The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Lead Effects on Plantlets Tissue Respiration
2.2. Lead Phytotoxicity
2.3. Lead Effects on the Root Cells Morphology
3. Discussion
4. Materials and Method
4.1. Growth Condition
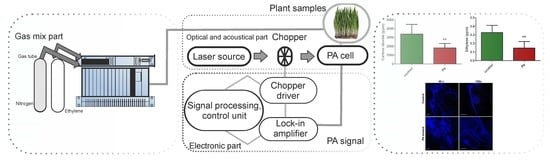
4.2. CO2 Laser-Based Photoacoustic Spectroscopy Experimental Procedure
4.3. Evaluation of Lead Phytotoxicity
4.4. Root Staining and Fluorescence Imaging Experimental Procedure
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Netw. Isrn Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Bieby, V.T.; Siti, R.S.A.; Hassan, B.; Mushrifah, I.; Nurina, A.; Muhammad, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Winecker, R.E.; Ropero-Miller, J.D.; Broussard, L.A.; Hammett-Stable, C.A. The Toxicology of Heavy Metals: Getting the Lead Out. Lab. Med. 2002, 33, 934–947. [Google Scholar] [CrossRef]
- Paul, B.T.; Clement, G.Y.; Anita, K.P.; Dwayne, J.S. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Monisha, J.; Tenzin, T.; Naresh, A.; Blessy, B.M.; Krishnamurthy, N.B. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Jiwan, S.; Ajay, S.K. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Env. 2011, 1, 15–21. [Google Scholar]
- Zu, Y.; Li, Y.; Christian, S.; Laurent, L.; Liu, F. Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China. Environ. Int. 2004, 30, 567–576. [Google Scholar]
- Nalini, P.; Chandra, P.S. Effect of Heavy Metals Co2+, Ni2+ and Cd2+ on Growth and Metabolism of Cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Małgorzata, N.; Andrzej, G. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.; Bratu, A.M.; Bacalum, M.; Prepelita, P. Application of the laser technology on the effect of Cd phytotoxicity in the detection of NH3, C2H4, C2H5OH and CO2 biomolecules at Triticumaestivum plantlets. Sustain. Chem. Pharm. 2020, 15, 100208. [Google Scholar] [CrossRef]
- Chitsan, L.; Naiwei, L.; Endy, S. Applications of open-PathFourier transform infrared for identification of volatile organic compound pollution sourcesand characterization of source emission behaviors. J. Air Waste Manag. Assoc. 2008, 58, 821–828. [Google Scholar] [CrossRef]
- Dewulf, J.; Van, L.H.; Wittmann, G. Analysis of volatile organic compounds using gas chromatography. Trac. Trends Anal. Chem. 2002, 21, 637–646. [Google Scholar] [CrossRef]
- Mogildea, G.; Mogildea, M. Experimental investigation of the microwave electrothermal thruster using metals as propellant. Optoelectron. Adv. Mater.–Rapid Commun. 2010, 4, 1826–1829. [Google Scholar]
- Mogildea, M.; Mogildea, G. Experimental research for the mass flow control of the metal vaporized and ionized with microwave used in electric propulsion. Optoelectron. Adv. Mater.–Rapid Commun. 2010, 12, 1157–1160. [Google Scholar]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Magureanu, A.M.; Petrus, M.; Popa, C. Laser photoacoustic spectroscopy: Principles, instrumentation, and characterization. J. Optoelectron. Adv. Mater. 2007, 9, 3655–3701. [Google Scholar]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Carlson, R.W.; Bazzaz, F.A.; Rolfe, G.L. The effect of heavy metals on plants: II. Net photosynthesis and transpiration of whole corn and sunflower plants treated with Pb, Cd, Ni, and Tl. Env. Res 1975, 10, 113–120. [Google Scholar] [CrossRef]
- Senesi, G.S.; Dell’Aglio, M.; Gaudiuso, R.; De Giacomo, A.; Zaccone, C.; De Pascale, O.; Miano, T.M.; Capitelli, M. Heavymetalconcentrations in soils as determined by laser-induced breakdown spectroscopy (LIBS), with special emphasis on chromium. Env. Res. 2009, 109, 413–420. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Austin, C.; Vincent, S.G. Heavy metals and Cancer. In Cancer Causing Substances; Atroshi, F., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.; Petrus, M. Heavy metals impact at plants using photoacoustic spectroscopy technology with tunable CO2 laser in the quantification of gaseous molecules. Microchem. J. 2017, 134, 390–399. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological Aspects of Cadmium and Lead Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Parys, E.; Romanowska, E.; Siedlecka, M.; Poskuta, J.W. The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol. Plant. 1998, 20, 313–322. [Google Scholar] [CrossRef]
- Schaller, G.E. Ethylene and the regulation of plant development. BMC Biol. 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Farouk, S.N.; Muhammad, A. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Eco. Env. Sci. 2018, 3, 265–268. [Google Scholar]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Gothberg, A.; Greger, M.; Holm, K.; Bengtsson, B.-E. Influence of Nutrient Levels on Uptake and Effects of Mercury, Cadmium, and Lead in Water Spinach. J. Env. Qual. 2004, 33, 1247–1255. [Google Scholar] [CrossRef]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtziaargyi. J. Hazard Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Guan, H.; Ma, L.; Chen, Z.; Gu, H.; Qu, L.J. Transcriptional profiling of Arabidopsis seedlings in response to heavy metal lead (Pb). Env. Exp. Bot. 2009, 67, 377–386. [Google Scholar] [CrossRef]
- Sengar, R.S.; Gautam, M.; Sengar, R.S.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead stress effects on physiobiochemical activities of higher plants. Rev. Env. Contam. Toxicol. 2008, 196, 73–93. [Google Scholar]
- Jiang, W.; Liu, D. Pb–induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. South Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Surjendu, K.D.; Jayashree, D.; Sanjukta, P.; Debasmita, P. Changes in the antioxidative enzyme activities and lipid peroxidation in wheat seedlings exposed to cadmium and lead stress. Braz. J. Plant Physiol. 2007, 19, 53–60. [Google Scholar]
- Tomulescu, I.M.; Radoviciu, E.M.; Merca, V.V.; Tuduce, A.D. Effect of Copper, Zinc and Lead and Their Combinations on the Germination Capacity of Two Cereals. Acta Agrar. Debr. 2004, 14, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Borlu, H.O.; Celiktas, V.; Duzenli, S.; Avci, M.; Hossain, A.; El Sabagh, A. Rowth, Physiology, and Biochemical Activities of Wheat (Triticum aestivum L.) Seedlings is Affected by Lead Stress. Fresenius Env. Bull. 2019, 28, 5122–5130. [Google Scholar]
- Kumar, S.; Sharma, P.; Misra, M.; Narayan Misra, A. Lead induced root and shoot growth reduction in wheat (Triticum aestivum L.) is due to increase in membrane lipid peroxidation. J. Pharmacogn. Phytochem. 2018, 7, 2080–2083. [Google Scholar]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 366, 1–11. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Bratu, A.M.; Popa, C. CO2 Laser Photoacoustic Spectroscopy: I. Principles. In CO2 Laser-Optimisation and Application; Dumitras, D.C., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Bratu, A.M.; Popa, C. CO2 Laser Photoacoustic Spectroscopy: II. Instrumentation and Applications. In CO2 Laser-Optimisation and Application; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ivascu, I.R.; Matei, C.E.; Patachia, M.; Bratu, A.M.; Dumitras, D.C. Multicomponent detection in photoacoustic spectroscopy applied to pollutants in the environmental air. Rom. Rep. Phys. 2015, 67, 1558–1564. [Google Scholar]
- Popa, C.; Petrus, M.; Bratu, A.M. Ammonia and ethylene biomarkers in the respiration of the people with schizophrenia using photoacoustic spectroscopy. J. Biomed. Opt. 2015, 20, 57006. [Google Scholar] [CrossRef]
- Popa, C. Ethylene Measurements from Sweet Fruits Flowers Using Photoacoustic Spectroscopy. Molecules 2019, 24, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples are available from the authors. |





