Peptides from Natural or Rationally Designed Sources Can Be Used in Overweight, Obesity, and Type 2 Diabetes Therapies
Abstract
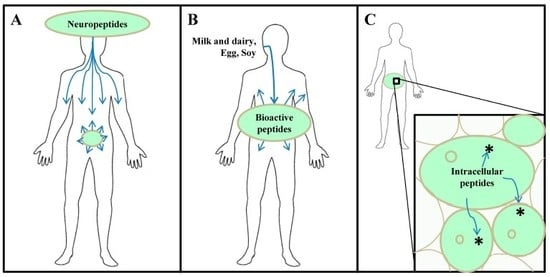
:1. Introduction
2. Brief Historical Perspective on Proteasome-Derived Intracellular Peptides: Possible Therapeutic Applications to Control and Treat Overweight, Obesity, and T2D
2.1. Therapeutics Pharmacology of Intracellular Peptides HP and Pep19 to Ameliorate Overweight, Obesity, and T2D
2.1.1. HP and HP-Containing Peptides Therapeutics Pharmacology Characterization
2.1.2. Pep19 Therapeutics Pharmacology Characterization
3. Molecular Physiology of Energy Expenditure
4. Neural and Endocrine Peptides Regulating Overweight, Obesity, and Energy Homeostasis
4.1. Neuropeptide Y
4.2. Peptide YY
4.3. Pancreatic Polypeptide (PP)
4.4. Glucagon-Like 1 (GLP-1)
4.5. Ghrelin
4.6. Amylin
5. Food-Derived Bioactive Peptides in Obesity and Related Metabolic Disturbances
5.1. Milk-Derived Bioactive Peptides
5.2. Egg-Derived Bioactive Peptides
5.3. Soy-Derived Bioactive Peptides
6. Undesired and Misuse of Therapeutic Peptides
7. Closing Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Da CostaLouzada, M.L.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.B.; Canella, D.S.; Moubarac, J.-C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. 2015, 81, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Flaquer, A.; Baumbach, C.; Kriebel, J.; Meitinger, T.; Peters, A.; Waldenberger, M.; Grallert, H.; Strauch, K. Mitochondrial genetic variants identified to be associated with BMI in adults. PLoS ONE 2014, 9, e105116. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, R.I.; Fabricatore, A.N. Obesity, psychiatric status, and psychiatric medications. Psychiatr Clin N. Am. 2011, 34, 747–764. [Google Scholar] [CrossRef]
- Rodulfo, J.I.A. Sedentarismo, la enfermedad del siglo xxi. Clínica E Investig. En Arterioscler. 2019, 31, 233–240. [Google Scholar] [CrossRef]
- Eleazu, C.O. The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. Afr Health Sci 2016, 16, 468–479. [Google Scholar] [CrossRef] [Green Version]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health--a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 2008, 87, 258s–268s. [Google Scholar] [CrossRef] [Green Version]
- Brand-Miller, J.C.; Holt, S.H.; Pawlak, D.B.; McMillan, J. Glycemic index and obesity. Am. J. Clin. Nutr. 2002, 76, 281s–285s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Pihlsgard, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl.J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switerland, 2000. [Google Scholar]
- Jones, D.S.; Podolsky, S.H.; Greene, J.A. The burden of disease and the changing task of medicine. N. Engl. J. Med. 2012, 366, 2333–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.N.; O’Sullivan, A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, A.K.; Graubard, B.I. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971-1975 to NHANES 1999-2002. Am. J. Clin. Nutr. 2006, 84, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Hannan, P.J.; Fulkerson, J.A.; Laska, M.N.; Eisenberg, M.E.; Neumark-Sztainer, D. Secular trends in fast-food restaurant use among adolescents and maternal caregivers from 1999 to 2010. Am. J. Public Health 2014, 104, e62–e69. [Google Scholar] [CrossRef]
- Smith, C.; Gray, A.R.; Mainvil, L.A.; Fleming, E.A.; Parnell, W.R. Secular changes in intakes of foods among New Zealand adults from 1997 to 2008/09. Public Health Nutr. 2015, 18, 3249–3259. [Google Scholar] [CrossRef] [Green Version]
- Tarnopolsky, M.A. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med. Sci. Sports Exerc. 2008, 40, 648–654. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [Green Version]
- Ochner, C.N.; Teixeira, J.; Geary, N.; Asarian, L. Greater short-term weight loss in women 20-45 versus 55-65 years of age following bariatric surgery. Obes. Surg. 2013, 23, 1650–1654. [Google Scholar] [CrossRef] [Green Version]
- Fuente-Martin, E.; Garcia-Caceres, C.; Diaz, F.; Argente-Arizon, P.; Granado, M.; Barrios, V.; Argente, J.; Chowen, J.A. Hypothalamic inflammation without astrogliosis in response to high sucrose intake is modulated by neonatal nutrition in male rats. Endocrinology 2013, 154, 2318–2330. [Google Scholar] [CrossRef] [PubMed]
- Fuente-Martin, E.; Garcia-Caceres, C.; Morselli, E.; Clegg, D.J.; Chowen, J.A.; Finan, B.; Brinton, R.D.; Tschop, M.H. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havel, P.J.; Kasim-Karakas, S.; Dubuc, G.R.; Mueller, W.; Phinney, S.D. Gender differences in plasma leptin concentrations. Nat. Med. 1996, 2, 949–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, M.; Leibel, R.L. The role of leptin in human physiology. N. Engl. J. Med. 1999, 341, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Nookaew, I.; Svensson, P.A.; Jacobson, P.; Jernas, M.; Taube, M.; Larsson, I.; Andersson-Assarsson, J.C.; Sjostrom, L.; Froguel, P.; Walley, A.; et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J. Clin. Endocrinol. Metab. 2013, 98, E370–E378. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Loh, D.H.; Colwell, C.S.; Tache, Y.; Reue, K.; Arnold, A.P. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm. Behav. 2015, 75, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Mayes, J.S.; Watson, G.H. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes. Rev. 2004, 5, 197–216. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. Clinical review 107: Role of gonadal steroids in the sexual dimorphisms in body composition and circulating concentrations of leptin. J. Clin. Endocrinol. Metab. 1999, 84, 1784–1789. [Google Scholar] [CrossRef]
- Greco, B.; Edwards, D.A.; Zumpe, D.; Clancy, A.N. Androgen receptor and mating-induced fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Res. 1998, 781, 15–24. [Google Scholar] [CrossRef]
- Kelly, M.J.; Qiu, J. Estrogen signaling in hypothalamic circuits controlling reproduction. Brain Res. 2010, 1364, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bianco-Borges, B.; Cabral, F.J.; Franci, C.R. Co-expression of leptin and oestrogen receptors in the preoptic-hypothalamic area. J. Neuroendocr. 2010, 22, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.; Ciechanover, A. THE UBIQUITIN SYSTEM. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. Intracellular protein degradation: From a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Pr. Res. Clin. Haematol. 2017, 30, 341–355. [Google Scholar] [CrossRef] [PubMed]
- de Duve, C. The lysosome turns fifty. Nat. Cell Biol. 2005, 7, 847–849. [Google Scholar] [CrossRef]
- Sha, Z.; Zhao, J.; Goldberg, A.L. Measuring the Overall Rate of Protein Breakdown in Cells and the Contributions of the Ubiquitin-Proteasome and Autophagy-Lysosomal Pathways. Methods Mol. Biol. 2018, 1844, 261–276. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Reits, E.; Griekspoor, A.; Neijssen, J.; Groothuis, T.; Jalink, K.; van Veelen, P.; Janssen, H.; Calafat, J.; Drijfhout, J.W.; Neefjes, J. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity 2003, 18, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Rock, K.L.; York, I.A.; Saric, T.; Goldberg, A.L. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 2002, 80, 1–70. [Google Scholar] [CrossRef]
