Tri(boryl)alkanes and Tri(boryl)alkenes: The Versatile Reagents
Abstract
:1. Introduction
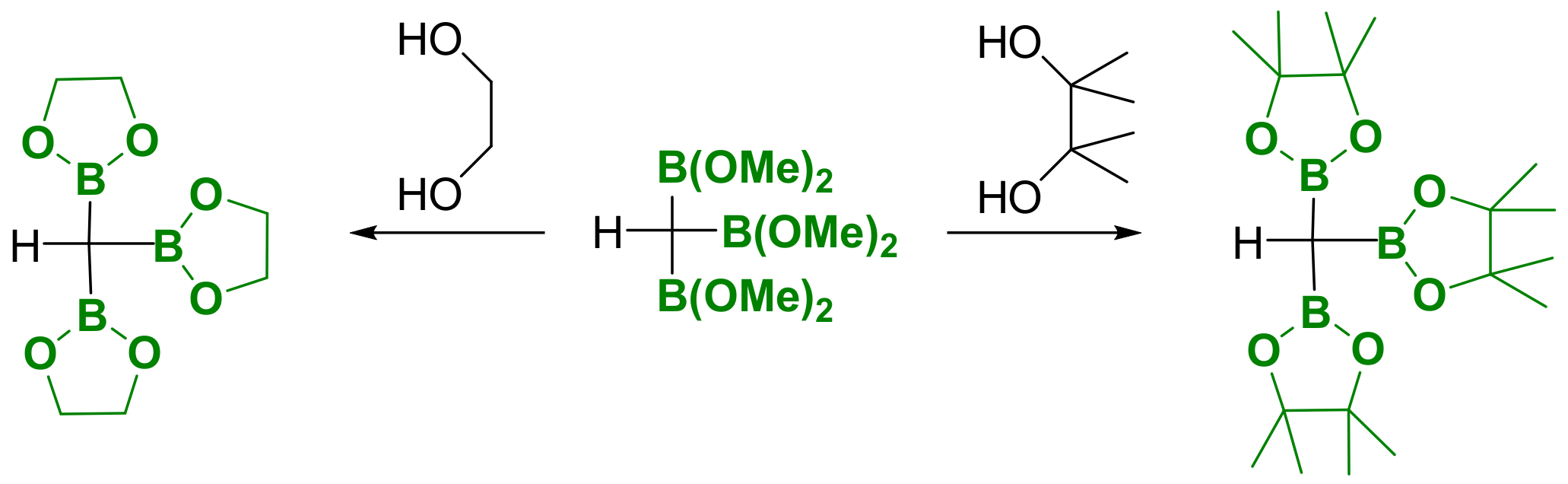
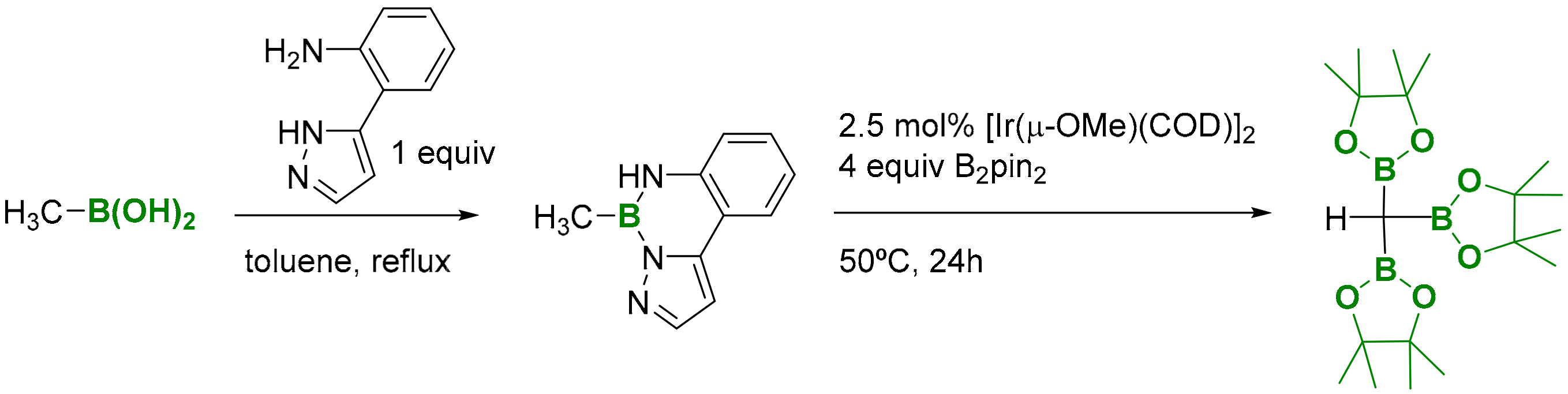
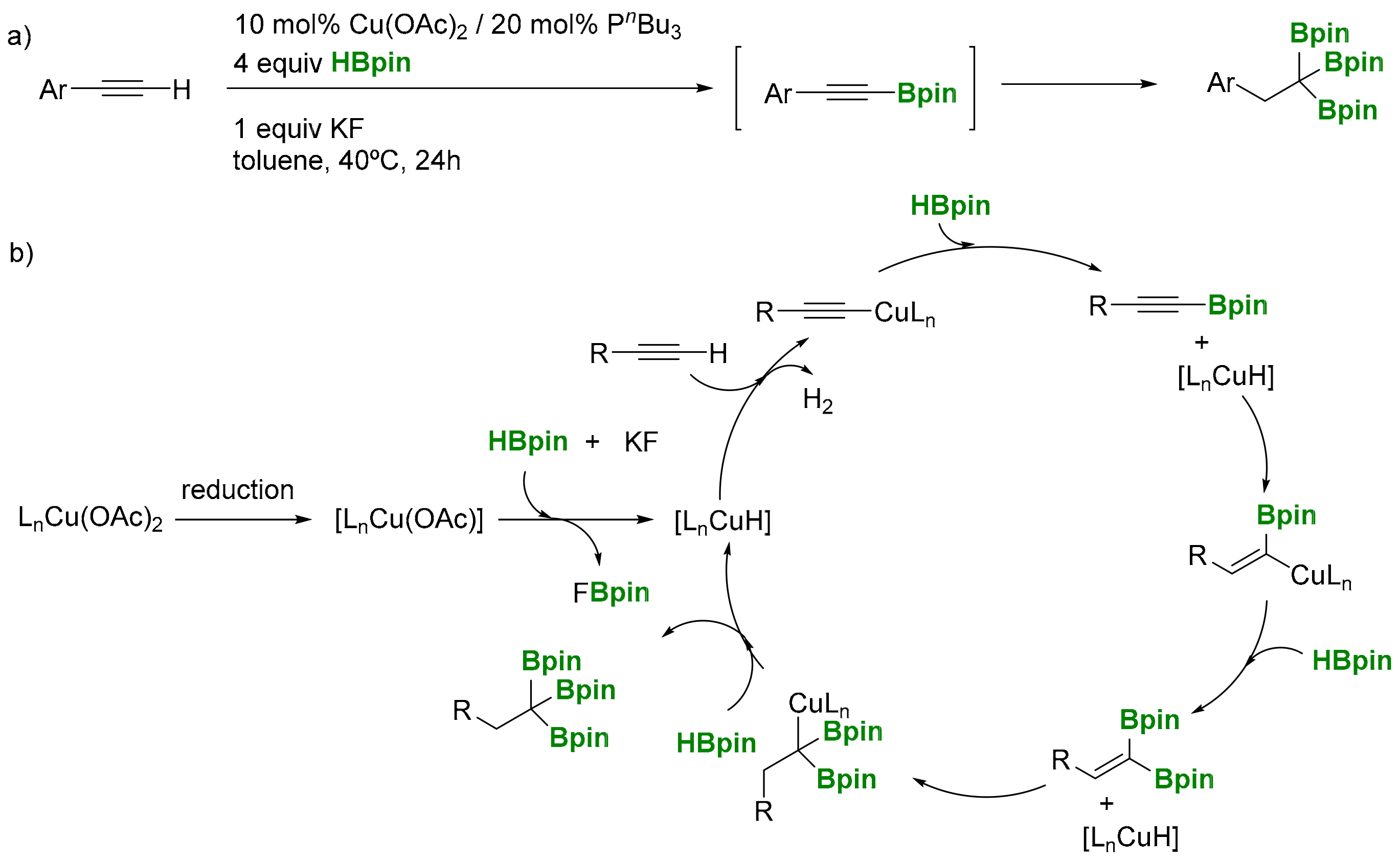
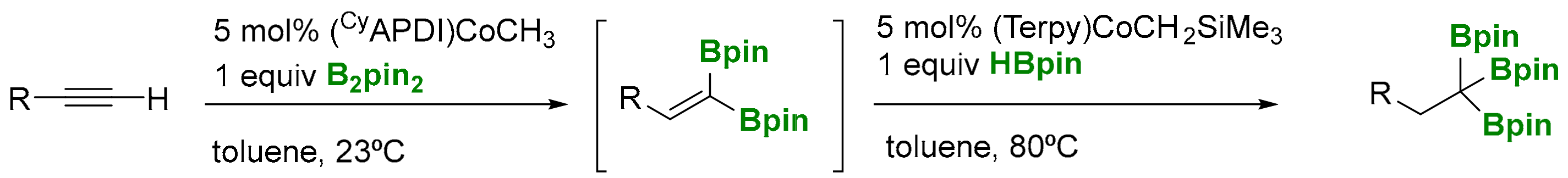
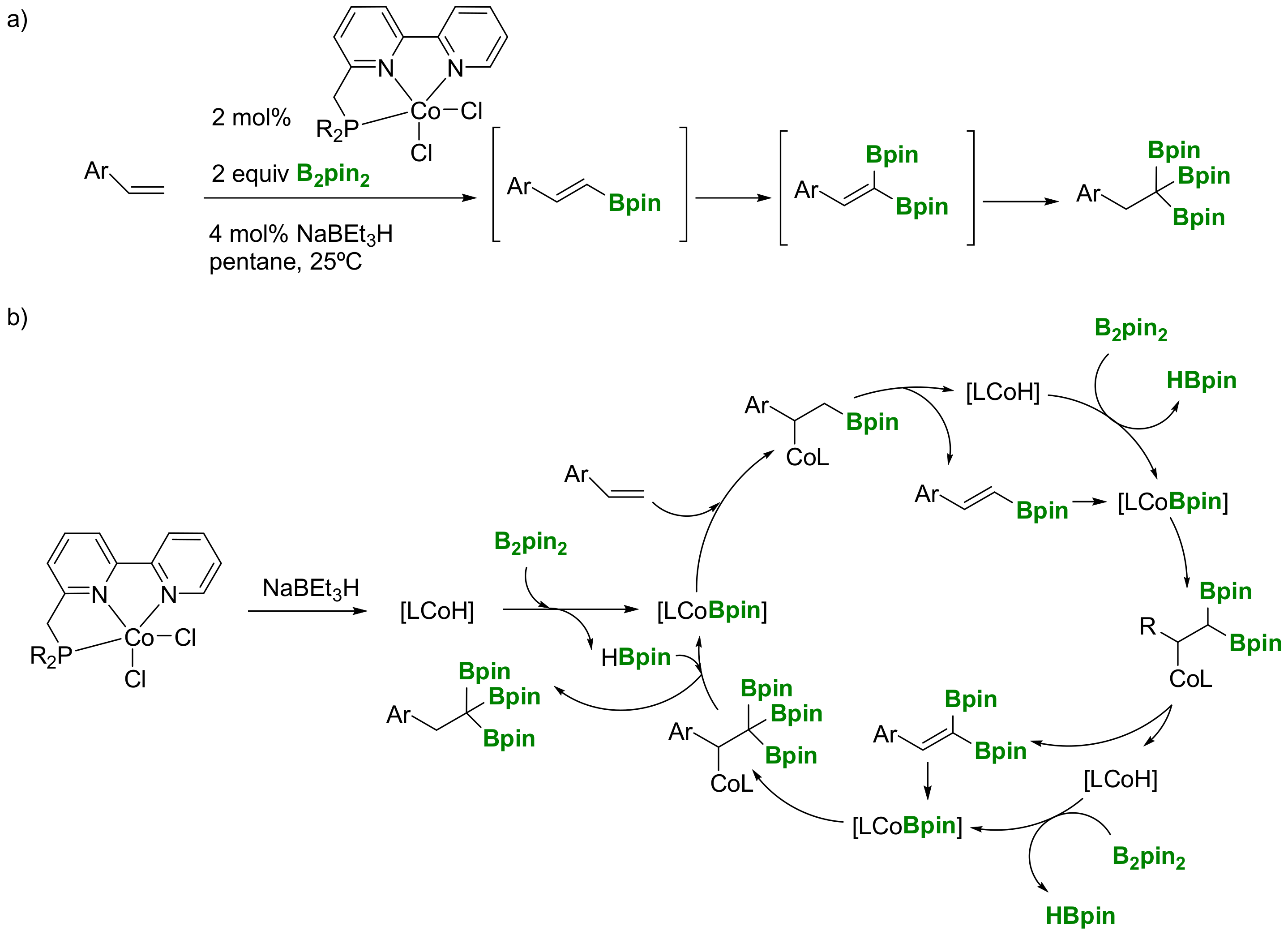
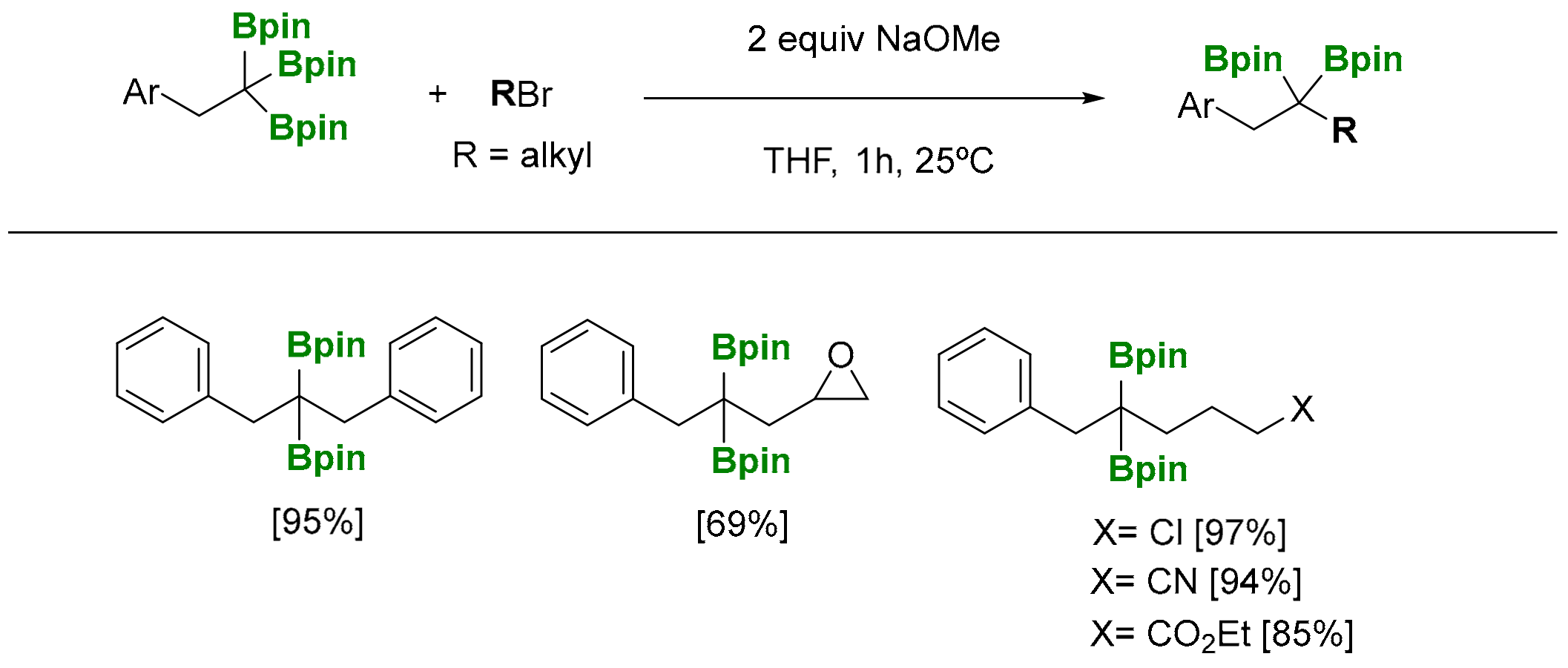
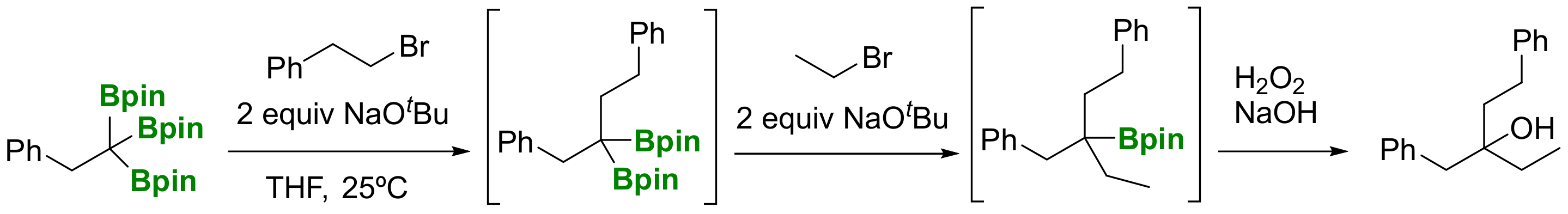
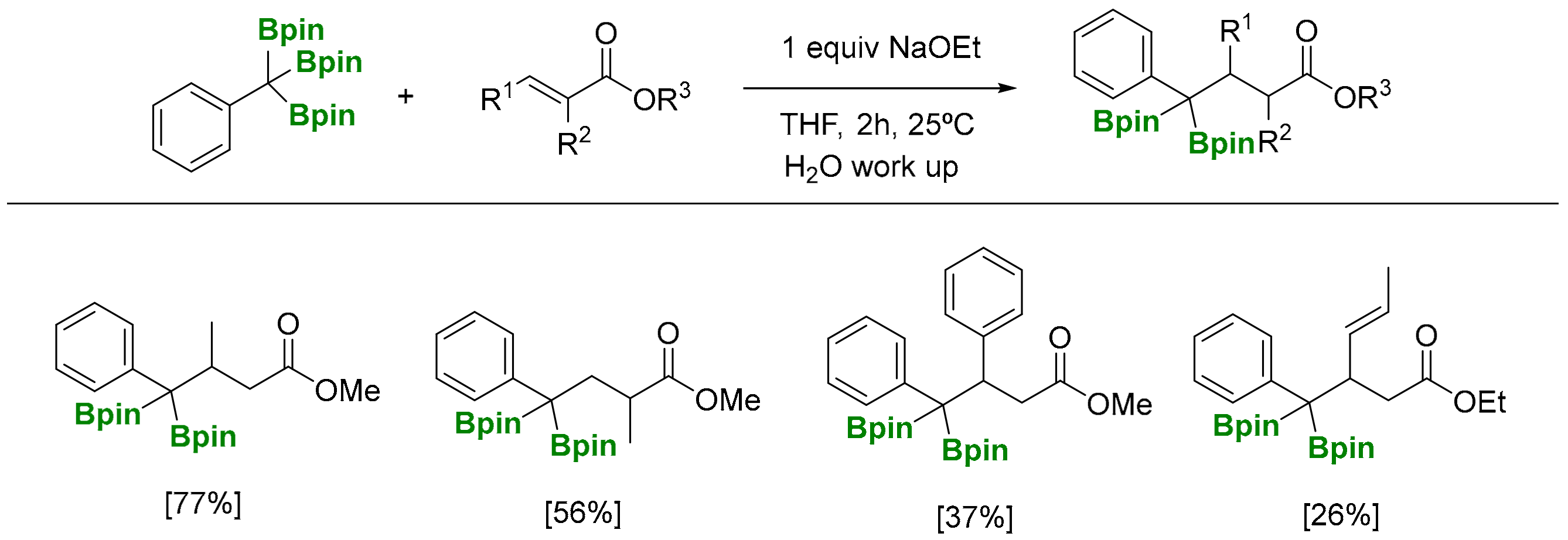
2. 1,1,1-Tri(boryl)alkanes
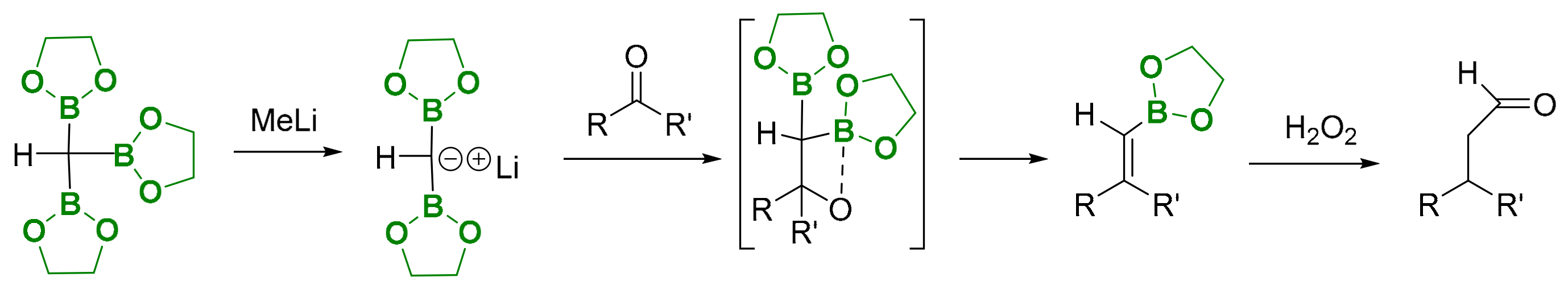
2.1. Synthetic Approaches
2.2. Synthetic Applications
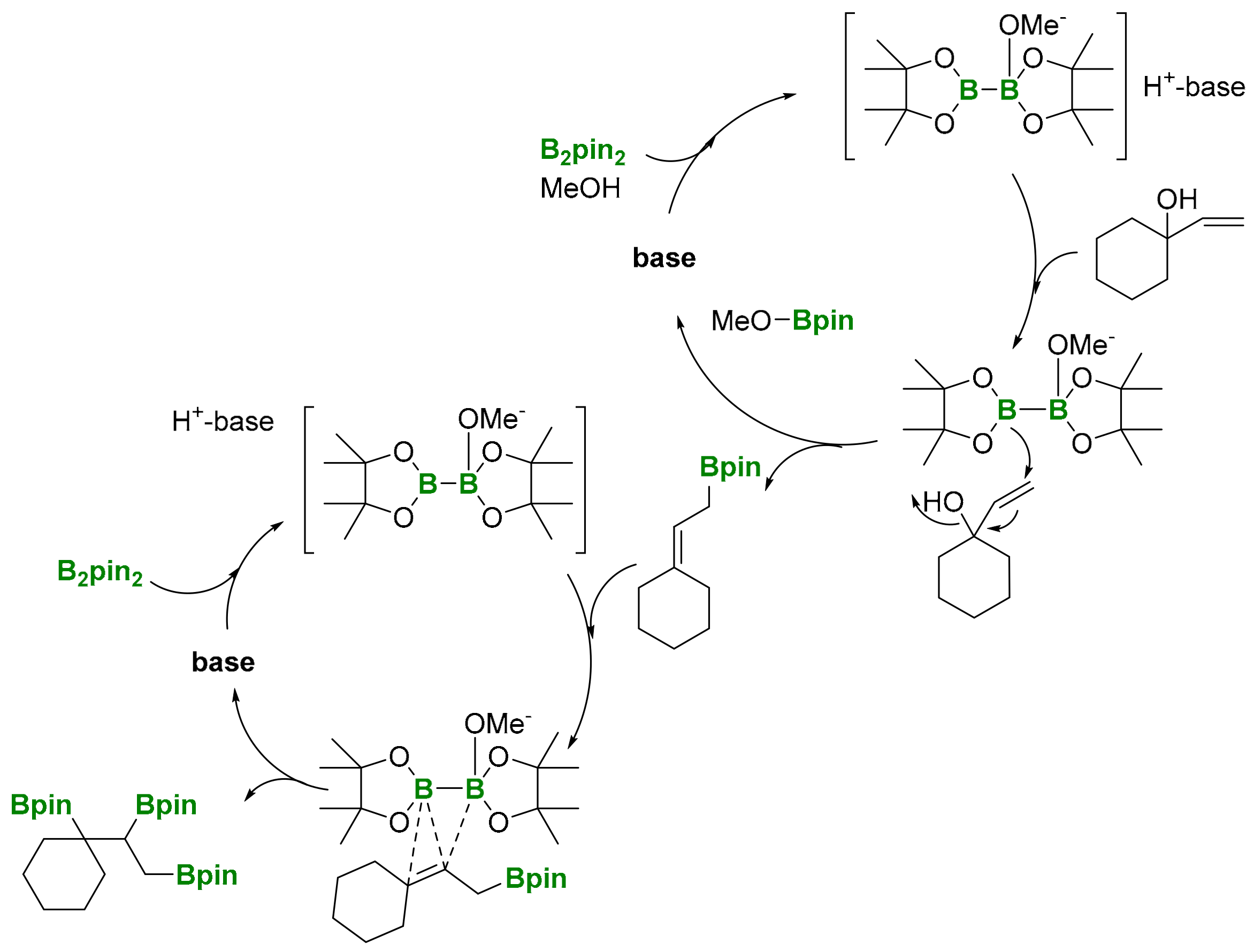
3. 1,2,3-Tri(boryl)alkanes
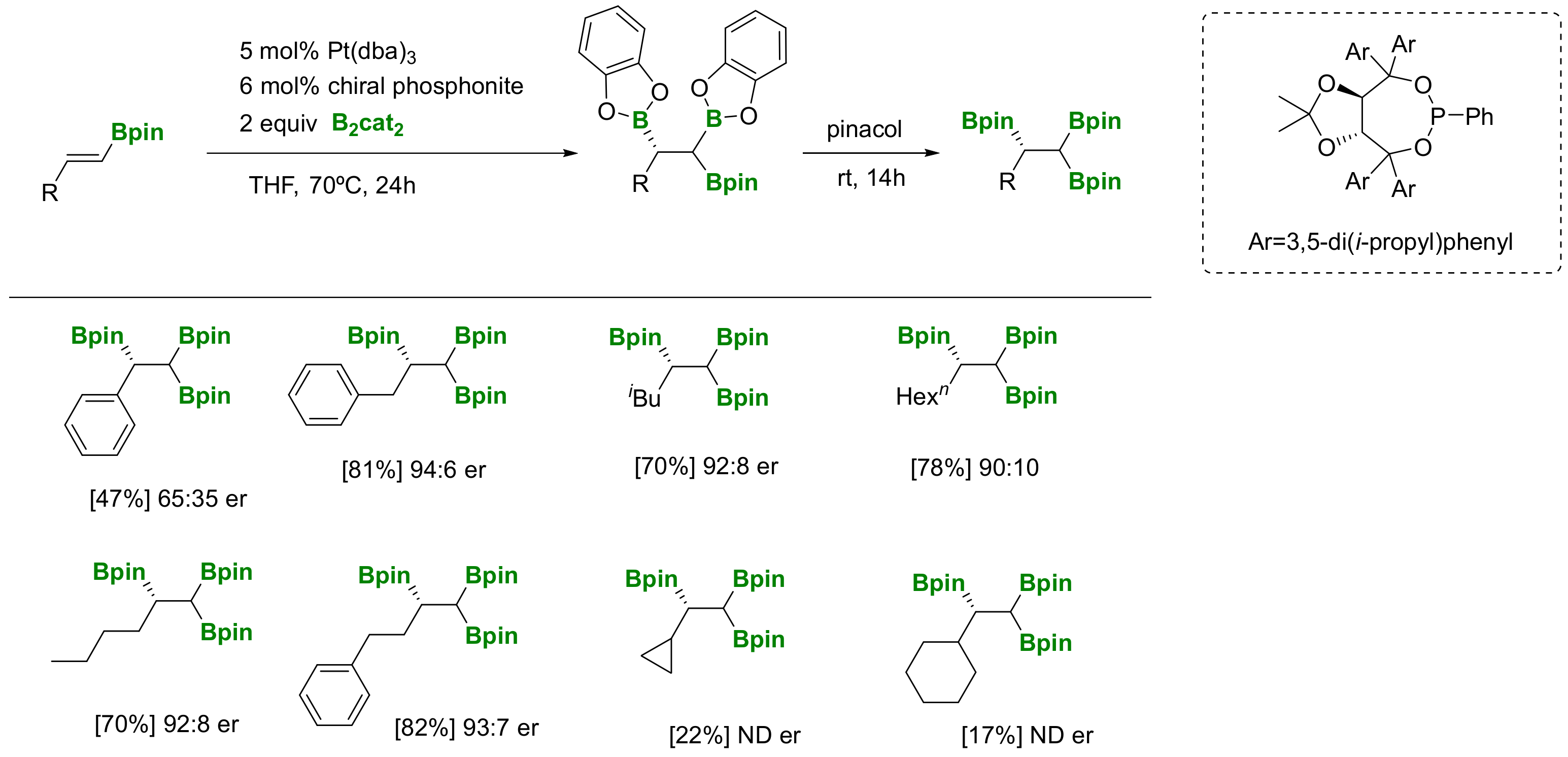
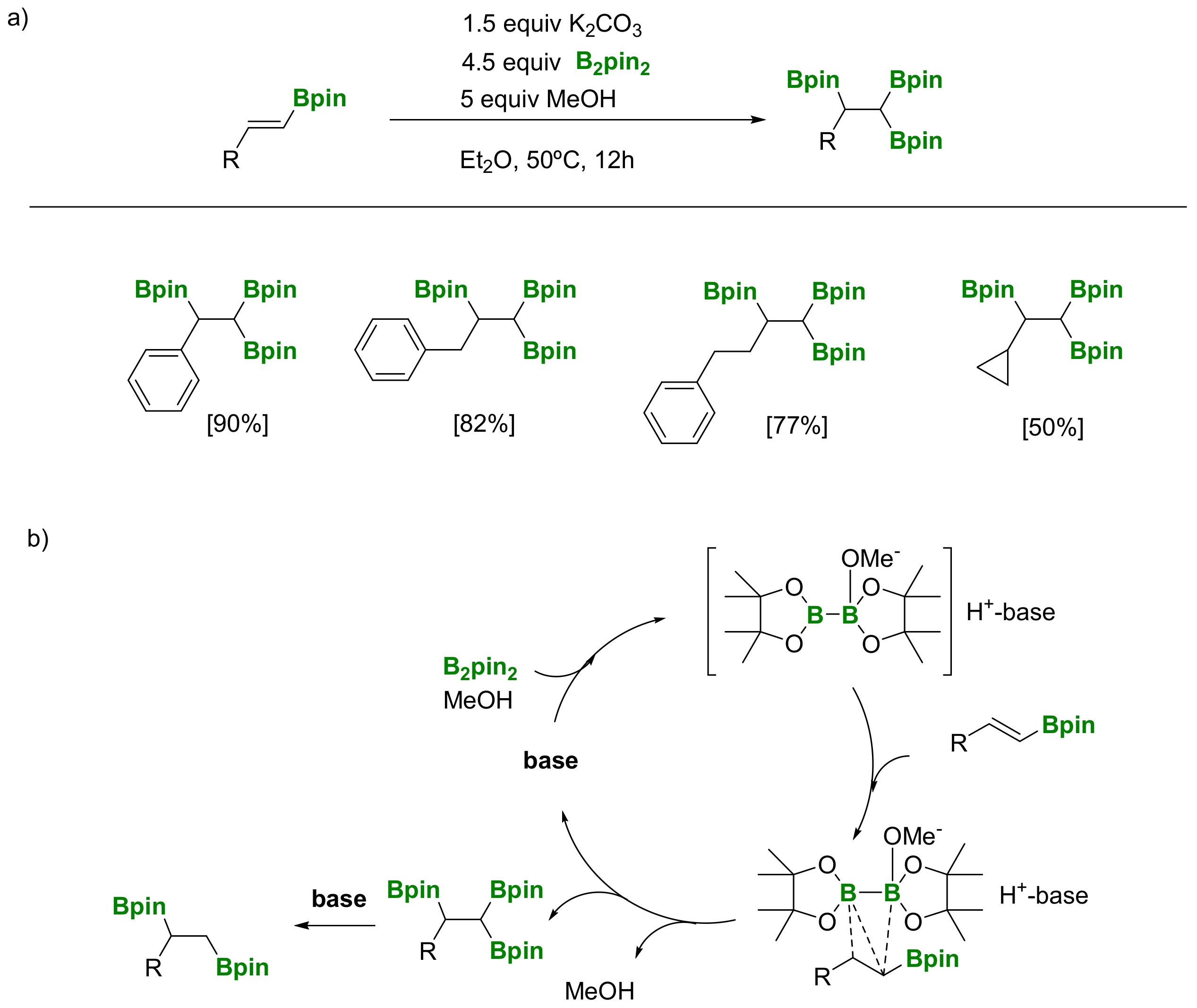
3.1. Synthetic Approaches
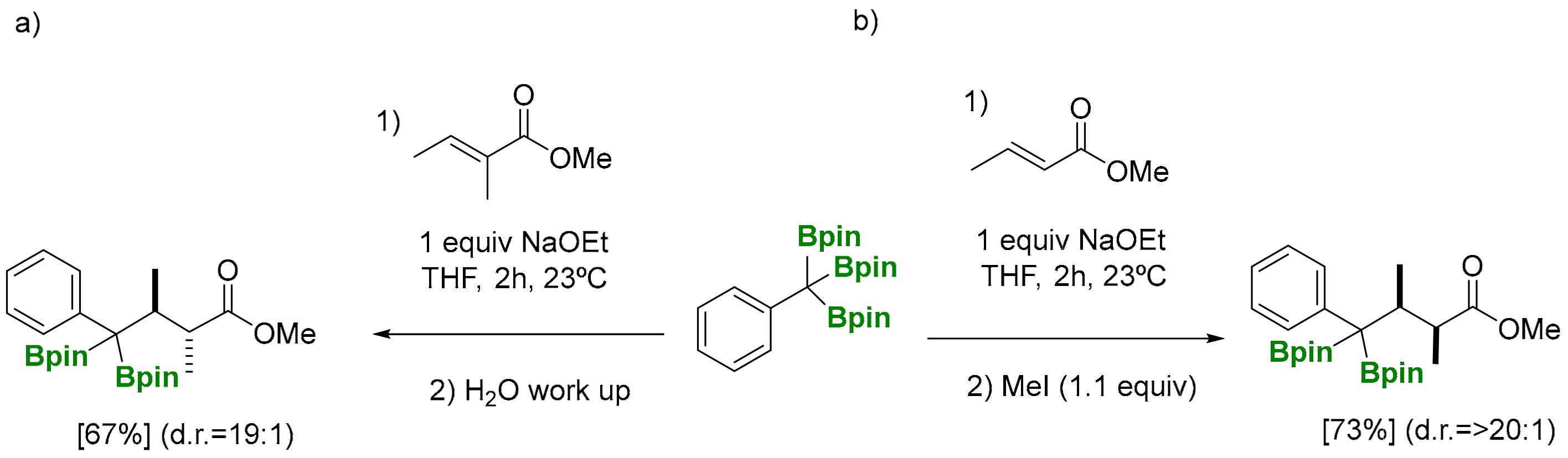
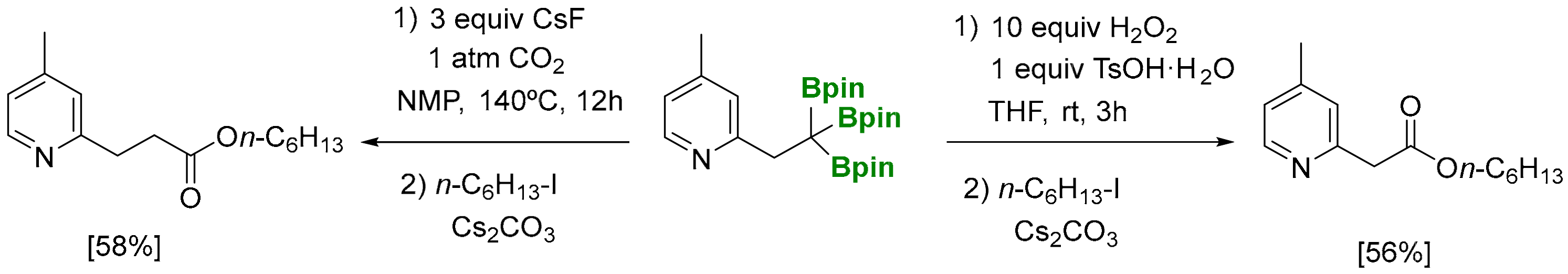
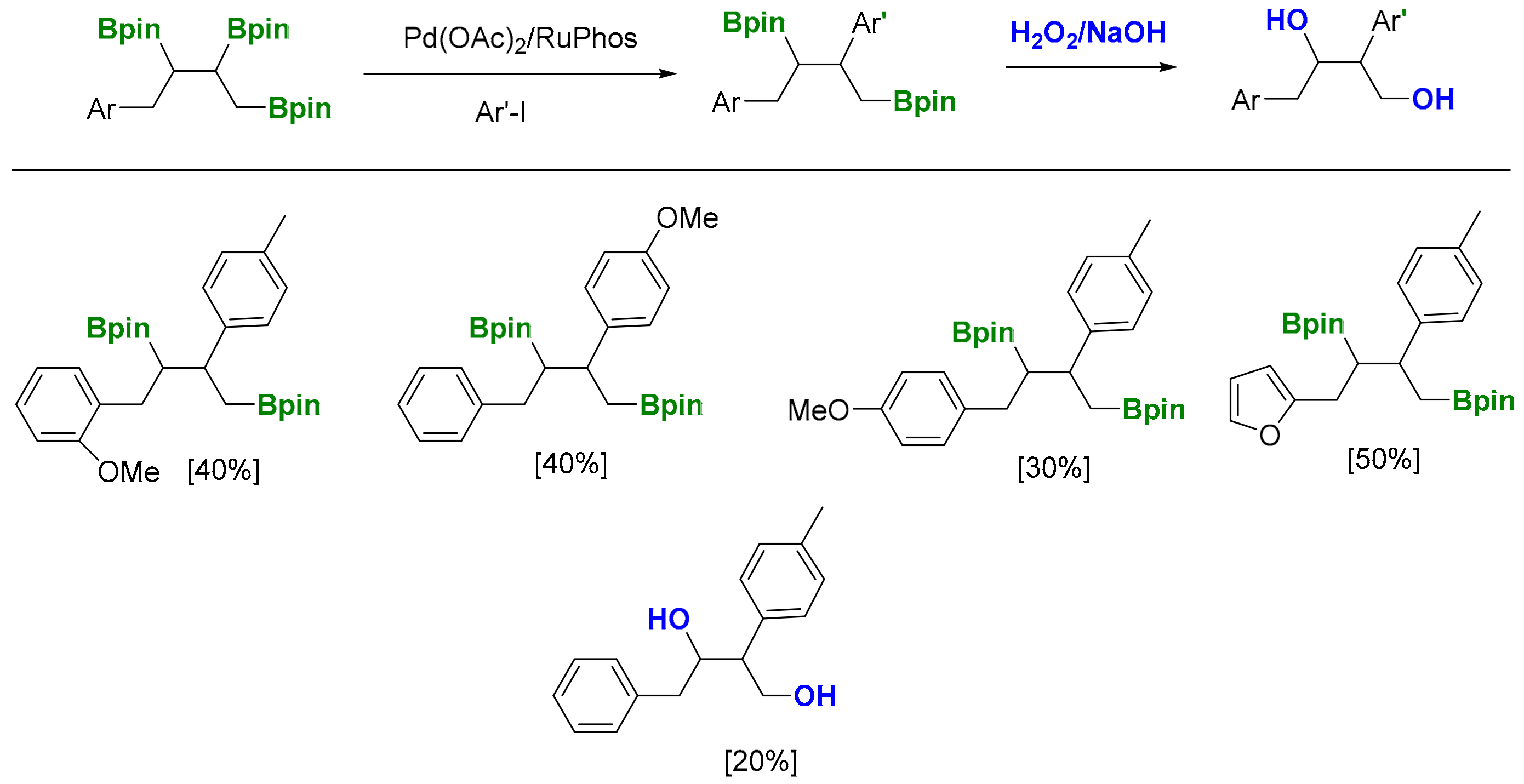
3.2. Synthetic Applications
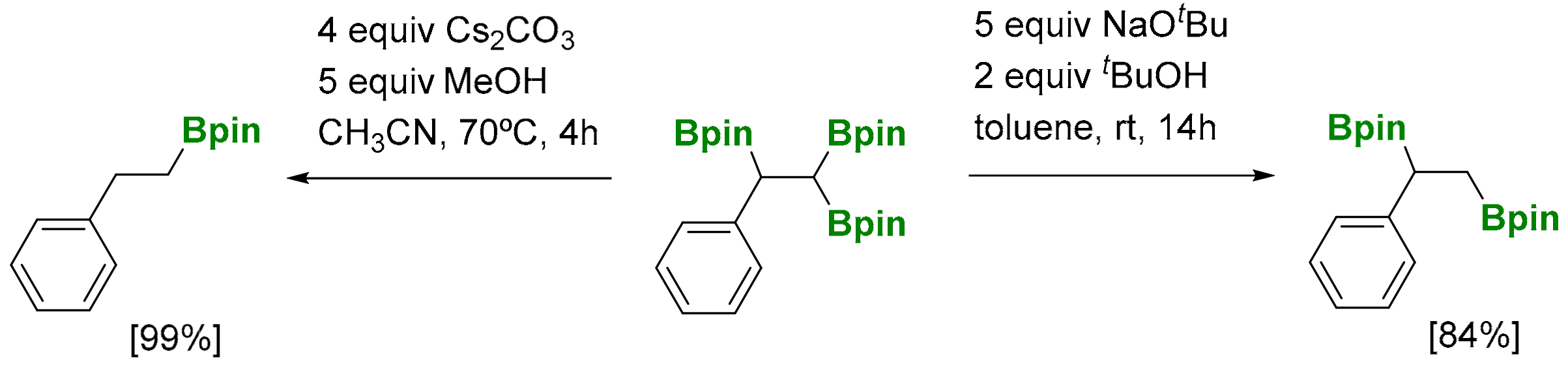
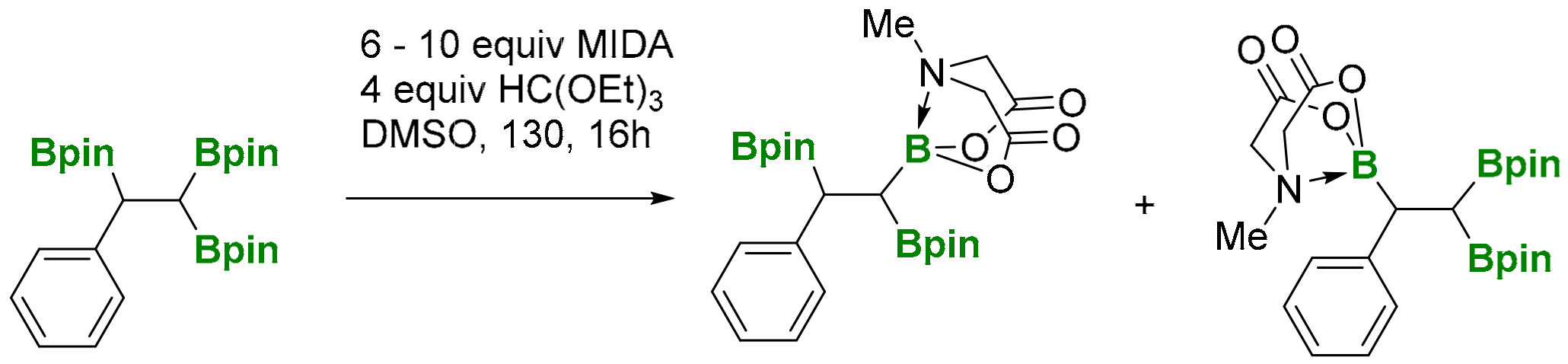
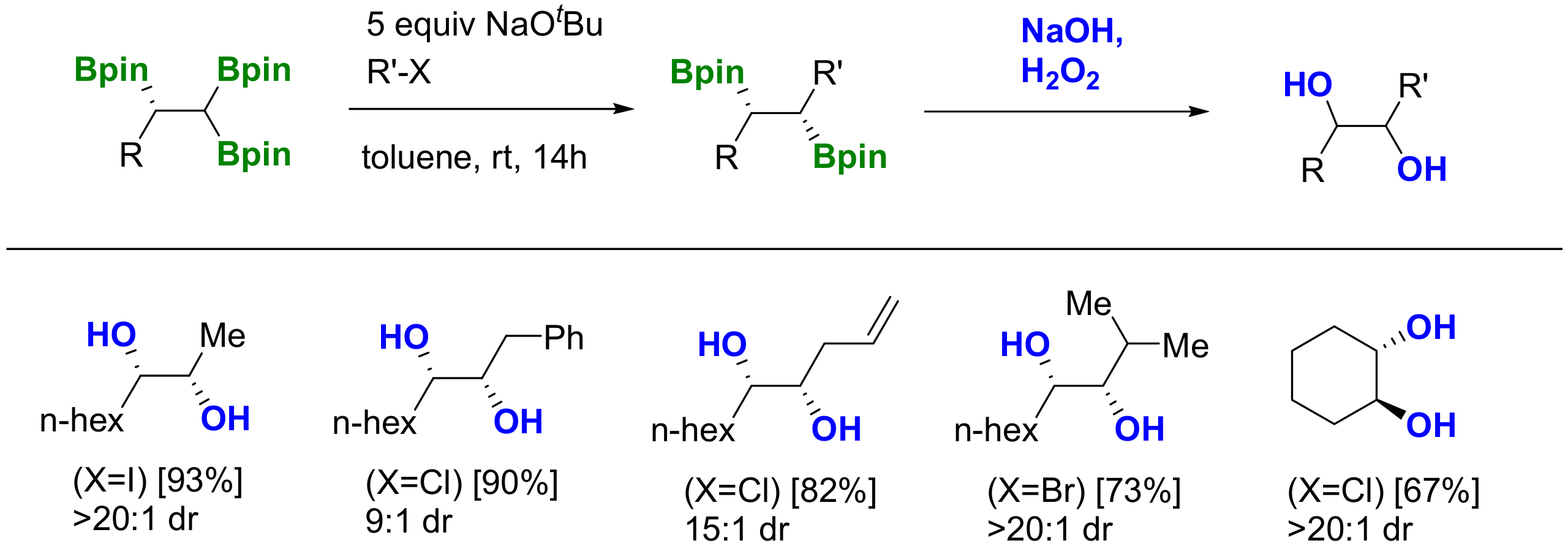
4. 1,1,2-Tri(boryl)alkanes
4.1. Synthetic Approaches
4.2. Synthetic Applications
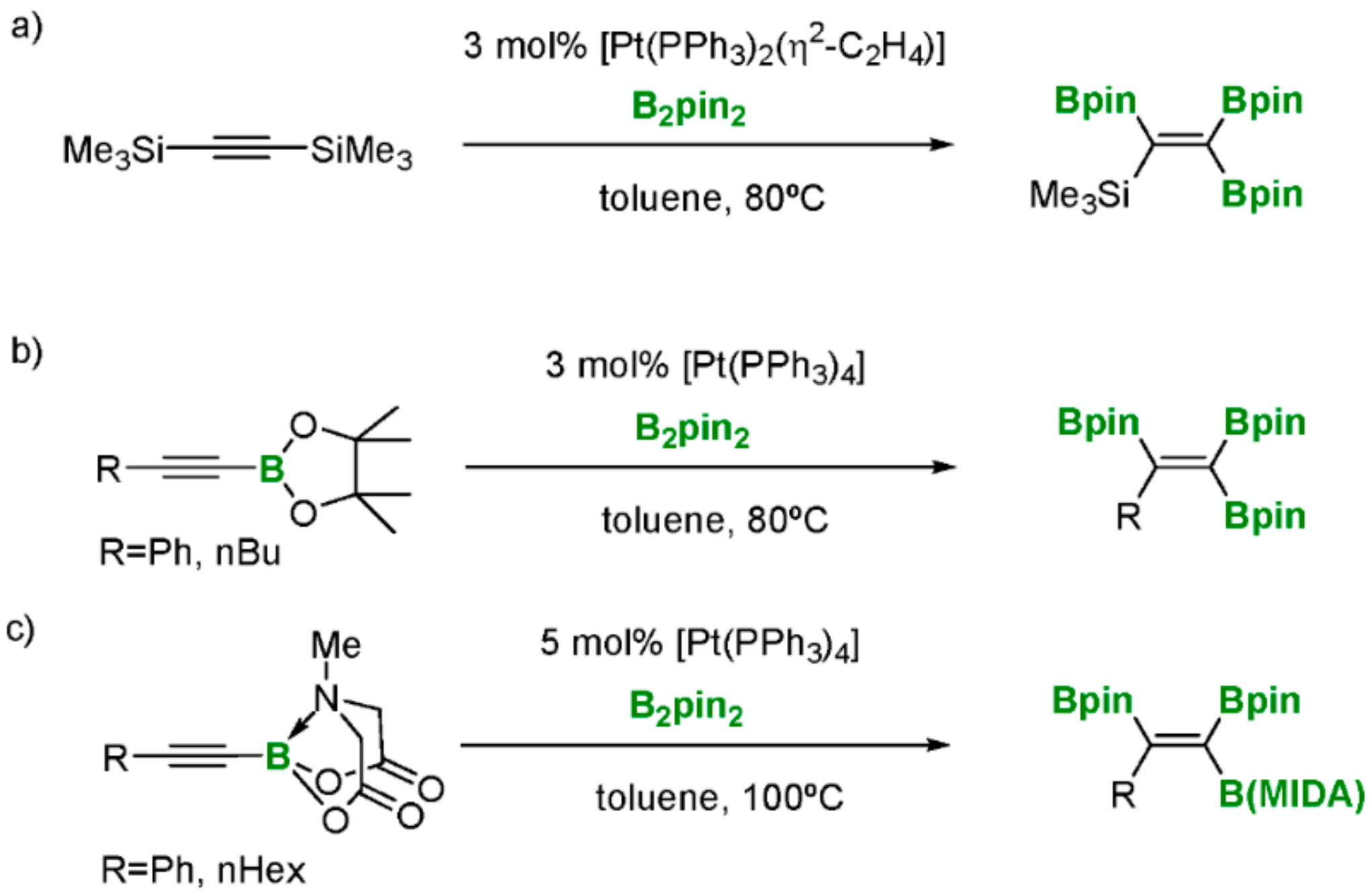
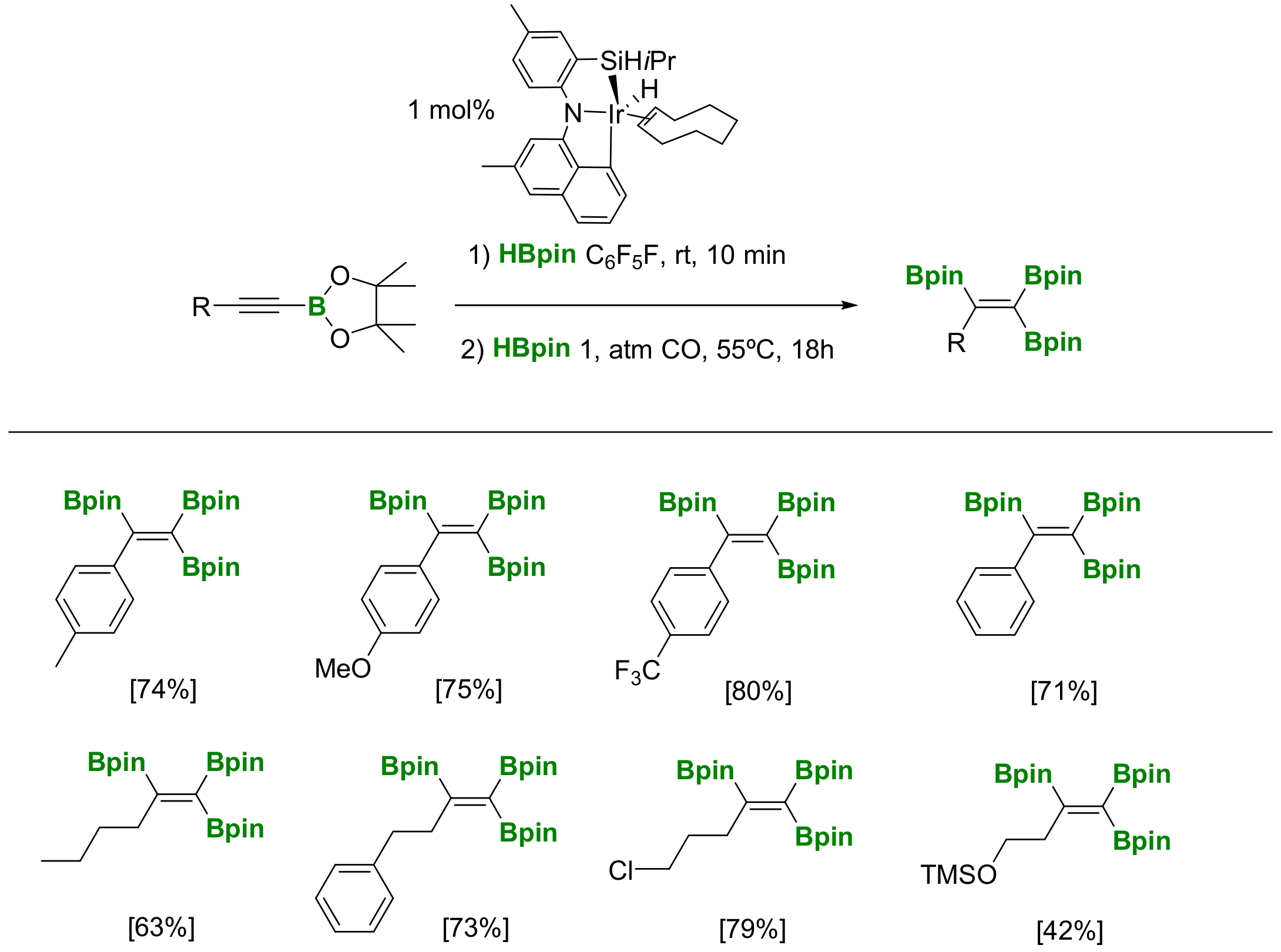
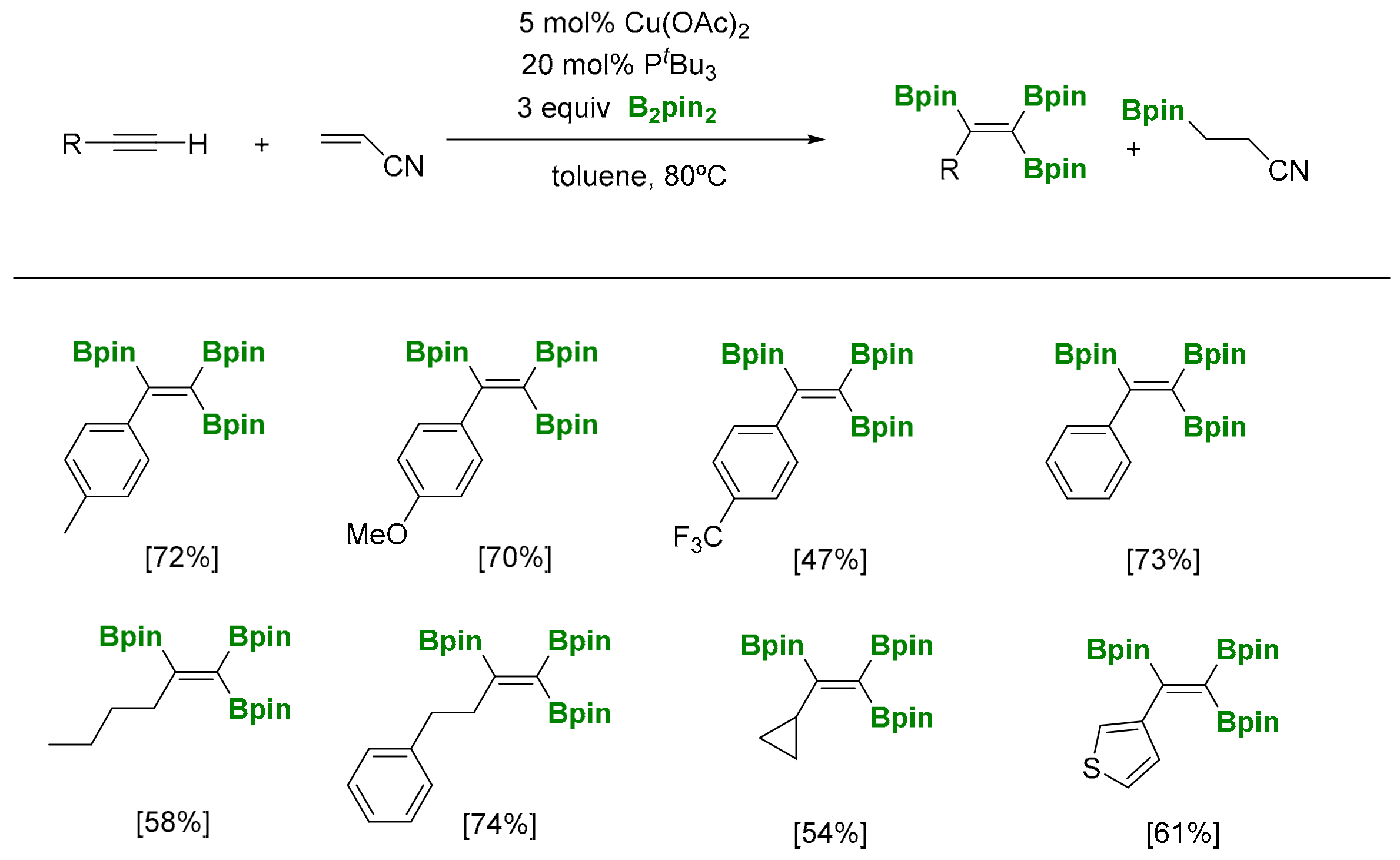
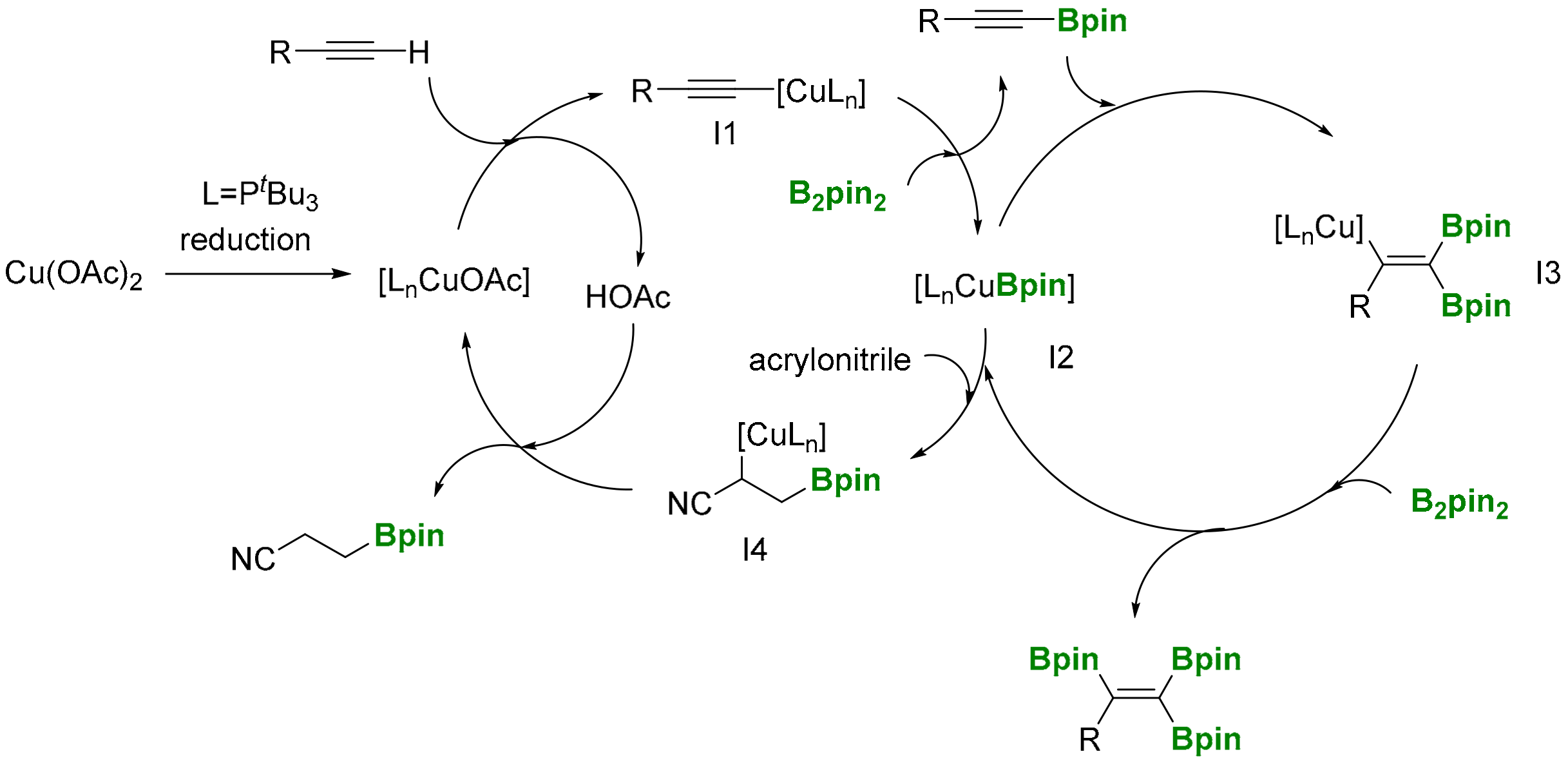
