Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design
Abstract
:1. Introduction
2. Results and Discussion
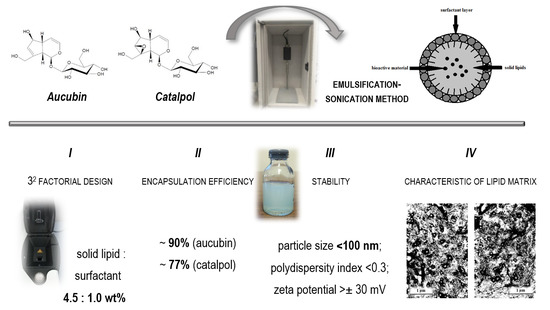
2.1. Optimization of Quantitative Composition of Studied Lipid Nanoparticles (32 Factorial Design)
2.2. Assessment of Encapsulation Efficiency and Loading Capacity of Iridoid Glycosides
2.3. Evaluation of Stability of Studied Lipid Nanoparticles
2.4. Analysis of Results of Characterization of Lipid Matrices
2.4.1. Transmission Electron Microscopy Analysis
2.4.2. Thermal Analysis
2.4.3. X-ray Studies
3. Materials and Methods
3.1. Materials
3.2. Experimental Factorial Design
3.3. Production of Lipid Nanoparticles
3.4. Physicochemical Characterization
3.5. Encapsulation Efficiency and Loading Capacity
3.6. Stability Test
3.7. Characterization of Lipid Matrices
3.7.1. Transmission electron microscopy (TEM)
3.7.2. Differential Scanning Calorimetry (DSC)
3.7.3. X-ray Diffraction (XRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro, J.A., Jr.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 115–141. [Google Scholar] [CrossRef]
- Keck, C.M. Lipid nanoparticles (SLN, NLC) for innovative consumer care & household products. Househ. Pers. Care Today 2014, 9, 18–24. [Google Scholar]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers) for cosmetic, dermal, and transdermal applications. In Nanoparticulate Drug Delivery Systems; Thassu, D., Ed.; CRC Press: New York, NY, USA, 2007; pp. 213–233. [Google Scholar]
- Wosicka, H.; Lulek, J. Stałe nanocząstki lipidowe i nanostrukturalne nośniki lipidowe w nowoczesnych kosmetykach. Pol. J. Cosmetol. 2009, 12, 23–37. [Google Scholar]
- Uner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006, 61, 375–386. [Google Scholar]
- Dinda, B. Pharmacokinetics of iridoids. In Pharmacology and Applications of Naturally Occurring Iridoids; Dinda, B., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 255–269. [Google Scholar]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ullah, H.; Zhang, L. Bioactive constituents form Buddleja species. Pak. J. Pharm. Sci. 2019, 32, 721–741. [Google Scholar]
- Krithika, R.; Srivastava, P.; Bajaj, R.; Kolet, S.; Chopade, M.; Soniya, M.; Thulasiram, H.V. Characterization of 10-hydroxygeraniol dehydrogenase from catharanthus roseus reveals cascaded enzymatic activity in iridoid biosynthesis. Sci. Rep. 2015, 5, 8258. [Google Scholar] [CrossRef]
- Sampaio-Santos, M.I.; Kaplan, M.A.C. Biosynthesis significance of iridoids in chemosystematics. J. Braz. Chem. Soc. 2001, 12, 144–153. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Herrmann, K.; Murray, R.D.H.; Ohloff, G.; Pattenden, G. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowska, M.; Nowak, I. Budleja Davida-charakterystyka, właściwości, zastosowanie. Pol. J. Cosmetol. 2016, 19, 104–109. [Google Scholar]