- Reits, E.; Neijssen, J.; Herberts, C.; Benckhuijsen, W.; Janssen, L.; Drijfhout, J.W.; Neefjes, J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity 2004, 20, 495–506. [Google Scholar] [CrossRef] [Green Version]
- York, I.A.; Mo, A.X.Y.; Lemerise, K.; Zeng, W.; Shen, Y.; Abraham, C.R.; Saric, T.; Goldberg, A.L.; Rock, K.L. The cytosolic endopeptidase, thimet oligopeptidase, destroys antigenic peptides and limits the extent of MHC class I antigen presentation. Immunity 2003, 18, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Ferro, E.S.; Hyslop, S.; Camargo, A.C. Intracellullar peptides as putative natural regulators of protein interactions. J. Neurochem. 2004, 91, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Rioli, V.; Gozzo, F.C.; Heimann, A.S.; Linardi, A.; Krieger, J.E.; Shida, C.S.; Almeida, P.C.; Hyslop, S.; Eberlin, M.N.; Ferro, E.S. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. J. Biol. Chem. 2003, 278, 8547–8555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, T.; Plaza, S.; Zanet, J.; Benrabah, E.; Valenti, P.; Hashimoto, Y.; Kobayashi, S.; Payre, F.; Kageyama, Y. Small peptides switch the transcriptional activity of Shavenbaby during Drosophila embryogenesis. Science 2010, 329, 336–339. [Google Scholar] [CrossRef] [Green Version]
- Slavoff, S.A.; Mitchell, A.J.; Schwaid, A.G.; Cabili, M.N.; Ma, J.; Levin, J.Z.; Karger, A.D.; Budnik, B.A.; Rinn, J.L.; Saghatelian, A. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat. Chem. Biol. 2013, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Dolan, B.P.; Bennink, J.R.; Yewdell, J.W. Translating DRiPs: Progress in understanding viral and cellular sources of MHC class I peptide ligands. Cell. Mol. Life Sci. 2011, 68, 1481–1489. [Google Scholar] [CrossRef] [Green Version]
- Fricker, L.D. Analysis of mouse brain peptides using mass spectrometry-based peptidomics: Implications for novel functions ranging from non-classical neuropeptides to microproteins. Mol. Biosyst. 2010, 6, 1355–1365. [Google Scholar] [CrossRef]
- Ribeiro, N.M.; Toniolo, E.F.; Castro, L.M.; Russo, L.C.; Rioli, V.; Ferro, E.S.; Dale, C.S. AGH is a new hemoglobin alpha-chain fragment with antinociceptive activity. Peptides 2013, 48, 10–20. [Google Scholar] [CrossRef]
- Cunha, F.M.; Berti, D.A.; Ferreira, Z.S.; Klitzke, C.F.; Markus, R.P.; Ferro, E.S. Intracellular peptides as natural regulators of cell signaling. J. Biol. Chem. 2008, 283, 24448–24459. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.M.M.; Correa, C.N.; Iwai, L.K.; Ferro, E.S.; Castro, L.M. Characterization of Intracellular Peptides from Zebrafish (Danio rerio) Brain. Zebrafish 2019, 16, 240–251. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, C.B.; Heimann, A.S.; Remer, R.A.; Russo, L.C.; Colquhoun, A.; Forti, F.L.; Ferro, E.S. Intracellular Peptides in Cell Biology and Pharmacology. Biomolecules 2019, 9, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, L.C.; Castro, L.M.; Gozzo, F.C.; Ferro, E.S. Inhibition of thimet oligopeptidase by siRNA alters specific intracellular peptides and potentiates isoproterenol signal transduction. FEBS Lett. 2012, 586, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, C.B.; Russo, L.C.; Castro, L.M.; Forti, F.L.; do Monte, E.R.; Rioli, V.; Gozzo, F.C.; Colquhoun, A.; Ferro, E.S. A novel intracellular peptide derived from g1/s cyclin d2 induces cell death. J. Biol. Chem. 2014, 289, 16711–16726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.; Yang, C.; Castro, L.M.; Tashima, A.K.; Ferro, E.S.; Moir, R.D.; Willis, I.M.; Fricker, L.D. Analysis of the Yeast Peptidome and Comparison with the Human Peptidome. PLoS ONE 2016, 11, e0163312. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Fishman, M.A.; Castro, L.M.; Tashima, A.K.; Ferro, E.S.; Fricker, L.D. Effect of Protein Denaturation and Enzyme Inhibitors on Proteasomal-Mediated Production of Peptides in Human Embryonic Kidney Cells. Biomolecules 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, D.A.; Russo, L.C.; Castro, L.M.; Cruz, L.; Gozzo, F.C.; Heimann, J.C.; Lima, F.B.; Oliveira, A.C.; Andreotti, S.; Prada, P.O.; et al. Identification of intracellular peptides in rat adipose tissue: Insights into insulin resistance. Proteomics 2012, 12, 2668–2681. [Google Scholar] [CrossRef] [PubMed]
- Heimann, A.S.; Favarato, M.H.; Gozzo, F.C.; Rioli, V.; Carreno, F.R.; Eberlin, M.N.; Ferro, E.S.; Krege, J.H.; Krieger, J.E. ACE gene titration in mice uncovers a new mechanism for ACE on the control of body weight. Physiol. Genom. 2005, 20, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Wang, F.; You, L.; Xu, P.; Cao, Y.; Chen, L.; Wen, J.; Guo, X.; Cui, X.; et al. Identification of intracellular peptides associated with thermogenesis in human brown adipocytes. J. Cell. Physiol. 2018, 234, 7104–7114. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharm. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Castro, L.M.; Dulman, R.; Yang, C.; Schmidt, M.; Ferro, E.S.; Fricker, L.D. Proteasome inhibitors alter levels of intracellular peptides in HEK293T and SH-SY5Y cells. PLoS ONE 2014, 9, e103604. [Google Scholar] [CrossRef]
- Gelman, J.S.; Sironi, J.; Berezniuk, I.; Dasgupta, S.; Castro, L.M.; Gozzo, F.C.; Ferro, E.S.; Fricker, L.D. Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib. PLoS ONE 2013, 8, e53263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricker, L.D.; Gelman, J.S.; Castro, L.M.; Gozzo, F.C.; Ferro, E.S. Peptidomic analysis of HEK293T cells: Effect of the proteasome inhibitor epoxomicin on intracellular peptides. J. Proteome Res. 2012, 11, 1981–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, E.S.; Rioli, V.; Castro, L.M.; Fricker, L.D. Intracellular peptides: From discovery to function. Eupa Open Proteom. 2014, 3, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Russo, L.C.; Asega, A.F.; Castro, L.M.; Negraes, P.D.; Cruz, L.; Gozzo, F.C.; Ulrich, H.; Camargo, A.C.; Rioli, V.; Ferro, E.S. Natural intracellular peptides can modulate the interactions of mouse brain proteins and thimet oligopeptidase with 14-3-3epsilon and calmodulin. Proteomics 2012, 12, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Gelman, J.S.; Dasgupta, S.; Berezniuk, I.; Fricker, L.D. Analysis of peptides secreted from cultured mouse brain tissue. Biochim. Et Biophys. Acta 2013, 1834, 2408–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reckziegel, P.; Festuccia, W.T.; Britto, L.R.G.; Jang, K.L.L.; Romao, C.M.; Heimann, J.C.; Fogaca, M.V.; Rodrigues, N.S.; Silva, N.R.; Guimaraes, F.S.; et al. A novel peptide that improves metabolic parameters without adverse central nervous system effects. Sci. Rep. 2017, 7, 14781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, S.C.; Ralvenius, W.T.; Gachet, M.S.; Fritschy, J.M.; Zeilhofer, H.U.; Gertsch, J. Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology 2015, 98, 78–89. [Google Scholar] [CrossRef]
- Ferro, E.S.; Tambourgi, D.V.; Gobersztejn, F.; Gomes, M.D.; Sucupira, M.; Armelin, M.C.; Kipnis, T.L.; Camargo, A.C. Secretion of a neuropeptide-metabolizing enzyme similar to endopeptidase 22.19 by glioma C6 cells. Biochem. Biophys. Res. Commun. 1993, 191, 275–281. [Google Scholar] [CrossRef]