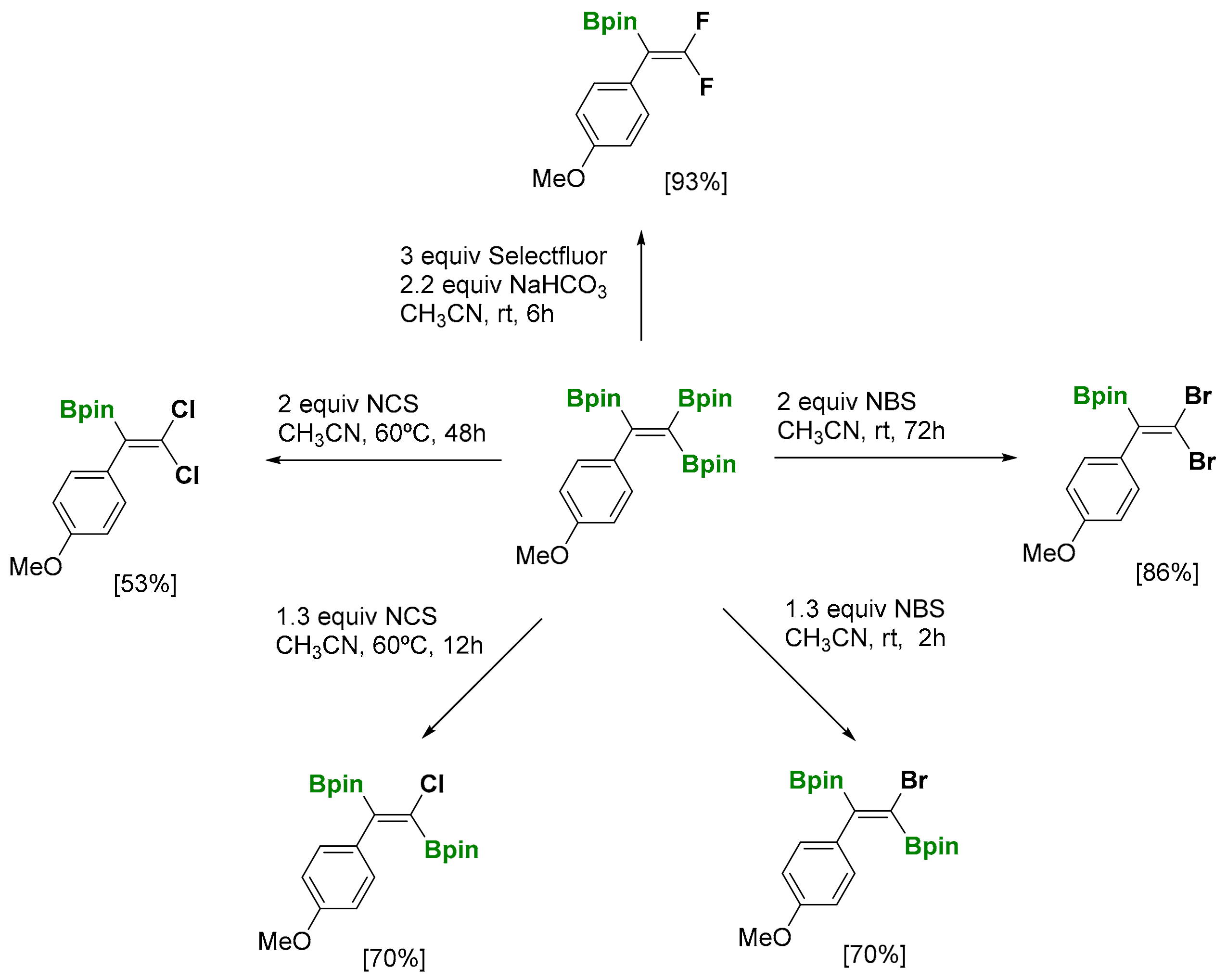
5. 1,1,2-Tri(boryl)alkenes
5.1. Synthetic Approaches
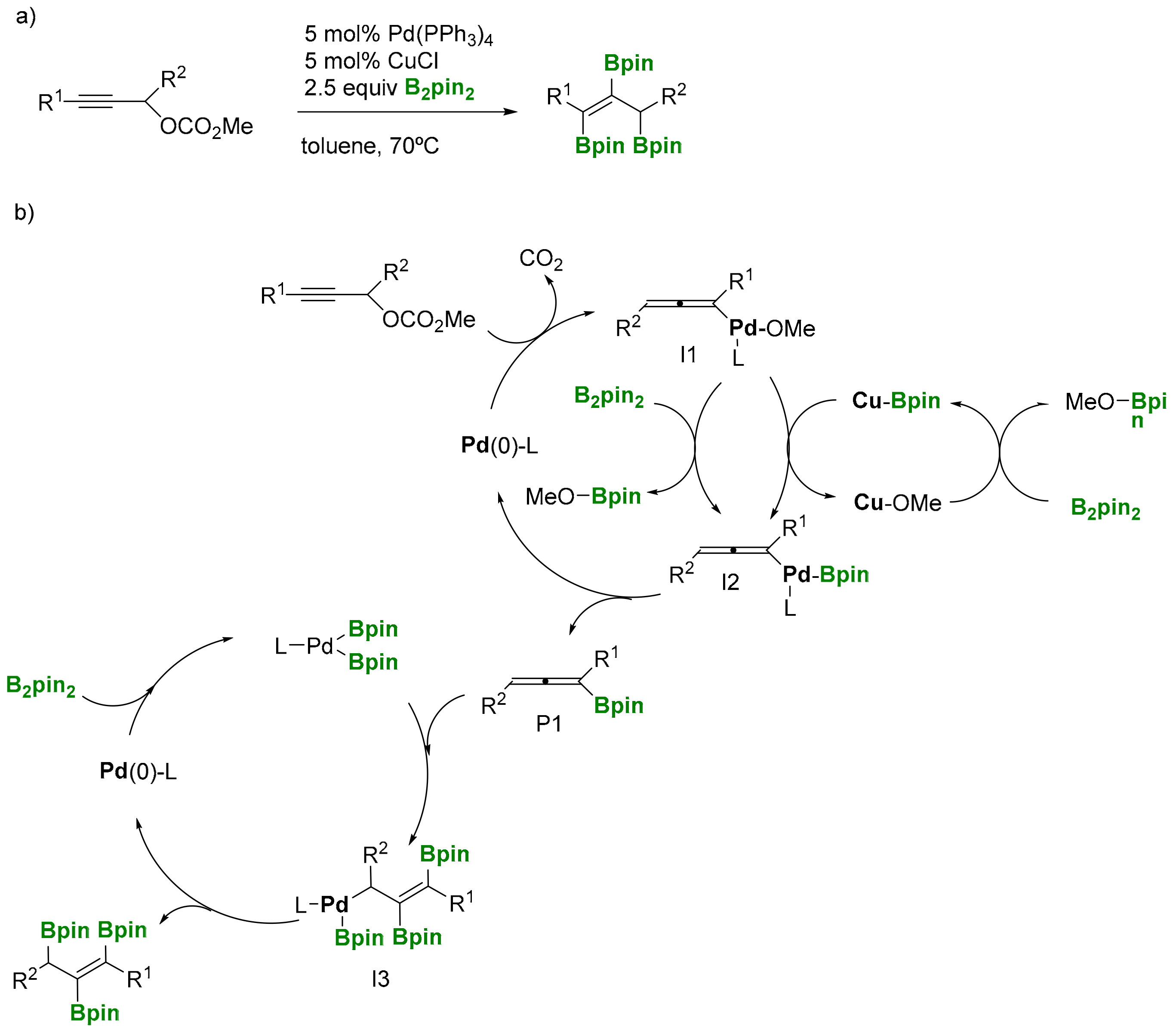
5.2. Synthetic Applications
6. Conclusions
Funding
Conflicts of Interest
References
- Castle, R.B.; Matteson, D.S. A Methanetetraboronic Ester. J. Am. Chem. Soc. 1968, 90, 2194. [Google Scholar] [CrossRef]
- Castle, R.B.; Matteson, D.S. Methanetetraboronic and methanetriboronic esters. J. Organomet. Chem. 1969, 20, 19–28. [Google Scholar] [CrossRef]
- Matteson, D.S.; Thomas, J.R. C-alkylation of methanetetraboronic and methanetriboronic esters. J. Organomet. Chem. 1970, 24, 263–271. [Google Scholar] [CrossRef]
- Matteson, D.S. Methanetetraboronic and methanetriboronic esters as synthetic intermediates. Synthesis 1975, 1975, 147–158. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ishibashi, A.; Suginome, M. Boryl-Directed, Ir-Catalyzed C(sp3)–H Borylation of Alkylboronic Acids Leading to Site-Selective Synthesis of Polyborylalkanes. Org. Lett. 2019, 21, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Ikeda, Y.; Michigami, K.; Sato, Y. Iridium-catalyzed triple C(sp3)–H borylations: Construction of triborylated sp3-carbon centers. Chem. Commun. 2013, 49, 5601–5603. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.N.; Obligacion, J.V.; Pappas, I.; Chirik, P.J. Cobalt-Catalyzed Benzylic Borylation: Enabling Polyborylation and Functionalization of Remote, Unactivated C(sp3)−H Bonds. J. Am. Chem. Soc. 2016, 138, 766–769. [Google Scholar] [CrossRef]
- Palmer, W.N.; Zarate, C.; Chirik, P.J. Benzyltriboronates: Building Blocks for Diastereoselective Carbon−Carbon Bond Formation. J. Am. Chem. Soc. 2017, 139, 2589–2592. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ming, W.; Zhang, Y.; Friedrich, A.; Marder, T.B. Copper-Catalyzed Triboration: Straightforward, Atom-Economical Synthesis of 1,1,1-Triborylalkanes from Terminal Alkynes and HBpin. Angew. Chem. Int. Ed. 2019, 58, 18923–18927. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.T.; Nguyen, P.; Marder, T.B.; Westcott, S.A. Transition Metal Catalyzed Diboration of Vinylarenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1336–1338. [Google Scholar] [CrossRef]
- Gu, Y.; Pritzkow, H.; Siebert, W. Synthesis and Reactivity of Monoborylacetylene Derivatives. Eur. J. Inorg. Chem. 2001, 200, 373–379. [Google Scholar] [CrossRef]
- Krautwald, S.; Bezdek, M.J.; Chirik, P.J. Cobalt-Catalyzed 1,1-Diboration of Terminal Alkynes: Scope, Mechanism, and Synthetic Applications. J. Am. Chem. Soc. 2017, 139, 3868–3875. [PubMed] [Green Version]
