Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate
Abstract
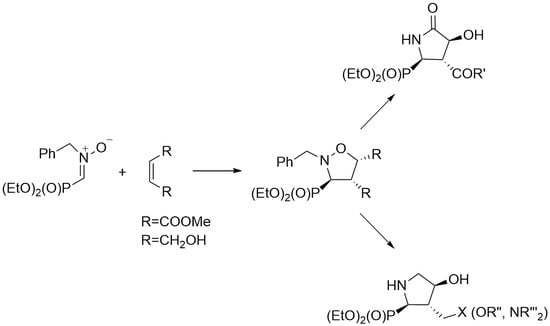
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Isoxazolidines 20, 21 and 23
3.3. Preparation of γ-Lactam 18
3.4. Ammonolysis of 18
3.5. Mesylation of (Isoxazolidin-3-yl)Phosphonate 21
3.6. The Synthesis of (Pyrrolidin-2-yl)Phosphonate 24 from Dimesylate 25
3.7. Synthesis of (Pyrrolidin-2-yl)Phosphonate 28
3.8. Synthesis of azide 29
3.9. Synthesis of (Pyrrolidin-2-yl)Phosphonate 30
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Massiot, G.; Delaude, C. Pyrrolidine Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Harcourt Brace Jovanovich: Bethesda, MD, USA, 1986; Volume 27, pp. 269–322. [Google Scholar]
- Ali, M.S.; Ahmad, F.; Ahmad, V.U.; Azhar, I.; Usmanghani, K. Unusual Chemical Constituents of Lotus garcinii (Fabaceae). Turk. J. Chem. 2001, 25, 107–112. [Google Scholar]
- Zhu, Y.; Wang, Y.; Gu, B.-B.; Yang, F.; Jiao, W.-H.; Hu, G.-H.; Yu, H.-B.; Han, B.-N.; Zhang, W.; Shen, Y.; et al. Antifungal bromopyrrole alkaloids from the South China Sea sponge Agelas sp. Tetrahedron 2016, 72, 2964–2971. [Google Scholar] [CrossRef]
- Walsh, C.T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075–3081. [Google Scholar] [CrossRef]
- Colegate, S.M.; Dorling, P.R.; Huxtable, C.R. A Spectroscopic Investigation of Swainsonine: An α-Mannosidase Inhibitor Isolated from Swainsona canescens. Aust. J. Chem. 1979, 32, 2257–2264. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Smith, L.W. The Alkaloids of Crotalaria Spectabilis roth. Aust. J. Chem. 1957, 10, 474–479. [Google Scholar] [CrossRef]
- Hay, D.G.; Mackay, M.F.; Culvenor, C.C.J. The structure of Lasiocarpine: Pyrrolizidine Alkaloid. Acta Crysallogr. 1982, 38, 155–159. [Google Scholar] [CrossRef]
- Hamid, H.K.; Kadhim, E.J. Extraction, isolation and characterization of Pyrrolizidine Alkaloids present in Senecio vulgaris Linn grown in Iraq. J. Pharmacogn. Phytochem. 2016, 5, 28–37. [Google Scholar]
- Szpak, P. Fish bone chemistry and ultrastructure: Implications for taphonomy and stable isotope analysis. J. Archaeol. Sci. 2011, 38, 3358–3372. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninge’s Principles of Biochemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2008; pp. 124–128. [Google Scholar]
- Abraham, G.N.; Podell, D.N. Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol. Cell. Biochem. 1981, 38, 181–190. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Malykh, A.G.; Sadaie, M.R. Piracetam and Piracetam–Like Drugs from Basic Science to Novel Clinical Applications to CNS Disorders. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef]
- Gouliaev, A.H.; Senning, A. Piracetam and other structurally related nootropics. Brain Res. Rev. 1994, 19, 180–222. [Google Scholar] [CrossRef]
- Winblad, B. Piracetam: A Review of Pharmacological Properties and Clinical Uses. CNS Drug Rev. 2005, 11, 169–182. [Google Scholar] [CrossRef]
- Shorvon, S. Pyrrolidone derivatives. Lancet 2001, 358, 1885–1892. [Google Scholar] [CrossRef]
- Weber, K.-H.; Walther, G.; Schneide, O.; Hinzen, D.; Kuhn, F.J.; Lehr, E. Preparation and formulation of piperazinyl and morpholinyl substituted 1-(benzyl or pyridylmethyl)-4-or-5-aminomethyl-pyrrolidin-2-ones and their nootropic use. U.S. Patent Application No. 4,767,759, 30 August 1988. [Google Scholar]