- Ferro, E.S.; Tullai, J.W.; Glucksman, M.J.; Roberts, J.L. Secretion of metalloendopeptidase 24.15 (EC 3.4.24.15). DNA Cell Biol. 1999, 18, 781–789. [Google Scholar] [CrossRef]
- Vincent, B.; Beaudet, A.; Dauch, P.; Vincent, J.P.; Checler, F. Distinct properties of neuronal and astrocytic endopeptidase 3.4.24.16: A study on differentiation, subcellular distribution, and secretion processes. J. Neurosci. 1996, 16, 5049–5059. [Google Scholar] [CrossRef]
- Jeske, N.A.; Berg, K.A.; Cousins, J.C.; Ferro, E.S.; Clarke, W.P.; Glucksman, M.J.; Roberts, J.L. Modulation of bradykinin signaling by EP24.15 and EP24.16 in cultured trigeminal ganglia. J. Neurochem. 2006, 97, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Garrido, P.A.G.; Rodrigues, C.C.; Colquhoun, A.; Castro, L.M.; Almeida, P.C.; Shida, C.S.; Juliano, M.A.; Juliano, L.; Camargo, A.C.M.; et al. Calcium modulates endopeptidase 24.15 (EC 3.4.24.15) membrane association, secondary structure and substrate specificity. FEBS J. 2005, 272, 2978–2992. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Wu, T.J.; Cummins, P.M.; Ferro, E.S.; Tullai, J.W.; Glucksman, M.J.; Roberts, J.L. The association of metalloendopeptidase EC 3.4.24.15 at the extracellular surface of the AtT-20 cell plasma membrane. Brain Res. 1999, 835, 113–124. [Google Scholar] [CrossRef]
- Gomes, I.; Dale, C.S.; Casten, K.; Geigner, M.A.; Gozzo, F.C.; Ferro, E.S.; Heimann, A.S.; Devi, L.A. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010, 12, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.; Grushko, J.S.; Golebiewska, U.; Hoogendoorn, S.; Gupta, A.; Heimann, A.S.; Ferro, E.S.; Scarlata, S.; Fricker, L.D.; Devi, L.A. Novel endogenous peptide agonists of cannabinoid receptors. Faseb J. 2009, 23, 3020–3029. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Heimann, A.S.; Gomes, I.; Devi, L.A. Antibodies against G-protein coupled receptors: Novel uses in screening and drug development. Comb. Chem High. Throughput Screen 2008, 11, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Décaillot, F.M.; Gomes, I.; Tkalych, O.; Heimann, A.S.; Ferro, E.S.; Devi, L.A. Conformation state-sensitive antibodies to G-protein-coupled receptors. J. Biol. Chem. 2007, 282, 5116–5124. [Google Scholar] [CrossRef] [Green Version]
- Heimann, A.S.; Gomes, I.; Dale, C.S.; Pagano, R.L.; Gupta, A.; de Souza, L.L.; Luchessi, A.D.; Castro, L.M.; Giorgi, R.; Rioli, V.; et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 20588–20593. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.; Ayoub, M.A.; Fujita, W.; Jaeger, W.C.; Pfleger, K.D.; Devi, L.A. G Protein-Coupled Receptor Heteromers. Annu. Rev. Pharm. Toxicol. 2016, 56, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Rozenfeld, R.; Devi, L.A. Receptor heteromerization and drug discovery. Trends Pharm. Sci. 2010, 31, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Dale, C.S.; De Lima Pagano, R.; Rioli, V. Hemopressin: A novel bioactive peptide derived from the α1-chain of hemoglobin. Mem. Inst. Oswaldo Cruz 2005, 100, 105–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Chicca, A.; Tamborrini, M.; Eisen, D.; Lerner, R.; Lutz, B.; Poetz, O.; Pluschke, G.; Gertsch, J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012, 287, 36944–36967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, G.T.; Mancini, G.; Lutz, B.; Luckman, S.M. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 7369–7376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, G.T.; Worth, A.A.; Hodkinson, D.J.; Srivastava, R.K.; Lutz, B.; Williams, S.R.; Luckman, S.M. Central functional response to the novel peptide cannabinoid, hemopressin. Neuropharmacology 2013, 71, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.S.; Pagano, R.D.L.; Rioli, V.; Hyslop, S.; Giorgi, R.; Ferro, E.S. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides 2005, 26, 431–436. [Google Scholar] [CrossRef]
- Petrucci, V.; Chicca, A.; Glasmacher, S.; Paloczi, J.; Cao, Z.; Pacher, P.; Gertsch, J. Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage. Sci. Rep. 2017, 7, 9560. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimizu, T.; Yanagita, T.; Nemoto, T.; Nakamura, K.; Taniuchi, K.; Dimitriadis, F.; Yokotani, K.; Saito, M. Brain RVD-haemopressin, a haemoglobin-derived peptide, inhibits bombesin-induced central activation of adrenomedullary outflow in the rat. Br. J. Pharm. 2014, 171, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Fogaca, M.V.; Sonego, A.B.; Rioli, V.; Gozzo, F.C.; Dale, C.S.; Ferro, E.S.; Guimaraes, F.S. Anxiogenic-like effects induced by hemopressin in rats. Pharm. Biochem. Behav. 2015, 129, 7–13. [Google Scholar] [CrossRef]
- Machado, M.F.M.; Cunha, F.M.; Berti, D.A.; Heimann, A.S.; Klitzke, C.F.; Rioli, V.; Oliveira, V.; Ferro, E.S. Substrate phosphorylation affects degradation and interaction to endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. Biochem. Biophys. Res. Commun. 2006, 339, 520–525. [Google Scholar] [CrossRef]
- van Eenige, R.; van der Stelt, M.; Rensen, P.C.N.; Kooijman, S. Regulation of Adipose Tissue Metabolism by the Endocannabinoid System. Trends Endocrinol. Metab. 2018, 29, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Perwitz, N.; Wenzel, J.; Wagner, I.; Büning, J.; Drenckhan, M.; Zarse, K.; Ristow, M.; Lilienthal, W.; Lehnert, H.; Klein, J. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. DiabetesObes. Metab. 2010, 12, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. -Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Hideaki, K.; Kunio, D.; Shigeru, S.; Hideyo, U.; Ryuji, S.; Umeji, M.; Shizume, T. Antihypertensive effect of tryptic hydrolysate of milk casein in spontaneously hypertensive rats. Comp. Biochem. Physiol. Part. C Comp. 1990, 96, 367–371. [Google Scholar] [CrossRef]
- Shu, C.; Shen, H.; Hopfer, U.; Smith, D.E. Mechanism of intestinal absorption and renal reabsorption of an orally active ace inhibitor: Uptake and transport of fosinopril in cell cultures. Drug Metab. Dispos. 2001, 29, 1307–1315. [Google Scholar]
- Sipola, M.; Finckenberg, P.; Santisteban, J.; Korpela, R.; Vapaatalo, H.; Nurminen, M.L. Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J. Physiol. Pharmacol. 2001, 52, 745–754. [Google Scholar] [CrossRef]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef]
- Piaggi, P.; Vinales, K.L.; Basolo, A.; Santini, F.; Krakoff, J. Energy expenditure in the etiology of human obesity: Spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J. Endocrinol. Investig. 2018, 41, 83–89. [Google Scholar] [CrossRef]
- Carneiro, I.P.; Elliott, S.A.; Siervo, M.; Padwal, R.; Bertoli, S.; Battezzati, A.; Prado, C.M. Is Obesity Associated with Altered Energy Expenditure? Adv. Nutr. 2016, 7, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Lenard, N.R.; Berthoud, H.-R. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity 2008, 16, S11–S22. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Charmandari, E.; Chrousos, G.P.; Kino, T. Circadian endocrine rhythms: The hypothalamic-pituitary-adrenal axis and its actions. Ann. N. Y. Acad. Sci. 2014, 1318, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.; Astiz, M.; Friedrichs, M.; Oster, H. Endocrine regulation of circadian physiology. J. Endocrinol. 2016, 230, R1–R11. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Sainsbury, A.; Zhang, L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obes. Rev. 2012, 13, 234–257. [Google Scholar] [CrossRef] [Green Version]