- Zhang, L.; Huang, Z. Synthesis of 1,1,1-Tris(boronates) from Vinylarenes by Co-Catalyzed Dehydrogenative Borylations−Hydroboration. J. Am. Chem. Soc. 2015, 137, 15600–15603. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Lloyd-Jones, G.C. Vinylborane Formation in Rhodium-catalysed Hydroborations; Ligand-free Homogeneous Catalysis. J. Chem. Soc. Chem. Commun. 1992, 710–712. [Google Scholar] [CrossRef]
- Hong, K.; Liu, X.; Morken, J.P. Simple Access to Elusive α-Boryl Carbanions and Their Alkylation: An Umpolung Construction for Organic Synthesis. J. Am. Chem. Soc. 2014, 136, 10581–10584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nave, S.; Sonawane, R.P.; Elford, T.G.; Aggarwal, V.K. Protodeboronation of Tertiary Boronic Esters: Asymmetric Synthesis of Tertiary Alkyl Stereogenic Centers. J. Am. Chem. Soc. 2010, 132, 17096–17098. [Google Scholar] [CrossRef]
- Matteson, D.S.; Moody, R.J.; Jesthi, P.K. Reaction of aldehydes and ketones with a boron-substituted carbanion, bis(ethylenedioxyboryl)methide. Simple aldehyde homologation. J. Am. Chem. Soc. 1975, 97, 5608–5609. [Google Scholar] [CrossRef]
