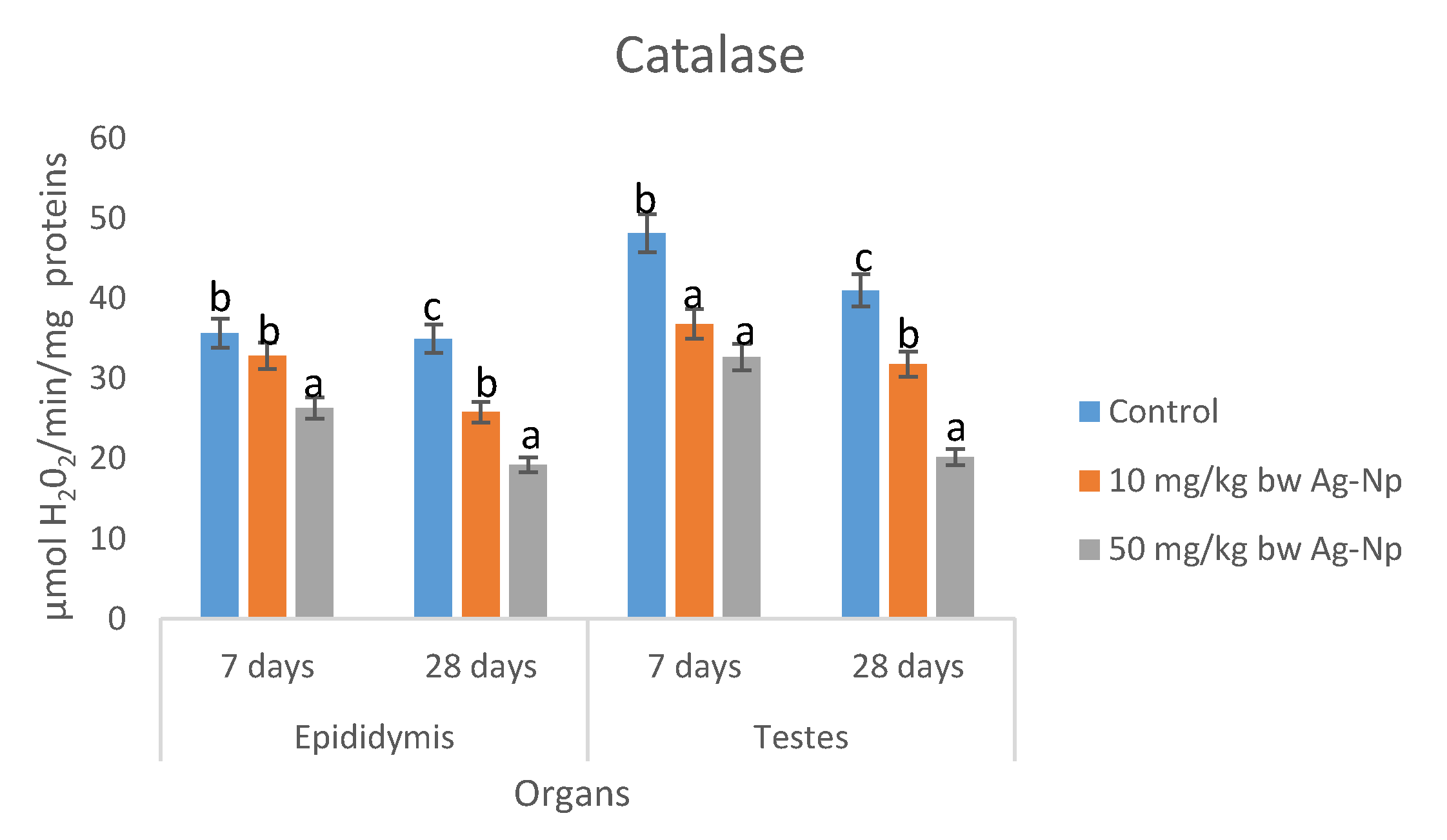
2.7. Testes and Epididymis Biochemical Parameters
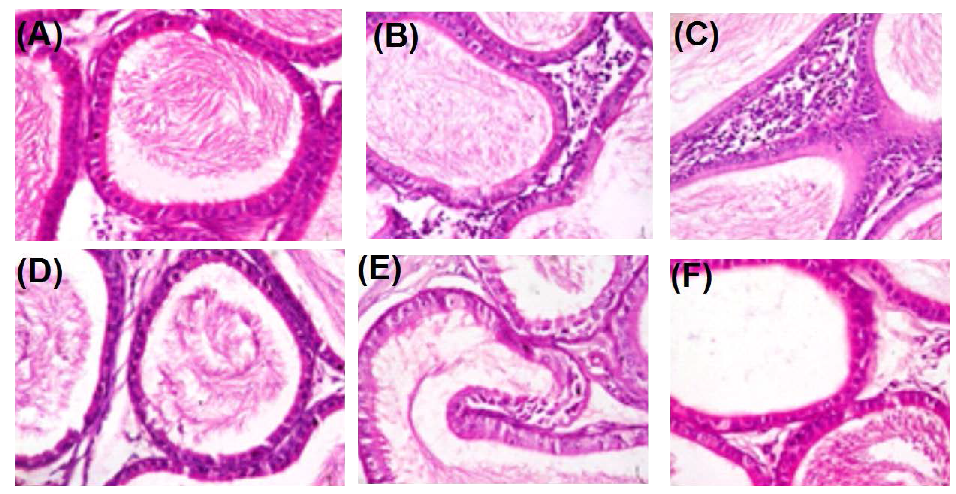
2.8. Histopathology
3. Discussion
4. Materials and Method
4.1. Synthesis and Characterization of Silver Nanoparticle (Ag-NP)
4.2. Experimental Animals
4.3. Experimental Design
4.4. Sample Collection and Preparation
4.5. Evaluation of Hematological Parameters.
4.6. Hormonal Analysis
4.7. Analysis of Testes and Epididymis Biochemical Parameters
4.8. Histological Procedures
4.9. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fathi, N.; Hoseinipanah, S.M.; Alizadeh, Z. The effect of silver nanoparticles on the reproductive system of adult male rats: A morphological, histological and DNA integrity study. Adv. Clin. Exp. Med. 2019, 28, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles–a novel aspect in cancer therapy. Mol. Cancer Ther. 2019, 13, 1740–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shittu, O.K.; Aaron, S.Y.; Oladuntoye, M.D.; Lawal, B. Diminazene aceturate modified nanocomposite for improved efficacy in acute trypanosome infection. J. Acute Dis. 2018, 7, 36–42. [Google Scholar]
- Shittu, O.K.; Lawal, B.; Ojo, A.A.; Yisa, A.S. Polyethylene glycol–modified nanocarrier encapsulation of diminazene aceturate improved haematobiochemical recovery in trypanosoma brucei brucei infected rats. Pol. J. Nat. Sci. 2019, 34, 317–332. [Google Scholar]
- Wu, J.; Wang, C.; Sun, J.; Xue, Y. Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 2011, 5, 4476–4489. [Google Scholar] [CrossRef]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 2012, 6, 8767–8777. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Sangodele, J.O.; Olaleye, M.T.; Monsees, T.K.; Akinmoladun, A.C. Redox status and sperm characteristics in 1,4-dinitrobenzene-induced reproductive toxicity in male Wistar rats. Toxicol. Environ. Health Sci. 2017, 9, 12–22. [Google Scholar] [CrossRef]
- Sharpe, R.M. The “oestrogen hypothesis”–where do we stand now? Int. J. Androl. 2003, 26, 2–15. [Google Scholar] [CrossRef]
- Yah, C.S. The toxicity of Gold Nanoparticles in relation to their physiochemical properties. Biomed. Res. 2013, 24, 400–413. [Google Scholar]
- Pothuraju, R.; Kaul, G. Effect of silver nanoparticles on functionalities of buffalo (Bubalus bubalis) spermatozoa. Adv. Sci. Eng. Med. 2013, 5, 91–95. [Google Scholar] [CrossRef]