- Bidaut-Russell, M.; Devane, W.A.; Howlett, A.C. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. J. Neurochem. 1990, 55, 21–26. [Google Scholar] [CrossRef]
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int. J. Obes. 2006, 30, S7–S12. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Marzo, V.D. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef]
- Bajzer, M.; Olivieri, M.; Haas, M.K.; Pfluger, P.T.; Magrisso, I.J.; Foster, M.T.; Tschöp, M.H.; Krawczewski-Carhuatanta, K.A.; Cota, D.; Obici, S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 2011, 54, 3121–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, C.; Bellocchio, L.; Mancini, G.; Mazza, R.; Cervino, C.; Braulke, L.J.; Fekete, C.; Latorre, R.; Nanni, C.; Bucci, M.; et al. CB1 Signaling in Forebrain and Sympathetic Neurons Is a Key Determinant of Endocannabinoid Actions on Energy Balance. Cell Metab. 2010, 11, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler-Laney, P.C.; Castaneda, E.; Pritchett, C.E.; Smith, M.L.; Giddings, M.; Artiga, A.I.; Boggiano, M.M. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol. Biochem. Behav. 2007, 87, 104–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahng, J.W.; Kim, J.G.; Kim, H.J.; Kim, B.-T.; Kang, D.-W.; Lee, J.-H. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007, 1150, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Singh Jaggi, A. An Integrative Review on Role and Mechanisms of Ghrelin in Stress, Anxiety and Depression. Curr. Drug Targets 2016, 17, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.J.; O’Neill, B.V.; Napolitano, A.; Bullmore, E.T. Neuropsychiatric Adverse Effects of Centrally Acting Antiobesity Drugs. CNS Neurosci. Ther. 2011, 17, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.L.; Rasmussen, E.B. Rimonabant’s reductive effects on high densities of food reinforcement, but not palatability, in lean and obese Zucker rats. Psychopharmacology 2014, 231, 2159–2170. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef]
- Wenger, T.; Moldrich, G. The role of endocannabinoids in the hypothalamic regulation of visceral function. ProstaglandinsLeukot. Essent. Fat. Acids 2002, 66, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justinova, Z.; Munzar, P.; Panlilio, L.V.; Yasar, S.; Redhi, G.H.; Tanda, G.; Goldberg, S.R. Blockade of THC-Seeking Behavior and Relapse in Monkeys by the Cannabinoid CB1-Receptor Antagonist Rimonabant. Neuropsychopharmacology 2008, 33, 2870–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huestis, M.A.; Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Frank, R.A. Blockade of Effects of Smoked Marijuana by the CB1-Selective Cannabinoid Receptor Antagonist SR141716. Arch. Gen. Psychiatry 2001, 58, 322. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Gamo, Y.; Sinclair, R.; Mitchell, S.E.; Morgan, D.G.; Clapham, J.C.; Speakman, J.R. Effects of Chronic Oral Rimonabant Administration on Energy Budgets of Diet-Induced Obese C57BL/6 Mice. Obesity 2012, 20, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Perrard, X.D.; Perrard, J.L.; Mansoori, A.; Smith, C.W.; Ballantyne, C.M.; Wu, H. Effect of the Cannabinoid Receptor-1 Antagonist Rimonabant on Inflammation in Mice With Diet-Induced Obesity. Obesity 2011, 19, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Jbilo, O.; Ravinet-Trillou, C.; Arnone, M.; Buisson, I.; Bribes, E.; Péleraux, A.; Pénarier, G.; Soubrié, P.; Le Fur, G.; Galiègue, S.; et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. Faseb J. 2005, 19, 1567–1569. [Google Scholar] [CrossRef]
- Motaghedi, R.; Lipman, E.G.; Hogg, J.E.; Christos, P.J.; Vogiatzi, M.G.; Angulo, M.A. Psychiatric adverse effects of rimonobant in adults with Prader Willi syndrome. Eur. J. Med. Genet. 2011, 54, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S.; Group, R.I.-E.S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Glandt, M.; Raz, I. Present and future: Pharmacologic treatment of obesity. J. Obes. 2011, 2011, 636181. [Google Scholar] [CrossRef] [Green Version]
- Flatt, P.R.; Conlon, J.M. Editorial: Newer peptide-based agents for treatment of patients with Type 2 diabetes. Peptides 2018, 100, 1–2. [Google Scholar] [CrossRef]
- Gribble, F.M.; Meek, C.L.; Reimann, F. Targeted intestinal delivery of incretin secretagogues—towards new diabetes and obesity therapies. Peptides 2018, 100, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A. Incretin-based therapies for type 2 diabetes mellitus: Properties, functions, and clinical implications. Am. J. Med. 2011, 124, S3–S18. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [Green Version]
- Spanswick, D.; Smith, M.A.; Mirshamsi, S.; Routh, V.H.; Ashford, M.L.J. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 2000, 3, 757–758. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting Appetite-Regulating Pathways in the Hypothalamic Regulation of Body Weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef]
- Broberger, C.; Landry, M.; Wong, H.; Walsh, J.N.; Hökfelt, T. Subtypes y1 and y2 of the neuropeptide y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 1997, 66, 393–408. [Google Scholar] [CrossRef]
- Hahn, T.M.; Breininger, J.F.; Baskin, D.G.; Schwartz, M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 2002, 1, 271–272. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Chronwall, B.M.; DiMaggio, D.A.; Massari, V.J.; Pickel, V.M.; Ruggiero, D.A.; O’Donohue, T.L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience 1985, 15, 1159–1181. [Google Scholar] [CrossRef]
- Morris, B.J. Neuronal localisation of neuropeptide Y gene expression in rat brain. J. Comp. Neurol. 1989, 290, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Kim, Y.J.; Zheng, F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides 2012, 46, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, P.T.; Yang, L.; Aja, S.; Moran, T.H.; Bi, S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011, 13, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Grandt, D.; Schimiczek, M.; Struk, K.; Shively, J.; Eysselein, V.E.; Goebell, H.; Reeve, J.R. Characterization of two forms of peptide YY, PYY(1-36) and PYY(3-36), in the rabbit. Peptides 1994, 15, 815–820. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Teubner, B.J.W.; Bartness, T.J. PYY(3–36) into the arcuate nucleus inhibits food deprivation-induced increases in food hoarding and intake. Peptides 2013, 47, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Adrian, T.E.; Ferri, G.L.; Bacarese-Hamilton, A.J.; Fuessl, H.S.; Polak, J.M.; Bloom, S.R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985, 89, 1070–1077. [Google Scholar] [CrossRef]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.L.; Qian, C.; Liu, L.Y.; Liu, Q.J.; Jin, Y.Q.; Li, S.X.; Liu, W.; Rayner, C.K.; Ma, J. Secretion of Gut Hormones and Expression of Sweet Taste Receptors and Glucose Transporters in a Rat Model of Obesity. Obes. Facts 2019, 12, 190–198. [Google Scholar] [CrossRef]