- Matteson, D.S.; Moody, R.J. Homologation of carbonyl compounds to aldehydes with lithium bis(ethylenedioxyboryl)methide. J. Org. Chem. 1980, 45, 1091–1095. [Google Scholar] [CrossRef]
- Matteson, D.S.; Davies, R.A.; Hagelee, L.A. A bromomethanetriboronic ester. J. Organomet. Chem. 1974, 69, 45–52. [Google Scholar] [CrossRef]
- Matteson, D.S.; Tripathy, P.B. Alkene-1,1-diboronic esters from the condensation of triborylmethide anions with ketones or aldehydes. J. Organomet. Chem. 1974, 69, 53–62. [Google Scholar]
- Yoshida, H.; Kawashima, S.; Takemoto, Y.; Okada, K.; Ohshita, J.; Takaki, K. Copper-Catalyzed Borylation Reactions of Alkynes and Arynes. Angew. Chem. Int. Ed. 2012, 51, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, T.; Han, Y.; Lin, W.; Liu, Q.; Tang, Y.; Zhai, Y.; Jia, M.; Zhang, W.; Zhu, T.; et al. Regio- and (E)-Stereoselective Triborylation of propargylic Carbonates. Chin. J. Chem. 2017, 35, 1251–1255. [Google Scholar] [CrossRef]
- Miralles, N.; Alam, R.; Szabó, K.; Fernández, E. Transition-Metal-Free Borylation of Allylic and Propargylic Alcohols. Angew. Chem. Int. Ed. 2016, 55, 4303–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonet, A.; Pubill-Ulldemolions, C.; Bo, C.; Gulyás, H.; Fernández, E. Transition-metal-free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 2011, 50, 7158. [Google Scholar] [CrossRef]
