Enhanced Immune Response Against the Thomsen-Friedenreich Tumor Antigen Using a Bivalent Entirely Carbohydrate Conjugate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of TACA-PS A1 Conjugates
2.2. ELISA Reveals Enhanced Antibody Production Against the TF antigen with the Tn-TF-PS A1 Conjugate
2.3. Polyclonal Antibodies Bind Human Tumor Cell Lines
2.4. Polyclonal Antibodies Mediate Tumor Cell Killing with Complement
2.5. Quantification of Cytokines Released from Splenocytes In Vitro
2.6. Quantification of Cytokine Producing Cells with ELISpot
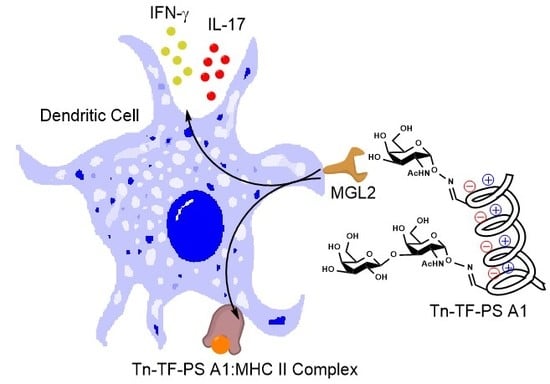
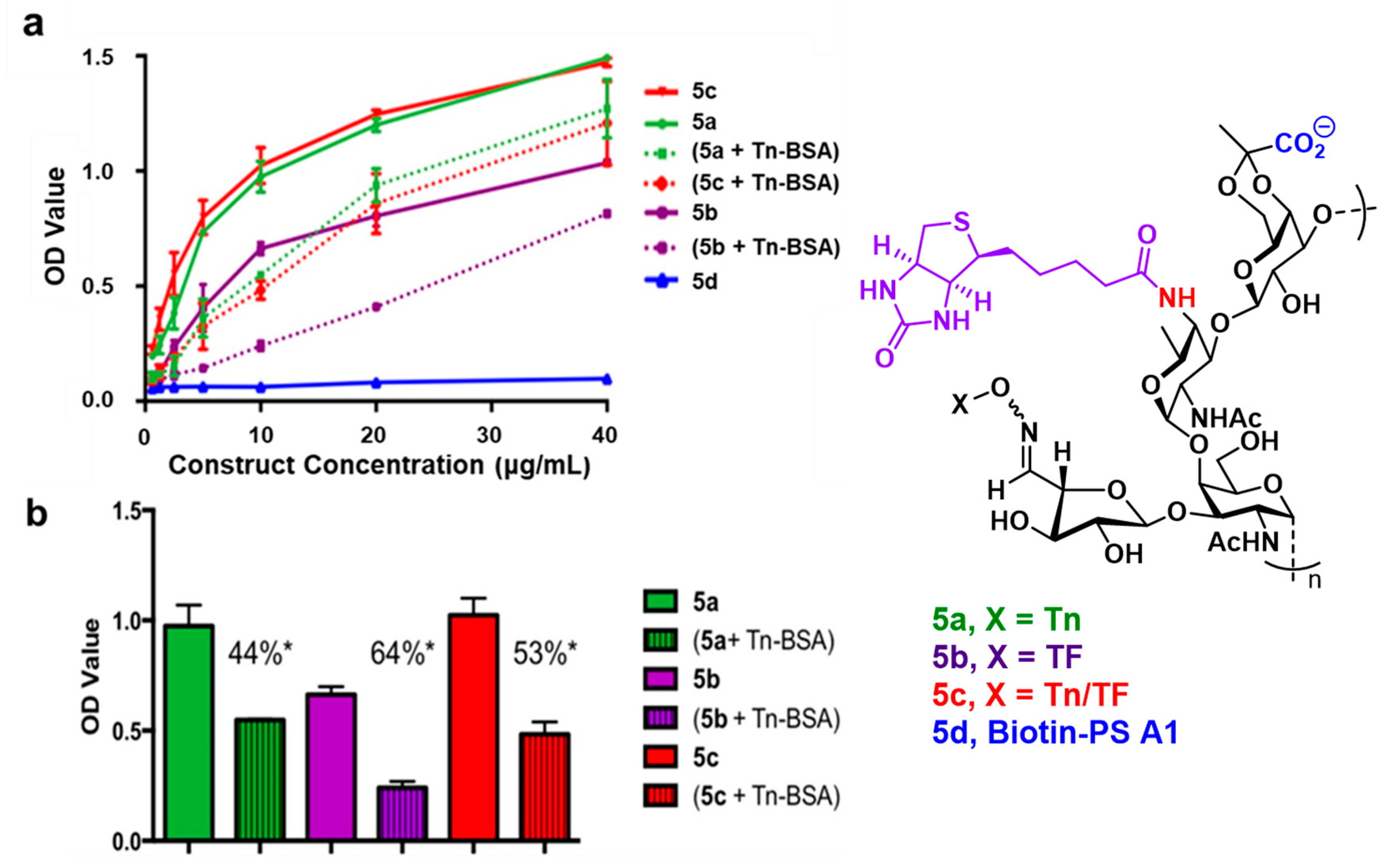
2.7. Biotinylated Conjugate Probes Bind to Recombinant MGL2
3. Materials and Methods
3.1. Vaccinations with TiterMax® Gold or Sigma Adjuvant System®
3.2. Enzyme Linked Immunosorbent Assay (ELISA)
3.3. Flow Cytometry
3.4. Complement Dependent Cytotoxicity Assay
3.5. MGL2 Binding Assay
3.6. Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef] [Green Version]
- Gassmann, P.; Kang, M.L.; Mees, S.T.; Haier, J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell-endothelial cell interaction. BMC Cancer 2010, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, F.; David, L.; Sobrinho-Simoes, M. Prognostic significance of T antigen expression in patients with gastric carcinoma. Cancer 1996, 78, 2448–2450. [Google Scholar] [CrossRef]
- Yu, L.-G.; Andrews, N.; Zhao, Q.; McKean, D.; Williams, J.F.; Connor, L.J.; Gerasimenko, O.V.; Hilkens, J.; Hirabayashi, J.; Kasai, K.; et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 2007, 282, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinsky, V.V.; Glinsky, G.V.; Rittenhouse-Olson, K.; Huflejt, M.E.; Glinskii, O.V.; Deutscher, S.L.; Quinn, T.P. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001, 61, 4851–4857. [Google Scholar] [PubMed]
- Kurtenkov, O.; Klaamas, K.; Mensdorff-Pouilly, S.; Miljukhina, L.; Shljapnikova, L.; Chuzmarov, V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: Relation to survival. Acta Oncol. 2007, 46, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kurtenkov, O.; Klaamas, K.; Rittenhouse-Olson, K.; Vahter, L.; Sergejev, B.; Miljukhina, L.; Shljapnikova, L. IgG immune response to tumor-associated carbohydrate antigens (TF, Tn, alphaGal) in patients with breast cancer: Impact of neoadjuvant chemotherapy and relation to the survival. Exp. Oncol. 2005, 27, 136–140. [Google Scholar]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [Green Version]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zheng, X.J.; Huo, C.X.; Song, C.; Li, Q.; Ye, X.S. Synthesis and evaluation of glycoconjugates comprising N-Acyl-modified Thomsen-Friedenreich antigens as anticancer vaccines. ChemMedChem 2016, 11, 1090–1096. [Google Scholar] [CrossRef]