- Chelikani, P.K.; Haver, A.C.; Reidelberger, R.D. Comparison of the inhibitory effects of PYY(3–36) and PYY(1-36) on gastric emptying in rats. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2004, 287, R1064–R1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persaud, S.J.; Bewick, G.A. Peptide YY: More than just an appetite regulator. Diabetologia 2014, 57, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Sam, A.H.; Gunner, D.J.; King, A.; Persaud, S.J.; Brooks, L.; Hostomska, K.; Ford, H.E.; Liu, B.; Ghatei, M.A.; Bloom, S.R.; et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology 2012, 143, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Brunner, H.R.; Seydoux, J.; Thorensa, B.; Pedrazzini, T. Increased insulin concentrations and glucose storage in neuropeptide Y Y1 receptor-deficient mice. Peptides 2001, 22, 421–427. [Google Scholar] [CrossRef]
- Khan, D.; Vasu, S.; Moffett, R.C.; Irwin, N.; Flatt, P.R. Islet distribution of Peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol. Cell. Endocrinol. 2016, 436, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Keire, D.A.; Mannon, P.; Kobayashi, M.; Walsh, J.H.; Solomon, T.E.; Reeve, J.R. Primary structures of PYY, [Pro34]PYY, and PYY-(3–36) confer different conformations and receptor selectivity. Am. J. Physiol. -Gastrointest. Liver Physiol. 2000, 279, G126–G131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, C.; Stephen, S.; Guitton, R.; Ramracheya, R.D. The Role of PYY in Pancreatic Islet Physiology and Surgical Control of Diabetes. Trends Endocrinol. Metab. 2017, 28, 626–636. [Google Scholar] [CrossRef]
- Alexiadou, K.; Anyiam, O.; Tan, T. Cracking the combination: Gut hormones for the treatment of obesity and diabetes. J. Neuroendocr. 2019, 31, e12664. [Google Scholar] [CrossRef] [Green Version]
- Neary, N.M.; Small, C.J.; Druce, M.R.; Park, A.J.; Ellis, S.M.; Semjonous, N.M.; Dakin, C.L.; Filipsson, K.; Wang, F.; Kent, A.S.; et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology 2005, 146, 5120–5127. [Google Scholar] [CrossRef] [Green Version]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [Green Version]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lonroth, H.; Fandriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Laferrere, B. Bariatric surgery and obesity: Influence on the incretins. Int. J. Obes. Suppl. 2016, 6, S32–S36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandekar, N.; Berning, B.A.; Sainsbury, A.; Lin, S. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol. Cell. Endocrinol. 2015, 418, 33–41. [Google Scholar] [CrossRef]
- Schwartz, T.W. Pancreatic Polypeptide: A Hormone Under Vagal Control. Gastroenterology 1983, 85, 1411–1425. [Google Scholar] [CrossRef]
- Simonian, H.P.; Kresge, k.m.; Boden, g.h.; Parkman, H.p. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: Evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol. Motility 2005, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Parkinson, J.R.C.; Minnion, J.S.; Addison, M.L.; Bloom, S.R.; Bell, J.D. Peptide YY3-36 and Pancreatic Polypeptide Differentially Regulate Hypothalamic Neuronal Activity in Mice In Vivo as Measured by Manganese-Enhanced Magnetic Resonance Imaging. J. Neuroendocrinol. 2011, 23, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Shi, Y.C.; Yulyaningsih, E.; Aljanova, A.; Zhang, L.; Macia, L.; Nguyen, A.D.; Lin, E.J.D.; During, M.J.; Herzog, H.; et al. Critical role of arcuate Y4 receptors and the melanocortin system in pancreatic polypeptide-induced reduction in food intake in mice. PLoS ONE 2009, 4, e8488. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.C.; Lin, Z.; Lau, J.; Zhang, H.; Yagi, M.; Kanzler, I.; Sainsbury, A.; Herzog, H.; Lin, S. PYY3-36 and pancreatic polypeptide reduce food intake in an additive manner via distinct hypothalamic dependent pathways in mice. Obesity 2013, 21, E669–E678. [Google Scholar] [CrossRef] [Green Version]
- Batterham, R.L.; Le Roux, C.W.; Cohen, M.A.; Park, A.J.; Ellis, S.M.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3989–3992. [Google Scholar] [CrossRef]
- Verschueren, S.; Janssen, P.; Van Oudenhove, L.; Hultin, L.; Tack, J. Effect of pancreatic polypeptide on gastric accommodation and gastric emptying in conscious rats. Am. J. Physiol. -Gastrointest. Liver Physiol. 2014, 307, G122–G128. [Google Scholar] [CrossRef]
- Brereton, M.F.; Vergari, E.; Zhang, Q.; Clark, A. Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J. Histochem. Cytochem. Off. J. Histochem. Soc. 2015, 63, 575–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Mari, A.; Nofrate, V.; Matoulek, M.; Kazdova, L.; Hill, M.; Pelikanova, T. Improvement in β-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. J. Diabetes Its Complicat. 2012, 26, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef]
- Vrang, N.; Larsen, P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of peripherally secreted and centrally produced peptides. Prog. Neurobiol. 2010, 92, 442–462. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Holst, J.J. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia 2004, 47, 357–366. [Google Scholar]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Toft-Nielson, M.b.; Madsbad, S.; Holst, J.J. The Effect of Glucagon-Like Peptide I (GLP-I) on Glucose Elimination in Healthy Subjects Depends on the Pancreatic Glucoregulatory Hormones. Diabetes 1996, 45, 552–556. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Veltman, D.J.; ten Kulve, J.S.; Groot, P.F.C.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. DiabetesObes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef]
- Nauck, M.A.; Kind, J.; Köthe, L.D.; Holst, J.J.; Deacon, C.F.; Broschag, M.; He, Y.L.; Kjems, L.; Foley, J. Quantification of the contribution of GLP-1 to mediating insulinotropic effects of DPP-4 inhibition with vildagliptin in healthy subjects and patients with type 2 diabetes using exendin [9–39] as a GLP-1 receptor antagonist. Diabetes 2016, 65, 2440–2447. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.J.; Nauck, M.A.; Kranz, D.; Holst, J.J.; Deacon, C.F.; Gaeckler, D.; Schmidt, W.E.; Gallwitz, B. Secretion, Degradation, and Elimination of Glucagon-Like Peptide 1 and Gastric Inhibitory Polypeptide in Patients with Chronic Renal Insufficiency and Healthy Control Subjects. Diabetes 2004, 53, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Vilsbøll, T.; Agersø, H.; Lauritsen, T.; Deacon, C.F.; Aaboe, K.; Madsbad, S.; Krarup, T.; Holst, J.J. The elimination rates of intact GIP as well as its primary metabolite, GIP 3-42, are similar in type 2 diabetic patients and healthy subjects. Regul. Pept. 2006, 137, 168–172. [Google Scholar] [CrossRef]
- Ali, S.; Lamont, B.J.; Charron, M.J.; Drucker, D.J. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J. Clin. Investig. 2011, 121, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans 2015–2020, 8th ed.; USA Office of Disease Prevention and Health Promotion: Washington, WA, USA, 2015.