- Maza, R.J.; Davenport, E.; Miralles, N.; Carbó, J.; Fernández, E. Transition-Metal-Free Allylic Borylation of 1,3-Dienes. Org. Lett. 2019, 21, 2251–2254. [Google Scholar] [CrossRef]
- Davenport, E.; Fernández, E. Transition-metal-free synthesis of vicinal triborated compounds and selective functionalisation of the internal C-B bond. Chem. Commun. 2018, 54, 10104–10107. [Google Scholar]
- Kaiser, D.; Noble, A.; Fasano, V.; Aggarwal, V.K. 1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 14104–14109. [Google Scholar] [CrossRef]
- Coombs, J.R.; Zhang, L.; Morken, J.P. Enantiomerically Enriched Tris(boronates): Readily Accessible Conjunctive Reagents for Asymmetric Synthesis. J. Am. Chem. Soc. 2014, 136, 16140–16143. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Yan, J.; Yang, K.; Chen, F.; Song, Q. Base-controlled highly selective synthesis of alkyl 1,2-bis(boronates) or 1,1,2-tris(boronates) from terminal alkynes. Green Chem. 2017, 19, 3997–4001. [Google Scholar] [CrossRef]
- Lin, S.; Wang, L.; Aminoleslami, N.; Lao, Y.; Yagel, C.; Sharma, A. A modular and concise approach to MIDA acylboronates via chemoselective oxidation of unsymmetrical geminal diborylalkane: Unlocking access to a novel class of acylborons. Chem. Sci. 2019, 10, 4684–4687. [Google Scholar] [CrossRef] [Green Version]
