The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator
Abstract
1. Introduction
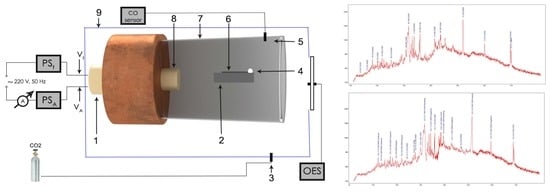
2. Materials and Methods
- a—radius of the cylindrical cavity (m);
- h—height of the cylindrical cavity (m);
- l—Longitudinal mode of the cavity;
- μ—Permeability of the medium within cavity (H/m);
- ε—Permittivity of the medium within the cavity (F/m);
- p01—First zero of the Bessel function (equal to approximately 2.405);
- fr—The resonant frequency of the cavity.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, X.; Li, Y.; Ge, W.; Wang, B. Degradation of CO2 through dielectric barrier discharge microplasma. Greenh. Gases: Sci. Technol. 2014, 5, 131–140. [Google Scholar] [CrossRef]
- Ray, D.; Saha, R.; Subrahmanyam, C. DBD Plasma Assisted CO2 Decomposition: Influence of Diluent Gases. Catalysts 2017, 7, 244. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, D.; Wang, N.; Liu, K.; Kleyn, A.W. Carbon dioxide dissociation in non-thermal radiofrequency and microwave plasma. J. Phys. D: Appl. Phys. 2017, 50, 294001. [Google Scholar] [CrossRef]
- Spencer, L.F.; Gallimore, A.D. Efficiency of CO2 Dissociation in a Radio-Frequency Discharge. Plasma Chem. Plasma Process. 2010, 31, 79–89. [Google Scholar] [CrossRef]
- Kwak, H.S.; Uhm, H.S.; Hong, Y.C.; Choi, E.H. Disintegration of Carbon Dioxide Molecules in a Microwave Plasma Torch. Sci. Rep. 2015, 5, 18436. [Google Scholar] [CrossRef] [PubMed]
- Uhm, H.S.; Hong, Y.C.; Shin, D.H. A microwave plasma torch and its applications. Plasma Sources Sci. Technol. 2006, 15, S26–S34. [Google Scholar] [CrossRef]
- Corvin, K.K. Dissociation of Carbon Dioxide in the Positive Column of a Glow Discharge. J. Chem. Phys. 1969, 50, 2570. [Google Scholar] [CrossRef]
- Lisovskiy, V.A.; Krol, H.H.; Dudin, S.V. Problems Of Atomic Science And Technology. Plasma Physics 2018, 118, 206–209. [Google Scholar]
- Nunnally, T.; Gutsol, K.; Rabinovich, A.; Fridman, A.; Gutsol, A.; Kemoun, A. Dissociation of CO2 in a low current gliding arc plasmatron. J. Phys. D: Appl. Phys. 2011, 44, 274009. [Google Scholar] [CrossRef]
- Sun, H.; Lee, J.; Do, H.; Im, S.-K.; Bak, M.S. Experimental and numerical studies on carbon dioxide decomposition in atmospheric electrodeless microwave plasmas. J. Appl. Phys. 2017, 122, 033303. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Li, X.; Kong, X.; Xu, R.; Tay, K.; Tu, X. Plasma-assisted CO2 conversion in a gliding arc discharge: Improving performance by optimizing the reactor design. J. CO2 Util. 2019, 29, 296–303. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, D.; Ma, C.; Yu, F.; Dai, B. DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures. Catalysts 2018, 8, 256. [Google Scholar] [CrossRef]
- Bekerom, D.C.M.V.D.; Linares, J.M.P.; Verreycken, T.; Van Veldhuizen, E.M.; Nijdam, S.; Berden, G.; A Bongers, W.; Van De Sanden, M.C.M.; Van Rooij, G.J. The importance of thermal dissociation in CO2 microwave discharges investigated by power pulsing and rotational Raman scattering. Plasma Sources Sci. Technol. 2019, 28, 055015. [Google Scholar] [CrossRef]
- Kanagasabay, S. Non-ionizing radiation. Curr. Approach. Occup. Health 1982, 2, 134–168. [Google Scholar]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Roy, R.; Agrawal, D. Experimental proof of major role of magnetic field losses in microwave heating of metal and metallic composites. J. Mater. Sci. Lett. 2001, 20, 1561–1563. [Google Scholar] [CrossRef]
- Mondal, A.; Agrawal, D.; Upadhyaya, A. Microwave heating of pure copper powder with varying particle size and porosity. J. Microw. Power Electromagn. Energy 2009, 43, 5–10. [Google Scholar] [CrossRef]
- Mogildea, G.; Mogildea, M. Experimental investigation of the metals vaporization and ionization with microwave used as propellant for ionic propulsion. J. Optoelectron. Adv. Mater.—Rapid Commun. 2010, 4, 352–356. [Google Scholar]