- Davies, M.J.; Bain, S.C.; Atkin, S.L.; Rossing, P.; Scott, D.; Shamkhalova, M.S.; Bosch-Traberg, H.; Syrén, A.; Umpierrez, G.E. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): A randomized clinical trial. Diabetes Care 2016, 39, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changes, M. See full prescribing information for complete boxed warning. Interactions 1998, 50, 1–25. [Google Scholar]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; Group, N.N.S. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes. JAMA 2015, 314, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.V.; Wilding, J.P.H.; Skjøth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.K.; Wu, J.C.Y. Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver 2013, 7, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef]
- Melissas, J.; Leventi, A.; Klinaki, I.; Perisinakis, K.; Koukouraki, S.; De Bree, E.; Karkavitsas, N. Alterations of global gastrointestinal motility after sleeve gastrectomy: A prospective study. Ann. Surg. 2013, 258, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J.; Daskalakis, M.; Koukouraki, S.; Askoxylakis, I.; Metaxari, M.; Dimitriadis, E.; Stathaki, M.; Papadakis, J.A. Sleeve gastrectomy - A “food limiting” operation. Obes. Surg. 2008, 18, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. New Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef]
- López, M.; Tovar, S.; Vázquez, M.J.; Nogueiras, R.; Seoane, L.M.; García, M.; Sẽarís, R.M.; Diéguez, C. Perinatal overfeeding in rats results in increased levels of plasma leptin but unchanged cerebrospinal leptin in adulthood. Int. J. Obes. 2007, 31, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezaki, K.; Hosoda, H.; Kakei, M.; Hashiguchi, S.; Watanabe, M.; Kangawa, K.; Yada, T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: Implication in the glycemic control in rodents. Diabetes 2004, 53, 3142–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezaki, K.; Sone, H.; Koizumi, M.; Nakata, M.; Kakei, M.; Nagai, H.; Hosoda, H.; Kangawa, K.; Yada, T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006, 55, 3486–3493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, J.C.; Sakata, I.; Kohno, D.; Perello, M.; Osborne-Lawrence, S.; Repa, J.J.; Zigman, J.M. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol. Endocrinol. 2011, 25, 1600–1611. [Google Scholar] [CrossRef]
- Korbonits, M.; Goldstone, A.P.; Gueorguiev, M.; Grossman, A.B. Ghrelin--a hormone with multiple functions. Front. Neuroendocrinol. 2004, 25, 27–68. [Google Scholar] [CrossRef]
- Katsuki, A.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Nakatani, K.; Togashi, K.; Yano, Y.; Adachi, Y.; Sumida, Y. Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2004, 151, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Esler, W.P.; Rudolph, J.; Claus, T.H.; Tang, W.; Barucci, N.; Brown, S.E.; Bullock, W.; Daly, M.; DeCarr, L.; Li, Y.; et al. Small-molecule Ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology 2007, 148, 5175–5185. [Google Scholar] [CrossRef] [Green Version]
- Pittner, R.A.; Albrandt, K.; Beaumont, K.; Gaeta, L.S.; Koda, J.E.; Moore, C.X.; Rittenhouse, J.; Rink, T.J. Molecular physiology of amylin. J. Cell. Biochem. 1994, 55, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kelly, L.; Heiman, M.; Greengard, P.; Friedman, J.M. Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metab. 2015, 22, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, P.M.; Albiston, A.; Morfis, M.; Tilakaratne, N. Receptor activity modifying proteins. Cell. Signal. 2001, 13, 73–83. [Google Scholar] [CrossRef]
- Young, A.A. Brainstem sensing of meal-related signals in energy homeostasis. Neuropharmacology 2012, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A. Control of energy homeostasis by amylin. Cell. Mol. Life Sci. 2012, 69, 1947–1965. [Google Scholar] [CrossRef] [Green Version]
- Wielinga, P.Y.; Löwenstein, C.; Muff, S.; Munz, M.; Woods, S.C.; Lutz, T.A. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol. Behav. 2010, 101, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Hieronymus, L.; Griffin, S. Role of Amylin in Type 1 and Type 2 Diabetes. Diabetes Educ. 2015, 41, 47S–56S. [Google Scholar] [CrossRef]
- Pillay, K.; Govender, P. Amylin uncovered: A review on the polypeptide responsible for type II diabetes. Biomed. Res. Int. 2013, 2013, 826706. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.J. Amylin compared with calcitonin gene-related peptide: Structure, biology, and relevance to metabolic disease. Endocr. Rev. 1994, 15, 163–201. [Google Scholar] [CrossRef]
- Whitehouse, F.; Kruger, D.F.; Fineman, M.; Shen, L.; Ruggles, J.A.; Maggs, D.G.; Weyer, C.; Kolterman, O.G. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 2002, 25, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Ratner, R.; Whitehouse, F.; Fineman, M.S.; Strobel, S.; Shen, L.; Maggs, D.G.; Kolterman, O.G.; Weyer, C. Adjunctive therapy with pramlintide lowers HbA1c without concomitant weight gain and increased risk of severe hypoglycemia in patients with type 1 diabetes approaching glycemic targets. Exp. Clin. Endocrinol. Diabetes 2005, 113, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Flier, J.S. The Adipocyte as an Active Participant in Energy Balance and Metabolism. Gastroenterology 2007, 132, 2103–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Fuller, M.F.; Han, K.-S.; Kies, A.K.; Miner-Williams, W. Food-derived bioactive peptides influence gut function. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S5–S22. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores, J.D.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-richwaste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health – A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Ratanakhanokchai, K. A novel multi-biofunctional protein from brown rice hydrolysed by endo/endo-exoproteases. Food Funct. 2016, 7, 2635–2644. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef]
- Meisel, H. Biochemical properties of peptides encrypted in bovine milk proteins. Curr. Med. Chem. 2005, 12, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Technological Options for the Production of Health-Promoting Proteins and Peptides Derived from Milk and Colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, B.; Dziuba, M. Milk proteins-derived bioactive peptides in dairy products: Molecular, biological and methodological aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Foltz, M.; Meynen, E.E.; Bianco, V.; van Platerink, C.; Koning, T.M.M.G.; Kloek, J. Angiotensin Converting Enzyme Inhibitory Peptides from a Lactotripeptide-Enriched Milk Beverage Are Absorbed Intact into the Circulation. J. Nutr. 2007, 137, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Enjoh, M.; Nakamura, Y.; Takano, T.; Kawamura, Y.; Arai, S.; Shimizu, M. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2002, 66, 378–384. [Google Scholar] [CrossRef]
- Regazzo, D.; Mollé, D.; Gabai, G.; Tomé, D.; Dupont, D.; Leonil, J.; Boutrou, R. The (193–209) 17-residues peptide of bovine β-casein is transported through caco-2 monolayer. Mol. Nutr. Food Res. 2010, 54, 1428–1435. [Google Scholar] [CrossRef]
- Foltz, M.; Cerstiaens, A.; van Meensel, A.; Mols, R.; van der Pijl, P.C.; Duchateau, G.S.M.J.E.; Augustijns, P. The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 2008, 29, 1312–1320. [Google Scholar] [CrossRef]
- Shimizu, M.; Tsunogai, M.; Arai, S. Transepithelial transport of oligopeptides in the human intestinal cell, Caco-2. Peptides 1997, 18, 681–687. [Google Scholar] [CrossRef]