- Lesley, G.; Nguyen, P.; Taylor, N.J.; Marder, T.B.; Scott, A.J.; Clegg, W.; Norman, N.C. Synthesis and Characterization of Platinum(II)-Bis(boryl) Catalyst Precursors for Diboration of Alkynes and Diynes: Molecular Structures of cis-[(PPh3)2Pt(B-4-Butcat)2], cis-[(PPh3)2Pt(Bcat)2], cis-[(dppe)Pt(Bcat)2], cis-[(dppb)Pt(Bcat)2], (E)-(4-MeOC6H4)C(Bcat)CH(Bcat), (Z)-(C6H5)C(Bcat)C(C6H5)(Bcat), and (Z,Z)-(4-MeOC6H4)C(Bcat)C(Bcat)C(Bcat)C(4-MeOC6H4)(Bcat) (cat = 1,2-O2C6H4; dppe ) Ph2PCH2CH2PPh2; dppb ) Ph2P(CH2)4PPh2. Organometallics 1996, 15, 5137–5154. [Google Scholar]
- Ali, H.A.; Quntar, A.E.A.A.; Goldberg, I.; Srebnik, M. Platinum(0)-Catalyzed Diboration of Alkynylboronates and Alkynylphosphonates with Bis(pinacolato)diborane(4): Molecular Structures of [((Me4C2O2)B)(C6H5)C=C(P(O)(OC2H5)2)(B(O2C2Me4))] and [((Me4C2O2)B)(C4H9)C=C(B(O2C2Me4))2]. Organometallics 2002, 21, 4533–4539. [Google Scholar] [CrossRef]
- Hyodo, K.; Suetsugu, M.; Nishihara, Y. Diborylation of Alkynyl MIDA Boronates and Sequential Chemoselective Suzuki−Miyaura Couplings: A Formal Carboborylation of Alkynes. Org. Lett. 2014, 16, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, T.; Contreras, R.; Wrackmeyer, B.J. New bicyclic organylboronicesters derived from iminodiacetic acids. J. Organomet. Chem. 1986, 307, 1–6. [Google Scholar] [CrossRef]