- Talebi, A.R.; Khorsandi, L.; Moridian, M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 1203–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, C.; Pool, E.; Somerset, V. Aggregation and dissolution of silver nanoparticles in a laboratory-based freshwater microcosm under simulated environmental conditions. Toxicol. Environ. Chem. 2013, 95, 1690–1701. [Google Scholar] [CrossRef]
- Bashir, L.; Shittu, O.K.; Busari, M.B.; Sani, S.; Aisha, M.I. Safety evaluation of giant African land snails (Archachatina maginata) haemolymph on hematological and biochemical parameters of Albino rats. J. Adv. Med. Pharm. Sci. 2015, 3, 122–130. [Google Scholar] [CrossRef]
- Adenubi, O.T.; Raji, Y.; Awe, E.O.; Makinde, J.M. The effect of the aqueous extract of the leaves of Boerhavia diffusa linn. on semen and testicular morphology of male Wistar rats. Sci. World J. 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.B.V.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci. Rep. 2018, 8, 1615. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.H.; Van Der, V.; Leo, T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Sung, J.H.; Ryu, H.R.; Song, K.S.; Song, N.W.; Park, H.M.; Shin, B.S.; Ahn, K.; Gulumian, M.; Faustman, E.M.; et al. Tissue distribution of gold and silver after subacute intravenous injection of co-administered gold and silver nanoparticles of similar sizes. Arch. Toxicol. 2018, 92, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Shittu, O.K.; Lawal, B.; Abubakar, N.A.; Berinyuy, B.E.; Busari, M.B.; Ibrahim, A.O. Toxicological Implications of Methanol Extract from Nigerian Bee Propolis on Some Selected Rat Tissues. J. Pharm. Biomed. Sci. 2015, 5, 499–506. [Google Scholar]
- Berinyuy, E.B.; Lawal, B.; Olalekan, A.A.; Olalekan, A.A.; Yusuf, A.A.; Sakpe, S.; Ossai, P.C. Hematological Status and Organs/Body-weight Parameters in Wister Rats during Chronic Administration of Cassia occidentalis. Int. Blood Res. Rev. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Watanabe, N. Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxicol. Lett. 2005, 155, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mary, G.; Elaine, M.; Faustman, T.W.; Ki SooJeon, K.; IlJe, Y.U. Blood Biochemical and Hematological Study after Subacute Intravenous Injection of Gold and Silver Nanoparticles and Co-administered Gold and Silver Nanoparticles of Similar Sizes. Hindawi BioMed. Res. Int. 2018, 8460910. [Google Scholar] [CrossRef] [Green Version]
