Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small “Non-Natural” Peptides
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Peptide Synthesis
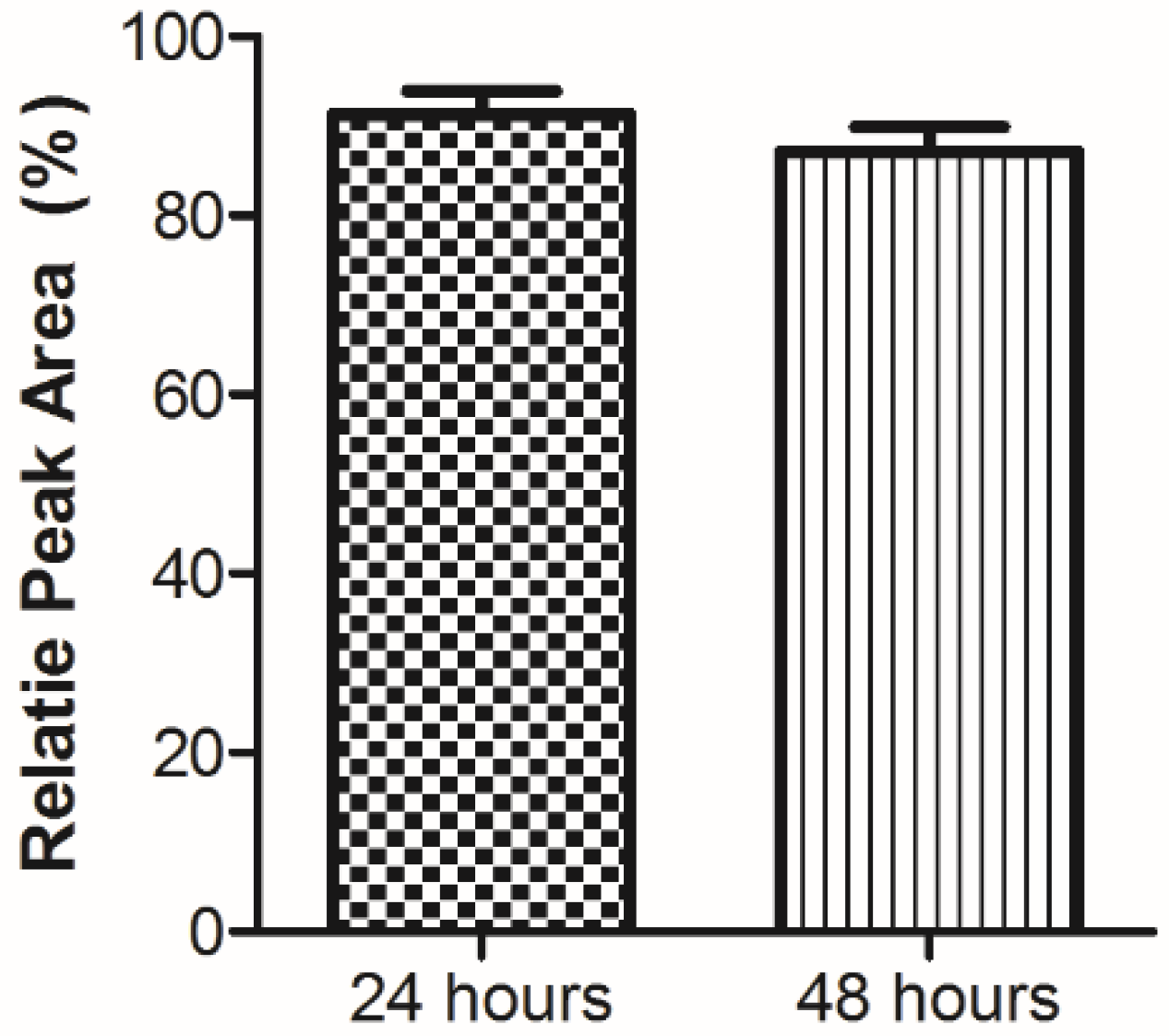
3.2. Plasma Stability Analysis
3.3. Formulation and Vaccine Preparation
3.4. In Vitro Studies
3.5. In Vivo Vaccination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Craik, D.J. Discovery and optimization of peptide macrocycles. Expert Opin. Drug Discov. 2016, 11, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Calvo Tardón, M.; Allard, M.; Dutoit, V.; Dietrich, P.Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef]
- Le, H.T.; Lemaire, I.; Gilbert, A.K.; Jolicoeur, F.; Lemaire, S. Bioactive peptidic analogues and cyclostereoisomers of the minimal antinociceptive histogranin fragment-(7-10). J. Med. Chem. 2003, 46, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Lemaire, I.; Gilbert, A.K.; Jolicoeur, F.; Leduc, N.; Yang, L.; Lemaire, S. Histogranin-like antinociceptive and anti-inflammatory derivatives of o-phenylenediamine and benzimidazole. J Pharmacol. Exp. Ther. 2004, 309, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumai, T.; Lee, S.; Cho, H.I.; Sultan, H.; Kobayashi, H.; Harabuchi, Y.; Celis, E. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol. Res. 2017, 5, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, Q.; Li, K.; Yin, H.; Zheng, J.N. Composite peptide-based vaccines for cancer immunotherapy (Review). Int. J. Mol. Med. 2015, 35, 17–23. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine 2014, 17, 2657–2669. [Google Scholar] [CrossRef]
- Bezu, L.; Kepp, O.; Cerrato, G.; Pol, J.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Peptide-based vaccines in anticancer therapy. Oncoimmunology 2018, 7, e15111506. [Google Scholar] [CrossRef]
- Combadière, B.; Beaujean, M.; Chaudesaigues, C.; Vieillard, V. Peptide-Based Vaccination for Antibody Responses Against HIV. Vaccines 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Rodriguez, J.; Meijerhof, T.; Niesters, H.G.; Stjernholm, G.; Hovden, A.O.; Sørensen, B.; Ökvist, M.; Sommerfelt, M.A.; Huckriede, A. A novel peptide-based vaccine candidate with protective efficacy against influenza A in a mouse model. Virology 2018, 515, 21–28. [Google Scholar] [CrossRef]
- Basirnejad, M.; Bolhassani, A. Development of HCV Therapeutic Vaccines Using Hp91 Peptide and Small Heat Shock Protein 20 as an Adjuvant. Protein Pept. Lett. 2018, 25, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Tuller, T.; Chor, B.; Nelson, N. Forbidden penta-peptides. Protein Sci. 2017, 16, 2251–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otaki, J.M.; Gotoh, T.; Yamamoto, H. Potential implications of availability of short amino acid sequences in proteins: An old and new approach to protein decoding and design. Biotechnol. Annu. Rev. 2008, 14, 109–141. [Google Scholar] [PubMed]
- Lucchese, G.; Stufano, A.; Trost, B.; Kusalik, A.; Kanduc, D. Peptidology: Short amino acid modules in cell biology and immunology. Amino Acids 2007, 33, 703–707. [Google Scholar] [CrossRef]