- Lima, E.C.; Domingos, J.L.O.; Dias, A.G.; Costa, P.R.R. First stereoselective synthesis and assignment of the absolute configuration of the nebracetam eutomer and derivatives. Tetrahedron Asymmetry 2008, 19, 1161–1165. [Google Scholar] [CrossRef]
- Cecioni, S.; Aouadi, K.; Guiard, J.; Parrot, S.; Strazielle, N.; Blondel, S.; Ghersi–Egea, J.-F.; Chapelle, C.; Denoroy, L.; Praly, J.-P. Novel routes to either racemic or enantiopure α-amino-(4-hydroxypyrrolidin-3-yl)acetic acid derivatives and biological evaluation of a new promising pharmacological scaffold. Eur. J. Med. Chem. 2015, 98, 237–249. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Best, D.; Saville, A.W.; Mui, J.; Martinez, R.F.; Nakagawa, S.; Kunimatsu, T.; Alonzi, D.S.; Butters, T.D.; Norez, C.; et al. C-Branched Iminosugars: Alpha-Glucosidase Inhibition by Enantiomers of isoDMDP, isoDGDP, and isoDAB–L–isoDMDP Compared to Miglitol and Miglustat. J. Org. Chem. 2013, 78, 7380–7397. [Google Scholar] [CrossRef]
- Aszodi, J.; Rowlands, D.A.; Mauvais, P.; Collette, P.; Bonnefoy, A.; Lampilas, M. Design and synthesis of bridged γ-lactams as analogues of β-lactam antibiotics. Bioorg. Med. Chem. Lett. 2004, 14, 2489–2492. [Google Scholar]
- Sugie, Y.; Inagaki, S.; Kato, Y.; Nishida, H.; Pang, C.-H.; Saito, T.; Sakemi, S.; Dib–Hajj, F.; Mueller, J.P.; Sutcliffe, J.; et al. CJ-21,058, a new SecA Inhibitor isolated from a fungus. J. Antibiot. 2002, 55, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Hilderbrand, R.L. The Role of Phosphonates in Living Systems; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Engel, R. Synthesis of Carbon-Phosphorus Bond; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Kukhar, V.P.; Hudson, H.P. (Eds.) Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Yamamoto, H.; Inokawa, S. Sugar Analogs Having Phosphorus in the Hemiacetal Ring. Adv. Carbohydr. Chem. Biochem. 1984, 42, 135–191. [Google Scholar]
- Denhel, A.; Lavielle, G. α-Aminophosphonates. II. Alkylation: Synthesis of α-aminophosphonic acids. Bull. Soc. Chim. Fr. 1978, 9, 95–96. [Google Scholar]
- Moonen, K.; Laureyn, I.; Stevens, C.V. Synthetic methods for azaheterocyclic phosphonates and their biological activity. Chem. Rev. 2004, 104, 6177–6215. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.A.; Wu, Y.; Xu, H.; Zhang, J. Asymmetric Synthesis of Cis-5-Substituted Pyrrolidine 2-Phosphonates Using Metal Carbenoid NH Insertion and δ-Amino β-Ketophosphonates. Org. Lett. 2004, 6, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Renaud, P.; Seebach, D. Enantiomerenreine Pyrrolidin-Derivate aus trans-4-Hydroxy-L-prolin durch elektrochemische oxidative Decarboxylierung und Titantetrachlorid-vermittelte Umsetzung mit Nukleophilen. Helv. Chem. Acta 1986, 69, 1704–1710. [Google Scholar] [CrossRef]
- El Khalabi, R.; El Hallaoui, A.; Ouazzani, F.; Elachqar, A.; Atmani, A.; Roumestant, M.L.; Viallefont, P.; Martinez, J. Syntheis of Phosphonic Analogues of 4-Hydroxyproline and 5-Hydroxypipecolic Acid. Prep. Biochem. Biotechnol. 2000, 30, 295–304. [Google Scholar] [CrossRef]
- Boto, A.; Gallardo, J.A.; Hernández, R.; Saavedra, C.J. One-pot synthesis of α-amino phosphonates from α-amino acids and β-amino alcohols. Tetrahedron Lett. 2005, 46, 7807–7811. [Google Scholar] [CrossRef]
- Chevrier, C.; Le Nouen, D.; Defoin, A.; Tarnus, C. Synthesis of Amino-L-Lyxose Phosphonates as Fucosyl-Phosphate Mimics. Eur. J. Org. Chem. 2006, 2384–2392. [Google Scholar] [CrossRef]