- Johannes, M.; Reindl, M.; Gerlitzki, B.; Schmitt, E.; Hoffmann-Röder, A. Synthesis and biological evaluation of a novel MUC1 glycopeptide conjugate vaccine candidate comprising a 4′-deoxy-4′-fluoro-Thomsen–Friedenreich epitope. Beilstein J. Org. Chem. 2015, 11, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, L.; Madani, R.; Gillig, A.; Kolympadi, M.; Philgren, M.; Muhs, A.; Gérard, C.; Vogel, P. AC-linked disaccharide analogue of Thomsen–Friedenreich epitope induces a strong immune response in mice. Chem. Eur. J. 2012, 18, 8578–8582. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Röder, A.; Kaiser, A.; Wagner, S.; Gaidzik, N.; Kowalczyk, D.; Westerlind, U.; Gerlitzki, B.; Schmitt, E.; Kunz, H. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen–Friedenreich Antigen and a fluorine-substituted analogue. Angew. Chem. Int. Ed. 2010, 49, 8498–8503. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Ragupathi, G.; Fernandez, C.; Diani, M.; Jefferson, M.P.; Wilton, A.; Kelly, W.K.; Morris, M.; Solit, D.; Clausen, H.; et al. A polyvalent vaccine for high-risk prostate patients: “Are more antigens better?”. Cancer Immunol. Immunother. 2007, 56, 1921–1930. [Google Scholar] [CrossRef]
- Ragupathi, G.; Koide, F.; Livingston, P.O.; Cho, Y.S.; Endo, A.; Wan, Q.; Spassova, M.K.; Keding, S.J.; Allen, J.; Ouerfelli, O.; et al. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: A synthetic route to anticancer vaccine candidates. J. Am. Chem. Soc. 2006, 128, 2715–2725. [Google Scholar] [CrossRef]
- Slovin, S.F.; Ragupathi, G.; Musselli, C.; Fernandez, C.; Diani, M.; Verbel, D.; Danishefsky, S.; Livingston, P.; Scher, H.I. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: Clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol. Immunother. 2005, 54, 694–702. [Google Scholar] [CrossRef]
- Shi, M.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.-P.; Andreana, P.R. Sialyl-Tn Polysaccharide A1 as an entirely carbohydrate immunogen: Synthesis and immunological evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef]
- De Silva, R.A.; Wang, Q.; Chidley, T.; Appulage, D.K.; Andreana, P.R. Immunological response from an entirely carbohydrate antigen: Design of synthetic vaccines based on Tn−PS A1 conjugates. J. Am. Chem. Soc. 2009, 131, 9622–9623. [Google Scholar] [CrossRef]
- Bourgault, J.P.; Trabbic, K.R.; Shi, M.; Andreana, P.R. Synthesis of the tumor associative alpha-aminooxy disaccharide of the TF antigen and its conjugation to a polysaccharide immune stimulant. Org. Biomol. Chem. 2014, 12, 1699–1702. [Google Scholar] [CrossRef]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide processing and presentation by the MHCII pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef]