- Kamran, F. Bioactive peptides from legumes: Functional and nutraceutical potential. Recent Adv. Food Sci. 2018, 1, 134–149. [Google Scholar]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Sefora Rutella, G.; Taneyo Saa, D.L.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Hu, J.; Xu, M.; Hang, B.; Wang, L.; Wang, Q.; Chen, J.; Song, T.; Fu, D.; Wang, Z.; Wang, S.; et al. Isolation and characterization of an antimicrobial peptide from bovine hemoglobin α-subunit. World J. Microbiol. Biotechnol. 2011, 27, 767–771. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct 2019, 10, 6244–6266. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.M.K.; Chang, E.; Li, J.; Burlage, C.; Zou, M.; Buhman, K.K.; Koser, S.; Donkin, S.S.; Teegarden, D. Dietary intervention with vitamin D, calcium, and whey protein reduced fat mass and increased lean mass in rats. Nutr. Res. 2008, 28, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, G.C.; Chaput, J.P.; Ledoux, M.; St-Pierre, S.; Anderson, G.H.; Zemel, M.B.; Tremblay, A. Recent developments in calcium-related obesity research. Obes. Rev. 2008, 9, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Sartang, M.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Brett, N.R.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Phelan, M.; Kerins, D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011, 2, 153–167. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011, 2, 18–27. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Cummings, D.E. Regulation of ghrelin in physiologic and pathophysiologic states. J. Nutr. 2005, 135, 1320–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey Protein but Not Soy Protein Supplementation Alters Body Weight and Composition in Free-Living Overweight and Obese Adults. J. Nutr. 2011, 141, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Leoncini, R.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; et al. Whey proteins reduce appetite, stimulate anorexigenic gastrointestinal peptides and improve glucometabolic homeostasis in young obese women. Nutrients 2019, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Nibber, T.; Chelikani, P.K. Whey Protein Components - Lactalbumin and Lactoferrin - Improve Energy Balance and Metabolism. Sci. Rep. 2017, 7, 9917. [Google Scholar] [CrossRef] [Green Version]
- Pilvi, T.K.; Harala, S.; Korpela, R.; Mervaala, E.M. Effects of high-calcium diets with different whey proteins on weight loss and weight regain in high-fat-fed C57BL/6J mice. Br. J. Nutr. 2009, 102, 337–341. [Google Scholar] [CrossRef]
- Morishita, S.; Ono, T.; Fujisaki, C.; Ishihara, Y.; Murakoshi, M.; Kato, H.; Hosokawa, M.; Miyashita, K.; Sugiyama, K.; Nishino, H. Bovine lactoferrin reduces visceral fat and liver triglycerides in ICR mice. J. Oleo Sci. 2013, 62, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, T.; Murakoshi, M.; Suzuki, N.; Iida, N.; Ohdera, M.; Iigo, M.; Yoshida, T.; Sugiyama, K.; Nishino, H. Potent anti-obesity effect of enteric-coated lactoferrin: Decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br. J. Nutr. 2010, 104, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, T.; Fujisaki, C.; Ishihara, Y.; Ikoma, K.; Morishita, S.; Murakoshi, M.; Sugiyama, K.; Kato, H.; Miyashita, K.; Yoshida, T.; et al. Potent Lipolytic Activity of Lactoferrin in Mature Adipocytes. Biosci. Biotechnol. Biochem. 2013, 77, 566–571. [Google Scholar] [CrossRef]
- Kushibiki, S.; Shingu, H.; Komatsu, T.; Itoh, F.; Moriya, N.; Touno, E.; Oshibe, A.; Hodate, K. Influence of orally administered bovine lactoferrin on lipid metabolism in lipopolysaccharide-injected preruminant calves. Anim. Sci. J. 2009, 80, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beynen, A.C. Lowering effect of dietary milk-whey protein v. casein on plasma and liver cholesterol concentrations in rats. Br. J. Nutr. 1993, 70, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Bovine lactoferrin reduces plasma triacylglycerol and NEFA accompanied by decreased hepatic cholesterol and triacylglycerol contents in rodents. Br. J. Nutr. 2004, 91, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Huettinger, M.; Retzek, H.; Eder, M.; Goldenberg, H. Characteristics of chylomicron remnant uptake into rat liver. Clin. Biochem. 1988, 21, 87–92. [Google Scholar] [CrossRef]
- Ziere, G.J.; Van Dijk, M.C.M.; Bijsterbosch, M.K.; Van Berkel, T.J.C. Lactoferrin uptake by the rat liver. Characterization of the recognition site and effect of selective modification of arginine residues. J. Biol. Chem. 1992, 267, 11229–11235. [Google Scholar]
- Crawford, S.E.; Borensztajn, J. Plasma clearance and liver uptake of chylomicron remnants generated by hepatic lipase lipolysis: Evidence for a lactoferrin-sensitive and apolipoprotein E-independent pathway. J. Lipid Res. 1999, 40, 797–805. [Google Scholar]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Bassols, J.; Ricart, W.; Fernández-Real, J.M. Decreased circulating lactoferrin in insulin resistance and altered glucose tolerance as a possible marker of neutrophil dysfunction in type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 4036–4044. [Google Scholar] [CrossRef] [Green Version]
- Mayeur, S.; Veilleux, A.; Pouliot, Y.; Lamarche, B.; Beaulieu, J.F.; Hould, F.S.; Richard, D.; Tchernof, A.; Levy, E. Plasma lactoferrin levels positively correlate with insulin resistance despite an inverse association with total adiposity in lean and severely obese patients. PLoS ONE 2016, 11, e0166138. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Bassols, J.; Castro, A.; Ricart, W.; Fernández-Real, J.M. Association of circulating lactoferrin concentration and 2 nonsynonymous LTF gene polymorphisms with dyslipidemia in men depends on glucose-tolerance status. Clin. Chem. 2008, 54, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Veldhorst, M.A.B.; Westerterp, K.R.; Engelen, M.P.K.J.; Brummer, R.J.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. Acute effects of breakfasts containing -lactalbumin, or gelatin with or without added tryptophan, on hunger, satiety hormones and amino acid profiles. Br. J. Nutr. 2009, 101, 1859–1866. [Google Scholar] [CrossRef]
- Hursel, R.; Van Der Zee, L.; Westerterp-Plantenga, M.S. Effects of a breakfast yoghurt, with additional total whey protein or caseinomacropeptide-depleted-lactalbumin-enriched whey protein, on diet-induced thermogenesis and appetite suppression. Br. J. Nutr. 2010, 103, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Froetschel, M.A.; Azain, M.J.; Edwards, G.L.; Barb, C.R.; Amos, H.E. Opioid and cholecystokinin antagonists alleviate gastric inhibition of food intake by premeal loads of casein in meal-fed rats. J. Nutr. 2001, 131, 3270–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; Schellekens, H.; Dinan, T.G.; Cryan, J.F.; Fitzgerald, R.J. Milk protein hydrolysates activate 5-HT 2C serotonin receptors: Influence of the starting substrate and isolation of bioactive fractions. Food Funct. 2013, 4, 728–737. [Google Scholar] [CrossRef] [Green Version]