- Wise, J.; Goodale, B.; Wise, S.; Craig, G.; Pongan, A.; Walter, R.; Thompson, W.; Aboueissa, A.; Mitani, H.; Spalding, M.J. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat. Toxicol. 2010, 97, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldbjerg, R.; Dang, D.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nano-particles induce toxicity in A549 cells via ROS-dependent and ROS-inde- pendent pathways. Toxicol. Vitro 2013, 2013. 27, 330–338. [Google Scholar] [CrossRef]
- Mohanty, J.; Nagababu, E.; Rifkind, J. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Shaluei, F.; Hedayati, A.; Jahanbakhshi, A.; Kolangi, H.; Fotovat, M. Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Human Exp. Toxicol. 2013, 32, 1270–1277. [Google Scholar] [CrossRef]
- Imani, M.; Halimi, M.; Khara, H. Effects of silver nanoparticles (AgNP) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp. Clin. Pathol. 2015, 24, 491–495. [Google Scholar] [CrossRef]
- Cheraghi, J.; Hosseini, E.; Hoshmandfar, R.; Sahraei, R. Hematologic parameters study of male and female rats administered different concentrations of silver nanoparticles. Int. J. Agric. Crop. Sci. 2013, 5, 789–796. [Google Scholar]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2011, 21, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef]
- Koskinen, L.O.; Collin, O.; Bergh, A. Cigarette smoke and hypoxia induce acute changes in the testicular and cerebral microcirculation. J. Med. Sci. 2000, 105, 215–226. [Google Scholar]
- Wiwanitkit, V.; Sereemaspun, A.; Rojanathanes, R. Effect of gold nanoparticles on spermatozoa: The first world report. Fertil. Steril. 2009, 91, e7–e8. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, K.I.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, T. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Abu, A.H.; Amuta, P.O.; Buba, E.; Inusa, T.R. Evaluation of antispermatogenic effect of Garcinia kola seed extract in albino rats. Asian Pac. J. Reprod. 2013, 2, 15–18. [Google Scholar] [CrossRef]
- Madan, Z. Effect of ethanol extract of Carica papaya seeds on the histology of the epididymis of adult male albino mice. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Reuben, A.; Wurochekke, A.U.; Mahmoud, S.J. Effect of methanol extract of Carica papaya seed on some hormone function in male albino rats. Int. J. Sci. Res. 2016, 5, 387–389. [Google Scholar]
- Obinna, V.C.; Agu, G.O. Effects of beta cypermethrin exposure on male F1 generation of albino rats during perinatal development. J. Appl. Life Sci. Int. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Lafuente, D.; Garcia, T.; Blanco, J.; Sánchez, D.J.; Sirvent, J.J.; Domingo, J.L.; Gómez, M. Effects of oral exposure to silver nanoparticles on the sperm of rats. Reprod. Toxicol. 2016, 60, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of Balb/c mice. Andrologia 2018, 50, e12920. [Google Scholar] [CrossRef] [PubMed]