- Fu, X.; Tao, L.; Zhang, X. A short polypeptide from the herpes simplex virus type 2 ICP10 gene can induce antigen aggregation and autophagosomal degradation for enhanced immune presentation. Hum. Gene Ther. 2010, 21, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Saenz, R.; Souza Cda, S.; Huang, C.T.; Larsson, M.; Esener, S.; Messmer, D. HMGB1-derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine 2010, 28, 7556–7562. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, E.F.; Madera, L.; Hancock, R.E. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann. N. Y. Acad. Sci. 2010, 1213, 46–61. [Google Scholar] [CrossRef]
- Patel, A.; Dong, J.C.; Trost, B.; Richardson, J.S.; Tohme, S.; Babiuk, S.; Kusalik, A.; Kung, S.K.; Kobinger, G.P. Pentamers not found in the universal proteome can enhance antigen specific immune responses and adjuvant vaccines. PLoS ONE 2012, 7, e43802. [Google Scholar] [CrossRef]
- Dong, J.C.; Kobinger, G.P. Hypothesis driven development of new adjuvants: Short peptides as immunomodulators. Hum Vaccin. Immunother. 2013, 9, 808–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Kobasa, D.; Kobinger, G.P.; Babiuk, S. Peptide Adjuvants. U.S. Patent 9,211,325, 15 December 2015. [Google Scholar]
- World Health Organization. Tobacco Fact Sheet. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 20 September 2019).
- Bremer, P.T.; Janda, K.D. Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials 2017, 138, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Thorn, J.M.; Bhattacharya, K.; Crutcher, R.; Sperry, J.; Isele, C.; Kelly, B.; Yates, L.; Zobel, J.; Zhang, N.; Davis, H.L.; et al. The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice. Vaccines 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Hybrid nanoparticle-based nicotine nanovaccines: Boosting the immunological efficacy by conjugation of potent carrier proteins. Nanomedicine 2018, 14, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Fraleigh, N.L.; Boudreau, J.; Bhardwaj, N.; Eng, N.F.; Murad, Y.; Lafrenie, R.; Acevedo, R.; Oliva, R.; Diaz-Mitoma, F.; Le, H.T. Evaluating the immunogenicity of an intranasal vaccine against nicotine in mice using the Adjuvant Finlay Proteoliposome (AFPL1). Heliyon 2016, 2, e00147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraleigh, N.L.; Oliva, R.; Lewicky, J.D.; Martel, A.L.; Acevedo, R.; Dagmar, G.R.; Le, H.T. Assessing the immunogenicity and toxicity of the AFPL1-conjugate nicotine vaccine using heterologous and homologous vaccination routes. PLoS ONE 2019, 14, e0221708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, H.T.; Le Foll, B.; Le, D.A.; Murad, Y. Development of novel lung mucosal vaccine for smoking cessation treatment. CIHR Project Grant PJT-148531, 2016-2020. Available online: http://webapps.cihr-irsc.gc.ca/decisions/p/project_details.html?applId=344049&lang=en (accessed on 1 March 2016).