- De Campos Zani, S.C.; Wu, J.; Chan, C.B. Egg and soy-derived peptides and hydrolysates: A review of their physiological actions against diabetes and obesity. Nutrients 2018, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Sumner, D.A.; Gow, H.; Hayes, D.; Matthews, W.; Norwood, B.; Rosen-molina, J.T.; Thurmanl, W. Economic and market issues on the sustainability of egg production in the United States: Analysis of alternative production systems. Poult. Sci. 2011, 90, 241–250. [Google Scholar] [CrossRef]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem. 2012, 135, 1245–1252. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Zhao, M.Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Li, X.; Lai, F.; Xiao, X. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochem. Biophys. Res. Commun. 2014, 452, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg white hydrolysate shows insulin mimetic and sensitizing effects in 3T3-F442A preadipocytes. PLoS ONE 2017, 12, e0185653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcés-Rimón, M.; González, C.; Uranga, J.A.; López-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin Egg White Hydrolysate Ameliorates Obesity-Related Oxidative Stress, Inflammation and Steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcés-Rimón, M.; González, C.; Vera, G.; Uranga, J.A.; López-Fandiño, R.; López-Miranda, V.; Miguel, M. Pepsin egg white hydrolysate improves glucose metabolism complications related to metabolic syndrome in zucker fatty rats. Nutrients 2018, 10, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Fernández, S.; Garcés-Rimón, M.; González, C.; Uranga, J.A.; López-Miranda, V.; Vera, G.; Miguel, M. Pepsin egg white hydrolysate ameliorates metabolic syndrome in high-fat/high-dextrose fed rats. Food Funct. 2018, 9, 78–86. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Requena, T.; Miguel, M.; Garcés-Rimón, M.; Martínez-Cuesta, M.C.; López-Fandiño, R.; Peláez, C. Pepsin egg white hydrolysate modulates gut microbiota in Zucker obese rats. Food Funct. 2017, 8, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Hara, H.; Tomita, F. Soybean β-Conglycinin Peptone Suppresses Food Intake and Gastric Emptying by Increasing Plasma Cholecystokinin Levels in Rats. J. Nutr. 2003, 133, 352–357. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Fujita, H.; Matoba, N.; Takenaka, Y.; Yamamoto, T.; Yamauchi, R.; Tsuruki, H.; Takahata, K. Bioactive peptides derived from food proteins preventing lifestyle-related diseases. BioFactors 2000, 12, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.J.; Juillerat, M.A.; Lee, C.H. Identification of LDL-receptor transcription stimulating peptides from soybean hydrolysate in human hepatocytes. J. Agric. Food Chem. 2008, 56, 4372–4376. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Nagaoka, S.; Nakamura, A.; Shibata, H.; Kanamaru, Y. Soystatin (VAWWMY), a Novel Bile Acid-Binding Peptide, Decreased Micellar Solubility and Inhibited Cholesterol Absorption in Rats. Biosci. Biotechnol. Biochem. 2010, 74, 1738–1741. [Google Scholar] [CrossRef] [Green Version]
- LLule, V.K.; Garg, S.; Pophaly, S.D.; Tomar, S.K. “Potential health benefits of lunasin: A multifaceted soy-derived bioactive peptide”. J. Food Sci. 2015, 80, C485–C494. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457. [Google Scholar] [CrossRef]
- Aoyama, T.; Fukui, K.; Nakamori, T.; Hashimoto, Y.; Yamamoto, T.; Takamatsu, K.; Sugano, M. Effect of Soy and Milk Whey Protein Isolates and Their Hydrolysates on Weight Reduction in Genetically Obese Mice. Biosci. Biotechnol. Biochem. 2000, 64, 2594–2600. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Oyaizu, S.; Fukuchi, Y.; Mizunoya, W.; Segawa, K.; Takahashi, M.; Mita, Y.; Fukuya, Y.; Fushiki, T.; Yasumoto, K. A soybean peptide isolate diet promotes postprandial carbohydrate oxidation and energy expenditure in type II diabetic mice. J. Nutr. 2003, 133, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, T.; Kohno, M.; Saito, T.; Fukui, K.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Hirotsuka, M.; Kito, M. Reduction by Phytate-reduced Soybean β-Conglycinin of Plasma Triglyceride Level of Young and Adult Rats. Biosci. Biotechnol. Biochem. 2001, 65, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.; Skakkebaek, N.E. Exposure to exogenous estrogens in food: Possible impact on human development and health. Eur. J. Endocrinol. 1999, 140, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deal, C.L.; Tony, M.; Hoybye, C.; Allen, D.B.; Tauber, M.; Christiansen, J.S. GrowthHormone Research Society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1072–E1087. [Google Scholar] [CrossRef] [PubMed]
- Quigley, C.A.; Child, C.J.; Zimmermann, A.G.; Rosenfeld, R.G.; Robison, L.L.; Blum, W.F. Mortality in Children Receiving Growth Hormone Treatment of Growth Disorders: Data From the Genetics and Neuroendocrinology of Short Stature International Study. J. Clin. Endocrinol. Metab. 2017, 102, 3195–3205. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Cooke, R.; Beckers, D.; Borgstrom, B.; Butler, G.; Carel, J.C.; Cianfarani, S.; Clayton, P.; Coste, J.; Deodati, A.; et al. Cancer Risks in Patients Treated with Growth Hormone in Childhood: The SAGhE European Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1661–1672. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Ho, K.K.Y. The Use and Abuse of Growth Hormone in Sports. Endocr. Rev. 2019, 40, 1163–1185. [Google Scholar] [CrossRef]
- Ferro, P.; Krotov, G.; Zvereva, I.; Rodchenkov, G.; Segura, J. Structure-activity relationship for peptidic growth hormone secretagogues. Drug Test. Anal. 2017, 9, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Krug, O.; Thomas, A.; Malerod-Fjeld, H.; Dehnes, Y.; Laussmann, T.; Feldmann, I.; Sickmann, A.; Thevis, M. Analysis of new growth promoting black market products. Growth Horm IGF Res. 2018, 41, 1–6. [Google Scholar] [CrossRef]
- Schally, A.V.; Wang, H.; He, J.; Cai, R.; Sha, W.; Popovics, P.; Perez, R.; Vidaurre, I.; Zhang, X. Agonists of growth hormone-releasing hormone (GHRH) inhibit human experimental cancers in vivo by down-regulating receptors for GHRH. Proc. Natl. Acad. Sci. USA 2018, 115, 12028–12033. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.M.; Huang, H.Y.; Schally, A.V.; Chao, A.; Chou, H.H.; Leung, P.C.; Wang, H.S. Growth hormone-releasing hormone antagonist inhibits the invasiveness of human endometrial cancer cells by down-regulating twist and N-cadherin expression. Oncotarget 2017, 8, 4410–4421. [Google Scholar] [CrossRef]
- Kanashiro-Takeuchi, R.M.; Tziomalos, K.; Takeuchi, L.M.; Treuer, A.V.; Lamirault, G.; Dulce, R.; Hurtado, M.; Song, Y.; Block, N.L.; Rick, F.; et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc. Natl. Acad. Sci. USA 2010, 107, 2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Konstantinopoulos, P.A.; Papailiou, J.; Kandarakis, S.A.; Andreopoulos, A.; Sykiotis, G.P. Erythropoietin abuse and erythropoietin gene doping: Detection strategies in the genomic era. Sports Med. 2005, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Salamin, O.; Kuuranne, T.; Saugy, M.; Leuenberger, N. Erythropoietin as a performance-enhancing drug: Its mechanistic basis, detection, and potential adverse effects. Mol. Cell Endocrinol. 2018, 464, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sgro, P.; Sansone, M.; Sansone, A.; Romanelli, F.; Di Luigi, L. Effects of erythropoietin abuse on exercise performance. Phys. Sportsmed. 2018, 46, 105–115. [Google Scholar] [CrossRef]
- Hardee, M.E.; Cao, Y.; Fu, P.; Jiang, X.; Zhao, Y.; Rabbani, Z.N.; Vujaskovic, Z.; Dewhirst, M.W.; Arcasoy, M.O. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS ONE 2007, 2, e549. [Google Scholar] [CrossRef] [Green Version]
- Trial, J.; Rice, L.; Alfrey, C.P. Erythropoietin withdrawal alters interactions between young red blood cells, splenic endothelial cells, and macrophages: An in vitro model of neocytolysis. J. Investig. Med. 2001, 49, 335–345. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gewehr, M.C.F.; Silverio, R.; Rosa-Neto, J.C.; Lira, F.S.; Reckziegel, P.; Ferro, E.S. Peptides from Natural or Rationally Designed Sources Can Be Used in Overweight, Obesity, and Type 2 Diabetes Therapies. Molecules 2020, 25, 1093. https://doi.org/10.3390/molecules25051093
Gewehr MCF, Silverio R, Rosa-Neto JC, Lira FS, Reckziegel P, Ferro ES. Peptides from Natural or Rationally Designed Sources Can Be Used in Overweight, Obesity, and Type 2 Diabetes Therapies. Molecules. 2020; 25(5):1093. https://doi.org/10.3390/molecules25051093
Chicago/Turabian StyleGewehr, Mayara C. F., Renata Silverio, José Cesar Rosa-Neto, Fabio S. Lira, Patrícia Reckziegel, and Emer S. Ferro. 2020. "Peptides from Natural or Rationally Designed Sources Can Be Used in Overweight, Obesity, and Type 2 Diabetes Therapies" Molecules 25, no. 5: 1093. https://doi.org/10.3390/molecules25051093