- Mathias, F.T.; Romano, R.M.; Kizys, M.M.; Kasamatsu, T.; Giannocco, G. Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology 2015, 9, 64–70. [Google Scholar] [CrossRef]
- Garcia, T.X.; Costa, G.M.; França, L.R.; Hofmann, M.C. Sub-acute intravenous administration of silver nanoparticles in male mice alters Leydig cell function and testosterone levels. Reprod. Toxicol. 2014, 45, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Kusemiju, T.O.; Osinubi, A.A.; Noronha, C.C.; Okanlawon, A.O. Effect of aqueous extract of the bark of Carica papaya on the testicular histology in Sprague-Dawley rats. Niger. Q. J. Hosp. Med. 2009, 20, 133–137. [Google Scholar]
- Salma, A.A.; Amer, H.A.; Shaemaa, H.A.; Abdulrahman, K.A. The effects of gold and silver nanoparticles on transaminase enzymes activities. Int. J. Chem. Res. 2011, 1, 2249–2329. [Google Scholar]
- Adeyemi, O.S.; Sulaiman, F.A. Biochemical and morphological changes in Trypanosoma brucei brucei-infected rats treated with homidium chloride and diminazene aceturate. J. Basic Clin. Physiol. Pharmacol. 2012. [Google Scholar] [CrossRef]
- Sangodele, J.O.; Olaleye, M.T.; Monsees, T.K.; Akinmoladun, A.C. Akinmoladun The para isomer of Dinitrobenzene disrupts redox homeostasis in liver and kidney of male wistar rats. Biochem. Biophys. Rep. 2017, 10, 297–302. [Google Scholar] [CrossRef]
- Lawal, B.; Shittu, O.K.; Ossai, P.C.; Abubakar, A.N.; Ibrahim, A.M. Antioxidant Activities of Giant African Snail (Achachatina maginata) Haemolymph against CCl4- Induced Hepatotoxicity in Albino Rats. Br. J. Pharm. Res. 2015, 6, 141–154. [Google Scholar] [CrossRef]
- Oyeyemi, M.O.; Akusu, M.O.; Olaoye, M.O.; Omobowale, O.T. Effect of frequent ejaculation on the semen characteristic of West African Dwarf Bucks. Troical. Vet. 1996, 14, 71–75. [Google Scholar]
- Chmielowiec-Korzeniowska, A.; Tymczyna, L.; Dobrowolska, M.; Banach, M.; Nowakowicz-Dębek, B.; Bryl, M.; Drabik, A.; Tymczyna-Sobotka, M.; Kolejko, M. Silver (Ag) in tissues and eggshells, biochemical parameters and oxidative stress in chickens. Open Chem. 2015, 13, 1269–1274. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, S.; Self, W.T. Exposure to silver nano- particles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. Environ. Health Perspect. 2011, 120. Available online: http://dx.doi.org/10.1289/ehp.1103928 (accessed on 9 August 2019).