- Beck, J.; Gharbi, S.; Herteg–Fernea, A.; Vercheval, L.; Bebrone, C.; Lassaux, P.; Zervosen, A.; Marchand–Brynaert, J. Aminophosphonic Acids and Aminobis(phosphonic acids) as Potential Inhibitors of Penicillin–Binding Proteins. Eur. J. Org. Chem. 2009, 1, 85–97. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Głowacka, I.E. Enantiomerically pure phosphonate analogues of cis- and trans-4-hydroxyprolines. Tetrahedron Asymmetry 2007, 18, 1351–1363. [Google Scholar] [CrossRef]
- Piotrowska, D.G. N-Substituted C-diethoxyphosphorylated nitrones as useful synthons for the synthesis of α-aminophosphonates. Tetrahedron Lett. 2006, 47, 5363–5366. [Google Scholar] [CrossRef]
- Piotrowska, D.G. Stereochemistry of substituted isoxazolidines derived from N-methyl C-diethyoxyphosphorylated nitrone. Tetrahedron 2006, 62, 12306–12317. [Google Scholar] [CrossRef]
- Merino, P.; Mates, J.A.; Revuelta, J.; Tejero, T.; Chiachio, U.; Romeo, G.; Iannazzo, D.; Romeo, R. Experimental and theoretical study of the 1,3-dipolar cycloaddition between D-glyceraldehyde nitrones and acrylates. Diastereoselective approach to 4-hydroxy pyroglutamic acid derivatives. Tetrahedron Asymmetry 2002, 13, 173–190. [Google Scholar] [CrossRef]
- Merion, P.; Pádár, P.; Delso, I.; Thirumalaikumar, M.; Tejero, T.; Kovács, L. Stereoselective synthesis of pyrrolidinyl glycines from nitrones: Complementarity of nucleophilic addition and 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 5013–5016. [Google Scholar] [CrossRef]
- Argyropoulos, N.G.; Gkiziss, P.; Coutouli-Argyropoulou, E. A convenient synthesis of new enantiomerically pure rihydroxypyrrolizidines using L-erythrose glycosylhydroxylamine as a masked acyclic chiral nitrone. Tetrahedron 2008, 64, 8752–8758. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Głowacka, I.E.; Schols, D.; Snoeck, R.; Andrei, G.; Gotkowska, J. Novel Isoxazolidine and γ-Lactam Analogues of Homonucleosides. Molecules 2019, 24, 4014. [Google Scholar] [CrossRef] [Green Version]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Benezra, C. NMR of phosphonates. VI. Variation of vicinal phosphorus-31-carbon-carbon-proton couplings with dihedral angle in phosphonates. J. Am. Chem. Soc. 1973, 95, 6890–6894. [Google Scholar] [CrossRef]
- Neeser, J.-R.; Tronchet, J.M.J.; Charollais, E.J. Structural analysis of 3-C-phosphonates, -phosphinates, and -phosphine oxides of branched-chain sugars. Can. J. Chem. 1983, 61, 2112–2120. [Google Scholar] [CrossRef]
- Adiwidjaja, G.; Meyer, B.; Thiem, J. Darstellung and Kristallstruktur von endo-2-Dimethylphosphono-exo-2-hydroxy-(–)-camphan zur Bestimmung von 3J(CCCP)-Vicinalkopplungen. Z. Naturforsch. 1979, 34, 1547–1551. [Google Scholar] [CrossRef]
- Buchanan, G.W.; Bourque, K.; Seeley, A. 13C- and 31P-NMR spectra of 1-diethylphosphono-1-hydroxycycloalkanes. Magn. Res. Chem. 1986, 24, 360–367. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka, I.E.; Hartwich, A.; Rozpara, I.; Piotrowska, D.G. Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate. Molecules 2021, 26, 3160. https://doi.org/10.3390/molecules26113160
Głowacka IE, Hartwich A, Rozpara I, Piotrowska DG. Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate. Molecules. 2021; 26(11):3160. https://doi.org/10.3390/molecules26113160
Chicago/Turabian StyleGłowacka, Iwona E., Anna Hartwich, Iwona Rozpara, and Dorota G. Piotrowska. 2021. "Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate" Molecules 26, no. 11: 3160. https://doi.org/10.3390/molecules26113160