- Huang, T.-L.; Yang, S.-H.; Chen, Y.-R.; Liao, J.-Y.; Tang, Y.; Yang, K.-C. The therapeutic effect of aucubin-supplemented hyaluronic acid on interleukin-1beta-stimulated human articular chondrocytes. Phytomedicine 2019, 53, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Sato, T.; Metori, K.; Koike, K.; Che, Q.; Takahashi, S. The promoting effects of geniposidic acid and aucubin in eucommia ulmoides oliver leaves on collagen synthesis. Biol. Pharm. Bull. 1998, 21, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Kartini, I.R.; Handojo, C.S. Wound healing activity of aucubin on hyperglycemic rat. J. Young Pharm. 2018, 10, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated suppression of NF-κB and MAPKs signaling pathways. Bioengineered 2021, 12, 183–195. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.M.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Souto, E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B Biointerfaces 2012, 100, 84–89. [Google Scholar] [CrossRef]
- Smucker, B.; Krzywinski, M.; Altman, N. Two-level factorial experiments. Nat. Methods 2019, 16, 211–212. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Jiang, Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin. Drug Deliv. 2011, 8, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC); Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–122. [Google Scholar]
- Joshi, S.A.; Ramteke, K.H. Solid lipid nanoparticle: A review. IOSR J. Pharm. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef]
- Farboud, E.S.; Nasrollahi, S.A.; Tabbakhi, Z. Novel formulation and evaluation of a Q10-loaded solid lipid nanoparticle cream: In vitro and in vivo studies. Int. J. Nanomed. 2011, 6, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004, 58, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control. Release 2016, 10, 337–351. [Google Scholar] [CrossRef]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control. Release 2004, 95, 217–227. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Rothen-Rutishauser, B.; Vanhecke, D.; Blank, F.; Gehr, P.; Ochs, M. Visualization and quantitative analysis of nanoparticles in the respiratory tract by transmission electron microscopy. Part. Fibre Toxicol. 2007, 4. [Google Scholar] [CrossRef] [Green Version]
- Graef, M.D. Introduction to Conventional Transmission Electron Microscopy; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Sousa Lobo, J.M. Characterization, sensorial evaluation and moisturizing efficacy of nanolipidgel formulations. Int. J. Cosmet. Sci. 2014, 36, 159–166. [Google Scholar] [CrossRef]
- Klancnik, G.; Medved, J.; Mrvar, P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation. RMZ Mater. Geoenviron. 2010, 57, 127–142. [Google Scholar]
- Allais, C.; Keller, G.; Lesieur, P.; Olivon, M.; Artzner, F. X-ray diffraction/Calorimetry coupling. J. Therm. Anal. Calorim. 2003, 74, 723–728. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Solid lipid nanoparticles and nanostructured lipid carriers as novel carriers for cosmetic ingredients. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 231–255. [Google Scholar]






| Sample | Mean Particle Size (nm, ±SD 1) | Polydispersity Index (-, ±SD 1) | Zeta Potential 2 (mV, ±SD 1) |
|---|---|---|---|
| A | 99.46 ± 0.40 | 0.247 ± 0.013 | 9.09 ± 2.15 |
| B | 92.58 ± 0.81 | 0.276 ± 0.006 | 26.97 ± 6.06 |
| C | 91.39 ± 1.97 | 0.313 ± 0.027 | 16.00 ± 4.33 |