- Farombi, E.O.; Adedara, I.A.; Abolaji, A.O.; Anamelechi, J.P.; Sangodele, J.O. Sperm characteristics, antioxidant status and hormonal profile in rats treated with artemisinin. First Int. J. Androl. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Wang, X.; Jia, G.; Gu, Y.; Wang, T.; Nie, H.; Ge, C.; Wang, H.; Liu, Y. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 2009, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induced oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Faniyan, T.O. Antioxidant status of rats administered silver nanoparticles orally. J. Taibah Univ. Sci. 2014, 9, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Dacie, J.V.; Lewis, S.M. Practical Haematology, 7th ed.; Churchill Livinstone: Edinburg, YX, USA; London, UK, 1995; pp. 12–17. [Google Scholar]
- Schwarzstein, L.; Aparicio, N.J.; Turner, M.D.; De Turner, E.A.; Coy, D.H.; Schally, A.V. Luteinizing Hormone (lh), follicle-stimulating hormone, and testosterone responses to consecutive injections of d-leucine-6-lh-releasing hormone ethylamide in normal men. Am. Fertil. Soc. 1984, 28, 4. [Google Scholar] [CrossRef]
- Vashney, R.; Kale, R.K. Effects of calmodulin antagonist. Int. J. Radiat. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef]
- Hu, M.L.; Dillard, C.J. Plasma SH and GSH measurement. Enzymol 1994, 233, 385–387. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role ofsuperoxide anion in the autoxidation ofepinephrine and a sample assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Igwebuike, U.M.; Eze, U.U. Morphology of the caeca of the African pied crow (Corvus albus). Anim. Res. Int. 2010, 7, 1121–1124. [Google Scholar]
Sample Availability: Samples of the compound is available from the authors. |







| Initial Body Weight (g) | 7 Days Final Body Weight (g) | Weight Gain/Loss | Initial Body Weight (g) | 28 Days Final Body Weight (g) | Weight Gain/Loss | |
|---|---|---|---|---|---|---|
| Control | 120.60 ± 12.24 | 145.50 ± 20.91 | 24.90 ± 3.45 b | 137.80 ± 4.40 | 170.50 ± 11.18 | 32.70 ± 5.43 c |
| 10 mg/kg bw | 150.45 ± 17.67 | 132.20 ± 8.72 | −18.25 ± 4.35 a | 175.45 ± 17.67 | 154.80 ± 7.85 | −20.65 ± 3.45 a |