- Lee, C.-I.; Shih, E.-C.; Zhou, J.; Reibenspies, J.H.; Ozerov, O.V. Synthesis of Triborylalkenes from Terminal Alkynes by Iridium-Catalyzed Tandem C-H Borylation and Diboration. Angew. Chem. Int. Ed. 2015, 54, 14003–14007. [Google Scholar] [CrossRef]
- Liu, X.; Ming, W.; Friedrich, A.; Kerner, F.; Marder, T.B. Copper-Catalyzed Triboration of Terminal Alkynes using B2pin2: Efficient synthesis of 1,1,2-triborylakenes. Angew. Chem. Int. Ed. 2020, 59, 304–309. [Google Scholar] [CrossRef] [Green Version]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvadó, O.; Fernández, E. Tri(boryl)alkanes and Tri(boryl)alkenes: The Versatile Reagents. Molecules 2020, 25, 1758. https://doi.org/10.3390/molecules25071758
Salvadó O, Fernández E. Tri(boryl)alkanes and Tri(boryl)alkenes: The Versatile Reagents. Molecules. 2020; 25(7):1758. https://doi.org/10.3390/molecules25071758
Chicago/Turabian StyleSalvadó, Oriol, and Elena Fernández. 2020. "Tri(boryl)alkanes and Tri(boryl)alkenes: The Versatile Reagents" Molecules 25, no. 7: 1758. https://doi.org/10.3390/molecules25071758