| D | 94.06 ± 0.61 | 0.214 ± 0.004 | 12.93 ± 1.87 |
| E | 93.32 ± 0.32 | 0.226 ± 0.004 | 39.30 ± 4.22 |
| F | 124.03 ± 2.32 | 0.370 ± 0.011 | 29.73 ± 2.61 |
| G | 116.63 ± 1.39 | 0.346 ± 0.008 | 17.57 ± 2.61 |
| H | 147.10 ± 2.44 | 0.402 ± 0.009 | 43.03 ± 2.06 |
| I | 112.07 ± 1.01 | 0.320 ± 0.007 | 39.23 ± 7.24 |
| Incorporated Active Substance | Concentration (μg/mL ± SD 1) | EE 2 (% ± SD 1) | LC2 (% ± SD 1) |
|---|---|---|---|
| aucubin | 10.476 ± 0.235 | 89.52 ± 0.72 | 19.89 ± 0.23 |
| catalpol | 20.683 ± 0.192 | 77.02 ± 0.93 | 15.41 + 0.46 |
| MEAN PARTICLE SIZE (nm, ±SD) | ||||
|---|---|---|---|---|
| Blank 1 | +aucubin | +catalpol | ||
| temp. 4 °C | day 1 | 100.97 ± 0.75 | 79.30 ± 1.73 | 82.55 ± 2.37 |
| day 30 | 139.10 ± 9.66 | 92.20 ± 3.85 | 84.78 ± 4.20 | |
| temp. 25 °C | day 1 | 93.32 ± 0.32 | 84.35 ± 1.84 | 84.79 ± 1.32 |
| day 30 | 103.93 ± 1.01 | 82.95 ± 2.65 | 85.86 ± 3.16 | |
| temp. 40 °C | day 1 | 139.57 ± 2.58 | 82.81 ± 1.56 | 81.84 ± 1.93 |
| day 30 | 101.73 ± 2.25 | 81.27 ± 2.31 | 82.66 ± 1.85 | |
| POLYDISPERSITY INDEX (-, ±SD) | ||||
| Blank 1 | +aucubin | +catalpol | ||
| temp. 4 °C | day 1 | 0.295 ± 0.021 | 0.236 ± 0.005 | 0.237 ± 0.003 |
| day 30 | 0.476 ± 0.085 | 0.297 ± 0.034 | 0.235 ± 0.003 | |
| temp. 25 °C | day 1 | 0.226 ± 0.004 | 0.240 ± 0.007 | 0.240 ± 0.014 |
| day 30 | 0.266 ± 0.018 | 0.232 ± 0.003 | 0.220 ± 0.009 | |
| temp. 40 °C | day 1 | 0.406 ± 0.010 | 0.237 ± 0.013 | 0.201 ± 0.013 |
| day 30 | 0.463 ± 0.021 | 0.216 ± 0.008 | 0.212 ± 0.013 | |
| ZETA POTENTIAL (mV, ±SD) | ||||
| Blank 1,2 | +aucubin 3 | +catalpol 3 | ||
| temp. 4 °C | day 1 | 9.37 ± 2.19 | 41.63 ± 6.44 | 50.77 ± 0.15 |
| day 30 | 8.82 ± 2.74 | 35.45 ± 1.91 | 41.60 ± 2.25 | |
| temp. 25 °C | day 1 | 39.30 ± 4.22 | 47.30 ± 1.47 | 55.53 ± 1.55 |
| day 30 | 15.50 ± 0.66 | 34.37 ± 2.31 | 37.07 ± 3.11 | |
| temp. 40 °C | day 1 | 15.30 ± 2.39 | 45.20 ± 0.95 | 53.43 ± 1.77 |
| day 30 | 13.17 ± 0.71 | 45.47 ± 1.10 | 46.23 ± 2.10 | |
| Quantity of ingredient (wt% ± 0.01) | |||||
|---|---|---|---|---|---|
| Sample | Softisan® 100 1 | Tween® 80 2 | Glycerol | CTAB 3 | Water Milli-Q® Plus |
| A | 4.00 | 0.50 | 37.50 | 0.50 | 56.00 |
| B | 4.00 | 1.00 | 37.50 | 0.50 | 55.50 |
| C | 4.00 | 1.50 | 37.50 | 0.50 | 55.00 |
| D | 4.50 | 0.50 | 37.50 | 0.50 | 55.50 |
| E | 4.50 | 1.00 | 37.50 | 0.50 | 55.00 |
| F | 4.50 | 1.50 | 37.50 | 0.50 | 54.50 |
| G | 5.00 | 0.50 | 37.50 | 0.50 | 55.00 |
| H | 5.00 | 1.00 | 37.50 | 0.50 | 54.50 |
| I | 5.00 | 1.50 | 37.50 | 0.50 | 54.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, M.; Souto, E.B.; Nowak, I. Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design. Molecules 2021, 26, 3161. https://doi.org/10.3390/molecules26113161
Dąbrowska M, Souto EB, Nowak I. Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design. Molecules. 2021; 26(11):3161. https://doi.org/10.3390/molecules26113161
Chicago/Turabian StyleDąbrowska, Marta, Eliana B. Souto, and Izabela Nowak. 2021. "Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design" Molecules 26, no. 11: 3161. https://doi.org/10.3390/molecules26113161