| 50 mg/kg bw | 165.32 ± 13.69 | 146.20 ± 7.00 | −19.12 ± 2.57 a | 205.55 ± 20.90 | 170.80 ± 4.80 | −34.75 ± 4.87 b |
| Epididymis (g) | Testes (g) | |||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 3.47 ± 1.19 b | 3.15 ± 0.92 a | 4.01 ± 1.51 b | 3.35 ± 0.86 a |
| 10 mg/kg bw | 2.47 ± 1.03 a | 2.96 ± 0.76 a | 2.78 ± 0.96 a | 3.75 ± 1.10 a |
| 50 mg/kg bw | 2.89 ± 0.97 a | 3.25 ± 1.03 a | 3.21 ± 1.49 a,b | 3.95 ± 1.513 a |
| 7 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Control | 10 mg/kg | 50 mg/kg | Control | 10 mg/kg | 50 mg/kg | |
| WBC (×103/mm3) | 11.77 ± 3.39 c | 10.68 ± 7.51 b | 6.23 ± 0.59 a | 6.18 ± 2.38 b | 5.99 ± 1.75 ab | 5.86 ± 2.81 a |
| NEU (%) | 10.54 ± 3.31 c | 5.67 ± 0.29 b | 3.41 ± 0.18 a | 3.14 ± 0.97 b | 3.21 ± 0.59 b | 2.167 ± 0.40 a |
| MID (%) | 0.96 ± 0.46 c | 0.87 ± 0.16 b | 0.63 ± 0.34 a | 0.61 ± 0.12 b | 0.57 ± 0.09 a | 0.67 ± 0.37 a |
| GRA | 0.27 ± 0.11 b | 0.23 ± 0.04 b | 0.13 ± 0.09 a | 0.07 ± 0.03 a | 0.21 ± 0.13 b | 0.20 ± 0.16 b |
| LYM (%) | 89.07 ± 4.30 c | 77.90 ± 1.21 b | 47.00 ± 2.6 a | 46.00 ± 3.6 b | 76.23 ± 1.28 b | 39.47 ± 4.19 c |
| MID (%) | 14.13 ± 0.40 b | 11.43 ± 2.22 a | 10.97 ± 2.6 a | 12.17 ± 3.1 b | 9.77 ± 1.47 a | 8.23 ± 4.70 a |
| GRA (%) | 3.43 ± 0.50 a | 3.17 ± 0.23 a | 5.60 ± 7.10 a | 3.43 ± 1.40 b | 1.91 ± 0.31 a | 3.40 ± 0.46 b |
| RBC (1012L) | 6.75 ± 0.13 a | 6.56 ± 1.19 a | 6.29 ± 2.85 a | 5.56 ± 1.47 a | 6.15 ± 0.73 b | 7.06 ± 1.79 c |
| HB (g/dl) | 161.33 ± 8.1 b | 156.0 ± 13.9 b | 134.00 ± 32 a | 128.3 ± 12.22 a | 138.33 ± 7.3 b | 153.67 ± 10.45 c |
| MCV (f/l) | 74.27 ± 1.42 b | 77.67 ± 1.53 b | 52.73 ± 0.51 a | 54.30 ± 1.66 a | 86.00 ± 1.00 b | 55.53 ± 1.76 a |
| MCH (pg) | 30.0 ± 0.50 b | 29.50 ± 0.78 b | 15.60 ± 3.1 a | 25.67 ± 4.38 a,b | 28.17 ± 0.73 b | 22.57 ± 5.16 a |
| RDW-SD | 32.30 ± 1.91 b | 30.03 ± 4.30 b | 25.40 ± 2.2 a | 26.27 ± 2.40 a | 28.43 ± 3.99 a | 29.97 ± 2.30 a |
| RDW-CV | 17.17 ± 0.25 | 15.70 ± 0.40 a | 15.30 ± 0.49 a | 13.60 ± 2.16 a | 14.73 ± 0.31 a | 14.17 ± 2.75 a |
| PLT (103 µL) | 286.00 ± 9.5 a | 305.0 ± 5.00 b | 261.33 ± 10 a | 272.33 ± 5.5 a | 334.67 ± 12 b | 296.67 ± 4 a |
| MPV (g/dl) | 7.49 ± 0.08 b | 7.30 ± 0.10 b | 6.83 ± 0.15 a | 6.67 ± 0.61 a | 7.27 ± 0.15 b | 6.63 ± 0.32 a |