| Work Parameters | Specifications |
|---|---|
| The total amount of seeds used for germinations | ≈5 g |
| Plant sample pressure | ≈1014 mb |
| Total amount of Pb used for germination | 15 mL |
| Total amount of distilled water used for germination | 25 mL |
| CO2 laser line for ethylene detection | 10P(14); λ = 10.53 μm; α = 30.4 cm−1 atm−1 |
| CO2 laser line for carbon dioxide detection | 9P(18); λ = 9.533 μm; α = 0.00301 cm−1 atm−1 |
| Synthetic air flow | Linde gas: 20% oxygen, 80% nitrogen (impurities: Hydrocarbons max. 0.1 ppmV, nitrogen oxides max. 0.1 ppmV) |
| Nitrogen flow | Linde gas 6.0, purity 99.9999% |
| Working temperature | ≈23–25 °C |
| Polycarbonate containers total volume | 0.83 cm3/g |
| Glass cuvette total volume | 150 cm3 |
| Photoacoustic total volume | 1000 cm3 |
| Responsivity of the photoacoustic cell | 330 cmV/W |
| Plant sample time analysis | ≈5 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, C.; Bratu, A.M.; Petrus, M.; Bacalum, M. The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy. Molecules 2020, 25, 1637. https://doi.org/10.3390/molecules25071637
Popa C, Bratu AM, Petrus M, Bacalum M. The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy. Molecules. 2020; 25(7):1637. https://doi.org/10.3390/molecules25071637
Chicago/Turabian StylePopa, Cristina, Ana Maria Bratu, Mioara Petrus, and Mihaela Bacalum. 2020. "The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy" Molecules 25, no. 7: 1637. https://doi.org/10.3390/molecules25071637


.jpg)


.jpg)