- Kalka-Moll, W.M.; Tzianabos, A.O.; Bryant, P.W.; Niemeyer, M.; Ploegh, H.L.; Kasper, D.L. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J. Immunol. 2002, 169, 6149–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, R.A.; Appulage, D.K.; Pietraszkiewicz, H.; Bobbitt, K.R.; Media, J.; Shaw, J.; Valeriote, F.A.; Andreana, P.R. The entirely carbohydrate immunogen Tn-PS A1 induces a cancer cell selective immune response and cytokine IL-17. Cancer Immunol. Immunother. 2012, 61, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamai, A.; Pignon, P.; Raimbaud, I.; Duperrier-Amouriaux, K.; Senellart, H.; Hiret, S.; Douillard, J.Y.; Bennouna, J.; Ayyoub, M.; Valmori, D. Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-gamma-secreting cells as they differentiate into effector T cells in vivo. Cancer Res. 2012, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Del Fresno, C.; Iborra, S.; Saz-Leal, P.; Martinez-Lopez, M.; Sancho, D. Flexible signaling of myeloid C-type lectin receptors in immunity and inflammation. Front. Immunol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Streng-Ouwehand, I.; Unger, W.W.; Van Kooyk, Y. C-type lectin receptors for tumor eradication: Future directions. Cancers 2011, 3, 3169–3188. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Hadjialirezaei, S.; Picco, G.; Beatson, R.; Burchell, J.; Stokke, B.T.; Sletmoen, M. Interactions between the breast cancer-associated MUC1 mucins and C-type lectin characterized by optical tweezers. PLoS ONE 2017, 12, e0175323. [Google Scholar] [CrossRef]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Weelij, D.R.; García-Vallejo, J.J.; van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Characterization of murine MGL1 and MGL2 C-type lectins: Distinct glycan specificities and tumor binding properties. Mol. Immunol. 2009, 46, 1240–1249. [Google Scholar] [CrossRef]
- Oo-Puthinan, S.; Maenuma, K.; Sakakura, M.; Denda-Nagai, K.; Tsuiji, M.; Shimada, I.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Bovin, N.V.; Irimura, T. The amino acids involved in the distinct carbohydrate specificities between macrophage galactose-type C-type lectins 1 and 2 (CD301a and b) of mice. Biochim. Biophys. Acta 2008, 1780, 89–100. [Google Scholar] [CrossRef]
- Tsuiji, M.; Fujimori, M.; Ohashi, Y.; Higashi, N.; Onami, T.M.; Hedrick, S.M.; Irimura, T. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J. Biol. Chem. 2002, 277, 28892–28901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibadullin, R.; Farnsworth, D.W.; Barchi, J.J., Jr.; Gildersleeve, J.C. GalNAc-tyrosine is a ligand of plant lectins, antibodies, and human and murine macrophage galactose-type lectins. ACS Chem. Biol. 2017, 12, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Denda-Nagai, K.; Aida, S.; Saba, K.; Suzuki, K.; Moriyama, S.; Oo-Puthinan, S.; Tsuiji, M.; Morikawa, A.; Kumamoto, Y.; Sugiura, D.; et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): Efficient uptake and presentation of glycosylated antigens by dendritic cells. J. Biol. Chem. 2010, 285, 19193–19204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggink, L.L.; Roby, K.F.; Cote, R.; Kenneth Hoober, J. An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides. J. Immunother. Cancer 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzari, I.G.; Napoletano, C.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Pierelli, L.; Nuti, M.; Rughetti, A. MGL receptor and immunity: When the ligand can make the difference. J. Immunol. Res. 2015, 2015, 450695. [Google Scholar] [CrossRef] [Green Version]
- Zizzari, I.G.; Martufi, P.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Bellati, F.; Nuti, M.; Rughetti, A.; Napoletano, C. The Macrophage Galactose-Type C-Type Lectin (MGL) modulates regulatory T cell functions. PLoS ONE 2015, 10, e0132617. [Google Scholar] [CrossRef] [Green Version]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Freire, T.; Lo-Man, R.; Bay, S.; Leclerc, C. Tn glycosylation of the MUC6 protein modulates its immunogenicity and promotes the induction of Th17-biased T cell responses. J. Biol. Chem. 2011, 286, 7797–7811. [Google Scholar] [CrossRef] [Green Version]
- Freire, T.; Zhang, X.; Deriaud, E.; Ganneau, C.; Vichier-Guerre, S.; Azria, E.; Launay, O.; Lo-Man, R.; Bay, S.; Leclerc, C. Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood 2010, 116, 3526–3536. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Saeland, E.; van Kooyk, Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer 2011, 128, 1371–1383. [Google Scholar] [CrossRef]
- Van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet preferences of MGL: Carbohydrate specificity and function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Nishat, S.; Ghosh, S.; Boga, S.; Hymel, G.T.; Andreana, P.R. Synthesis of glycoimmunogen Tn-Thr-PS A1 via hydrazone bond and stability optimization of PS A1 monosaccharide mimics under vaccine development conditions. J. Carbohydr. Chem. 2020. [Google Scholar] [CrossRef]
- Trabbic, K.R.; Bourgault, J.P.; Shi, M.; Clark, M.; Andreana, P.R. Immunological evaluation of the entirely carbohydrate-based Thomsen-Friedenreich-PS B conjugate. Org. Biomol. Chem. 2016, 14, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Stils, H.F. Adjuvants and antibody production: Dispelling the myths associated with freund’s complete and other adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Chentouh, R.; Fitting, C.; Cavaillon, J.-M. Specific features of human monocytes activation by monophosphoryl lipid A. Sci. Rep. 2018, 8, 7096. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Powlesland, A.S.; Hitchen, P.G.; Parry, S.; Graham, S.A.; Barrio, M.M.; Elola, M.T.; Mordoh, J.; Dell, A.; Drickamer, K.; Taylor, M.E. Targeted glycoproteomic identification of cancer cell glycosylation. Glycobiology 2009, 19, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Musrap, N.; Karagiannis, G.S.; Saraon, P.; Batruch, I.; Smith, C.; Diamandis, E.P. Proteomic analysis of cancer and mesothelial cells reveals an increase in Mucin 5AC during ovarian cancer and peritoneal interaction. J. Proteom. 2014, 103, 204–215. [Google Scholar] [CrossRef]
- Snijdewint, F.G.M.; von Mensdorff-Pouilly, S.; Karuntu-Wanamarta, A.H.; Verstraeten, A.A.; van Zanten-Przybysz, I.; Hummel, P.; Nijman, H.W.; Kenemans, P.; Hilgers, J. Cellular and humoral immune responses to MUC1 mucin and tandem-repeat peptides in ovarian cancer patients and controls. Cancer Immunol. Immunother. 1999, 48, 47–55. [Google Scholar] [CrossRef]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D., Jr.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Mitsdoerffer, M.; Lee, Y.; Jager, A.; Kim, H.J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Boukhvalova, M.S.; Prince, G.A.; Soroush, L.; Harrigan, D.C.; Vogel, S.N.; Blanco, J.C. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 2006, 24, 5027–5035. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- Malyguine, A.M.; Strobl, S.; Dunham, K.; Shurin, M.R.; Sayers, T.J. ELISPOT assay for monitoring Cytotoxic T Lymphocytes (CTL) activity in cancer vaccine clinical trials. Cells 2012, 1, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Ragupathi, G.; Koide, F.; Sathyan, N.; Kagan, E.; Spassova, M.; Bornmann, W.; Gregor, P.; Reis, C.A.; Clausen, H.; Danishefsky, S.J.; et al. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol. Immunother. 2003, 52, 608–616. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleski, K.A.; Trabbic, K.R.; Shi, M.; Bourgault, J.-P.; Andreana, P.R. Enhanced Immune Response Against the Thomsen-Friedenreich Tumor Antigen Using a Bivalent Entirely Carbohydrate Conjugate. Molecules 2020, 25, 1319. https://doi.org/10.3390/molecules25061319
Kleski KA, Trabbic KR, Shi M, Bourgault J-P, Andreana PR. Enhanced Immune Response Against the Thomsen-Friedenreich Tumor Antigen Using a Bivalent Entirely Carbohydrate Conjugate. Molecules. 2020; 25(6):1319. https://doi.org/10.3390/molecules25061319
Chicago/Turabian StyleKleski, Kristopher A., Kevin R. Trabbic, Mengchao Shi, Jean-Paul Bourgault, and Peter R. Andreana. 2020. "Enhanced Immune Response Against the Thomsen-Friedenreich Tumor Antigen Using a Bivalent Entirely Carbohydrate Conjugate" Molecules 25, no. 6: 1319. https://doi.org/10.3390/molecules25061319