| PCW | 9.90 ± 0.10 a | 10.20 ± 0.12 a | 14.0 ± 2.21 b | 12.57 ± 0.40 b | 10.50 ± 0.61 a | 12.93 ± 1.10 b |
| HCT | 0.21 ± 0.01 a | 0.22 ± 0.01 a | 0.27 ± 0.17 a | 0.36 ± 0.11 a | 0.34 ± 0.03 a | 0.35 ± 0.07 a |
| LH (mIU/mL) | FSH (mIU/mL) | Testosterone (ng/mL) | ||||
|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 1.2 ± 0.22 c | 0.7 ± 0.3 c | 2.8 ± 0.24 c | 1.10 ± 0.21 c | 3.10 ± 0.51 c | 2.40 ± 0.53 c |
| 10 mg/kg bw | 0.8 ± 0.32 b | 0.6 ± 0.2 b | 1.2 ± 0.21 b | 0.89 ± 0.10 b | 2.80 ± 0.19 b | 1.00 ± 0.51 b |
| 50 mg/kg bw | 0.69 ± 0.4 a | 0.5 ± 0.22 a | 0.9 ± 0.6 a | 0.75 ± 0.12 a | 2.20 ± 0.71 a | 0.52 ± 0.23 a |
| Total Motility (%) | Progressive Motility (%) | Non-Progressive (%) | Immobility (%) | |
|---|---|---|---|---|
| 7-day treatment | ||||
| Control | 60.29 ± 5.78 c | 62.02 ± 5.46 c | 30.34 ± 3.57 a | 35.61 ± 1.91 a |
| 10 mg/kg | 40.71 ± 3.35 b | 34.44 ± 3.97 b | 44.36 ± 8.60 b | 56.24 ± 2.53 b |
| 50 mg/kg | 20.23 ± 1.69 a | 9.20 ± 0.69 a | 46.19 ± 4.49 b | 66.49 ± 4.10 c |
| 28-day treatment | ||||
| Control | 57.16 ± 4.49 c | 62.76 ± 5.33 c | 44.29 ± 9.12 a | 29.99 ± 5.24 a |
| 10 mg/kg | 34.13 ± 0.57 b | 25.10 ± 6.52 b | 52.76 ± 4.71 b | 63.43 ± 2.90 b |
| 50 mg/kg | 9.65 ± 0.53 a | 3.88 ± 1.33 a | 59.05 ± 0.51 b | 76.81 ± 4.31 c |
| Straight-Line Velocity (µm/s) | Curvilinear Velocity (µm/s) | Average Path Velocity (µm/s) | |
|---|---|---|---|
| 7-day treatment | |||
| Control | 6.22 ± 0.16 c | 19.52 ± 2.25 c | 12.54 ± 0.57 c |
| 10 mg/kg bw | 2.15 ± 0.10 b | 3.14 ± 0.35 b | 3.02 ± 0.59 b |
| 50 mg/kg bw | 0.74 ± 0.06 a | 0.70 ± 0.27 a | 1.46 ± 0.47 a |
| 28-day treatment | |||
| Control | 4.77 ± 0.49 c | 21.74 ± 2.64 b | 0.43 ± 0.08 a |
| 10 mg/kg bw | 1.84 ± 0.52 b | 1.84 ± 0.52 a | 0.63 ± 0.21 b |
| 50 mg/kg bw | 0.43 ± 0.08 a | 2.10 ± 0.08 a | 0.61 ± 0.13 b |
| ALH (µm) | Beat Cross Frequency (Hz) | Linearity (%) | Straightness (%) | |
|---|---|---|---|---|
| 7-day treatment | ||||
| Control | 4.09 ± 0.61 c | 6.80 ± 0.52 c | 56.75 ± 8.26 c | 61.35 ± 4.33 c |
| 10 mg/kg bw | 0.91 ± 0.04 b | 3.40 ± 0.18 b | 34.18 ± 4.34 b | 42.69 ± 1.41 b |
| 50 mg/kg bw | 0.61 ± 0.13 a | 1.52 ± 0.20 a | 22.88 ± 4.57 a | 7.17 ± 0.93 a |
| 28-day treatment | ||||