- Mogildea, M.; Mogildea, G. Experimental research for the mass flow control of the metal vaporized and ionized with microwave used in electric propulsion. J. Optoelectron. Adv. Mater. 2010, 12, 1157–1160. [Google Scholar]
- Mogildea, G.; Mogildea, M. Experimental investigation of the microwave electrothermal thruster using metals as propellant. J. Optoelectron. Adv. Mater.—Rapid Commun. 2010, 4, 1826–1829. [Google Scholar]
- Madorsky, S.; Hart, V.; Straus, S.; Sedlak, V. Thermal degradation of tetrafluoroethylene and hydrofluoroethylene polymers in a vacuum. J. Res. Natl. Inst. Stand. Technol. 1953, 51, 327. [Google Scholar] [CrossRef]
- Chryssomallis, M.; Christodoulou, C. Antenna Radiation Patterns. In Wiley Encyclopedia of Electrical and Electronics Engineering; Wiley: Hoboken, NJ, USA, 1999; Volume 2. [Google Scholar]
- Leins, M.; Gaiser, S.; Schulz, A.; Walker, M.; Schumacher, U.; Hirth, T. How to Ignite an Atmospheric Pressure Microwave Plasma Torch without Any Additional Igniters. J. Vis. Exp. 2015, 98. [Google Scholar] [CrossRef] [PubMed]
- Nordling, N.; Micci, M. Low-power Microwave Arcjet Performance Testing. IEPC 1997, 97, 89. [Google Scholar]
- Dudley, D.G. The IEEE Series on Electromagnetic Wave Theory. IEEE Antennas Propag. Mag. 2006, 48, 126–127. [Google Scholar] [CrossRef]
- Popescu, S.; Jerby, E.; Meir, Y.; Barkay, Z.; Ashkenazi, D.; Mitchell, J.B.A.; Le Garrec, J.-L.; Narayanan, T. Plasma column and nano-powder generation from solid titanium by localized microwaves in air. J. Appl. Phys. 2015, 118, 023302. [Google Scholar] [CrossRef]
- Mogildea, G.; Mogildea, M.; Jivanescu, I.; Porosincu, C.; Lungu, C.; Chiritoi, G.; Mingireanu, F. Direct vaporization and ionization of the metals wires using microwave field. J. Optoelectron. Adv. Mater. 2015, 7, 367–371. [Google Scholar]
- Isola, L.M.; Gomez, B.J.A.; Guerra, V. Determination of the electron temperature and density in the negative glow of a nitrogen pulsed discharge using optical emission spectroscopy. J. Phys. D: Appl. Phys. 2009, 43, 015202. [Google Scholar] [CrossRef]
- Bazavan, M.; Teodorescu, M.; Dinescu, G. Confirmation of OH as good thermometric species for gas temperature determination in an atmospheric pressure argon plasma jet. Plasma Sources Sci. Technol. 2017, 26, 075001. [Google Scholar] [CrossRef]
- Navrátil, Z.; Trunec, D.; Šmíd, R.; Lazar, L. A software for optical emission spectroscopy-problem formulation and application to plasma diagnostics. Czechoslov. J. Phys. 2006, 56, B944–B951. [Google Scholar] [CrossRef]
- Dobrea, S.; Mihaila, I.; Tiron, V.; Popa, G. Optical and mass spectrometry diagnosis of a CO2 microwave plasma discharge. Rom. Rep. Physics. 2014, 66, 1147–1154. [Google Scholar]
- Lisovskiy, V.A.; Krol, H.H.; Dudin, S.V. Investigation of DC glow discharge in CO2 using Optical Emission Spectroscopy. Probl. Atomic Sci. Technol. 2018, 6, 206–209. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |







| Tungsten Wire | First Ionization Energy (eV) | Vaporization Rate (mg/s) | Electrical Conductivity (S/m) |
|---|---|---|---|
| (0.5 mm diameter) | 7.98 | 2 | 2 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogildea, G.; Mogildea, M.; Popa, C.; Chiritoi, G. The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator. Molecules 2020, 25, 1558. https://doi.org/10.3390/molecules25071558
Mogildea G, Mogildea M, Popa C, Chiritoi G. The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator. Molecules. 2020; 25(7):1558. https://doi.org/10.3390/molecules25071558
Chicago/Turabian StyleMogildea, George, Marian Mogildea, Cristina Popa, and Gabriel Chiritoi. 2020. "The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator" Molecules 25, no. 7: 1558. https://doi.org/10.3390/molecules25071558
APA StyleMogildea, G., Mogildea, M., Popa, C., & Chiritoi, G. (2020). The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator. Molecules, 25(7), 1558. https://doi.org/10.3390/molecules25071558



.jpg)