- Pentel, P.R.; LeSage, M.G. New directions in nicotine design and use. Adv. Pharmacol. 2014, 69, 553–580. [Google Scholar]
- McCluskie, M.J.; Thorn, J.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Cartier, J.; Kim, I.J.; Bhattacharya, K.; Finneman, J.I.; et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharamacol. 2015, 29, 663–671. [Google Scholar] [CrossRef]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef]
- Zeigler, D.F.; Roque, R.; Clegg, C.H. Optimization of a multivalent peptide vaccine for nicotine addiction. Vaccine 2019, 37, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Zhang, R.Y.; Wang, X.F.; Yin, X.G.; Wang, J.; Wang, Y.C.; Liu, X.; Du, J.J.; Liu, Z.; Guo, J. Peptide-free Synthetic Nicotine Vaccine Candidates with α-Galactosylceramide as Adjuvant. Mol. Pharm. 2019, 16, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.A.; Nilsson, B.L. Multicomponent peptide assemblies. Chem. Soc. Rev. 2018, 47, 3659–3720. [Google Scholar] [CrossRef] [PubMed]
- Lewicky, J.D.; Martel, A.L.; Fraleigh, N.L.; Boraman, A.; Nguyen, T.M.D.; Schiller, P.W.; Shiao, T.C.; Roy, R.; Le, H.T. Strengthening peptide-based drug activity with novel glyconanoparticle. PLoS ONE 2018, 13, e0204472. [Google Scholar] [CrossRef]
- Daw, K.; Baghdayan, A.S.; Awasthi, S.; Shankar, N. Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitro. FEMS Immunol. Med. Microbiol. 2012, 65, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Staats, H.F.; Ennis, E.A., Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 1999, 162, 6141–6147. [Google Scholar]
- Gwinn, W.M.; Kirwan, S.M.; Wang, S.H. Effective induction of protective systemic immunity with nasally-administered vaccines adjuvanted with IL-1. Vaccine 2010, 28, 6901–6914. [Google Scholar] [CrossRef] [Green Version]
- Khoruts, A.; Osness, R.E.; Jenkins, M.K. IL-1 acts on antigen-presenting cells to enhance the in vivo proliferation of antigen-stimulated naïve CD4 T cells via a CD28-dependent mechansism that does not involve increased expression of CD28 ligands. Eur. J. Immunol. 2004, 34, 1085–1090. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J. IL-1 acts directly on CD4 T cells to enhance their antigen-drived expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Immunological and inflammatory function of the interleukin-1 family. Annu. Rev. Immunol. 2009, 106, 519–550. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from the authors. |



| Peptide | Amino Acid Sequence |
|---|---|
| 1 | KWCEC |
| 2 | KWCECKFFKFFG |
| 3 | KWCECEFFEFFG |
| 4 | Succinamic-KWCEC |
| Peptide | Ninhydrin Test | Scale and Yield | HPLC tR (min) | Mass Spectrometry | |
|---|---|---|---|---|---|
| 1 | Positive | 300 nmol 100 mg (50.0%) | 14.8 | Calculated: Measured: | 667.25 668.1 (M + H)+ |
| 2 | Positive | 200 nmol 240 mg (76.5%) | 20.8 | Calculated: Measured: | 1568.73 785.5 (M + 2H)2+ |
| 3 | Positive | 200 nmol 45 mg (14.3%) | 23.7 | Calculated: Measured: | 1571.8 786.4 (M + 2H)2+ |
| 4 | Positive | 100 nmol 37 mg (48.2%) | 16.5 | Calculated: Measured: | 766.87 767.3 (M + H)+ |
| Vaccine | Composition |
|---|---|
| 2 | BDA + Nicotine |
| 3 | BDA + Nicotine/Chitosan |
| 4 | BDA + Nicotine + Peptide 1 |
| 5 | BDA + Nicotine + Peptide 1/Chitosan |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.-T.; Fraleigh, N.L.; Lewicky, J.D.; Boudreau, J.; Dolinar, P.; Bhardwaj, N.; Diaz-Mitoma, F.; Montaut, S.; Fallahi, S.; Martel, A.L. Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small “Non-Natural” Peptides. Molecules 2020, 25, 1290. https://doi.org/10.3390/molecules25061290
Le H-T, Fraleigh NL, Lewicky JD, Boudreau J, Dolinar P, Bhardwaj N, Diaz-Mitoma F, Montaut S, Fallahi S, Martel AL. Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small “Non-Natural” Peptides. Molecules. 2020; 25(6):1290. https://doi.org/10.3390/molecules25061290
Chicago/Turabian StyleLe, Hoang-Thanh, Nya L. Fraleigh, Jordan D. Lewicky, Justin Boudreau, Paul Dolinar, Nitin Bhardwaj, Francisco Diaz-Mitoma, Sabine Montaut, Sarah Fallahi, and Alexandrine L. Martel. 2020. "Enhancing the Immune Response of a Nicotine Vaccine with Synthetic Small “Non-Natural” Peptides" Molecules 25, no. 6: 1290. https://doi.org/10.3390/molecules25061290