| Control | 6.14 ± 1.33 c | 8.09 ± 1.45 c | 66.46 ± 9.50 c | 88.08 ± 11.94 c |
| 10 mg/kg bw | 0.63 ± 0.22 b | 4.20 ± 0.55 b | 30.95 ± 1.65 b | 30.93 ± 1.13 b |
| 50 mg/kg bw | 0.33 ± 0.09 a | 2.61 ± 0.41 a | 10.15 ± 0.70 a | 7.24 ± 1.62 a |
| Proteins | Epididymis (g/dL) | Testes (g/dL) | ||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 1.41 ± 0.32 c | 1.07 ± 0.36 c | 1.52 ± 0.04 a | 1.26 ± 0.01 a |
| 10 mg/kg bw | 0.89 ± 0.03 b | 0.93 ± 0.39 b | 1.02 ± 0.02 a | 1.20 ± 0.02 a |
| 50 mg/kg bw | 0.21 ± 0.03 a | 0.27 ± 0.0 a | 1.08 ± 0.02 a | 1.23 ± 0.05 a |
| MDA (Units/g Tissue × 106) | Epididymis (Unit/g Tissue × 106) | Testes (Unit/g Tissue × 106) | ||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 17.68 ± 10.13 a | 15.42 ± 6.03 a | 23.43 ± 2.86 a | 16.75 ± 8.30 a |
| 10 mg/kg bw | 20.65 ± 9.46 a | 16.93 ± 8.80 a | 27.34 ± 9.20 a | 18.19 ± 3.51 a |
| 50 mg/kg bw | 35.49 ± 8.52 b | 33.48 ± 5.69 b | 36.90 ± 11.10 b | 34.09 ± 9.89 b |
| H2O2(μmol/mg Protein) | Epididymis | Testes | ||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 33.95 ± 0.37 a | 37.1 ± 0.29 a | 34.63 ± 0.34 a | 34.5 ± 0.40 a |
| 10 mg/kg bw | 34.65 ± 0.24 a | 38.75 ± 0.32 a | 36.66 ± 0.24 ab | 36.4 ± 0.57 a |
| 50 mg/kg bw | 38.05 ± 0.33 a | 40.13 ± 0.60 b | 38.95 ± 0.21 b | 39.78 ± 0.40 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olugbodi, J.O.; David, O.; Oketa, E.N.; Lawal, B.; Okoli, B.J.; Mtunzi, F. Silver Nanoparticles Stimulates Spermatogenesis Impairments and Hematological Alterations in Testis and Epididymis of Male Rats. Molecules 2020, 25, 1063. https://doi.org/10.3390/molecules25051063
Olugbodi JO, David O, Oketa EN, Lawal B, Okoli BJ, Mtunzi F. Silver Nanoparticles Stimulates Spermatogenesis Impairments and Hematological Alterations in Testis and Epididymis of Male Rats. Molecules. 2020; 25(5):1063. https://doi.org/10.3390/molecules25051063
Chicago/Turabian StyleOlugbodi, Janet Olayemi, Oladipupo David, Ene Naomi Oketa, Bashir Lawal, Bamidele Joseph Okoli, and Fanyana Mtunzi. 2020. "Silver Nanoparticles Stimulates Spermatogenesis Impairments and Hematological Alterations in Testis and Epididymis of Male Rats" Molecules 25, no. 5: 1063. https://doi.org/10.3390/molecules25051063









