Cytoprotective Mechanisms of DJ-1: Implications in Cardiac Pathophysiology
Abstract
:1. Introduction
2. Structure
3. Role in Mitochondrial Function
4. Cytoprotective Activities of DJ-1
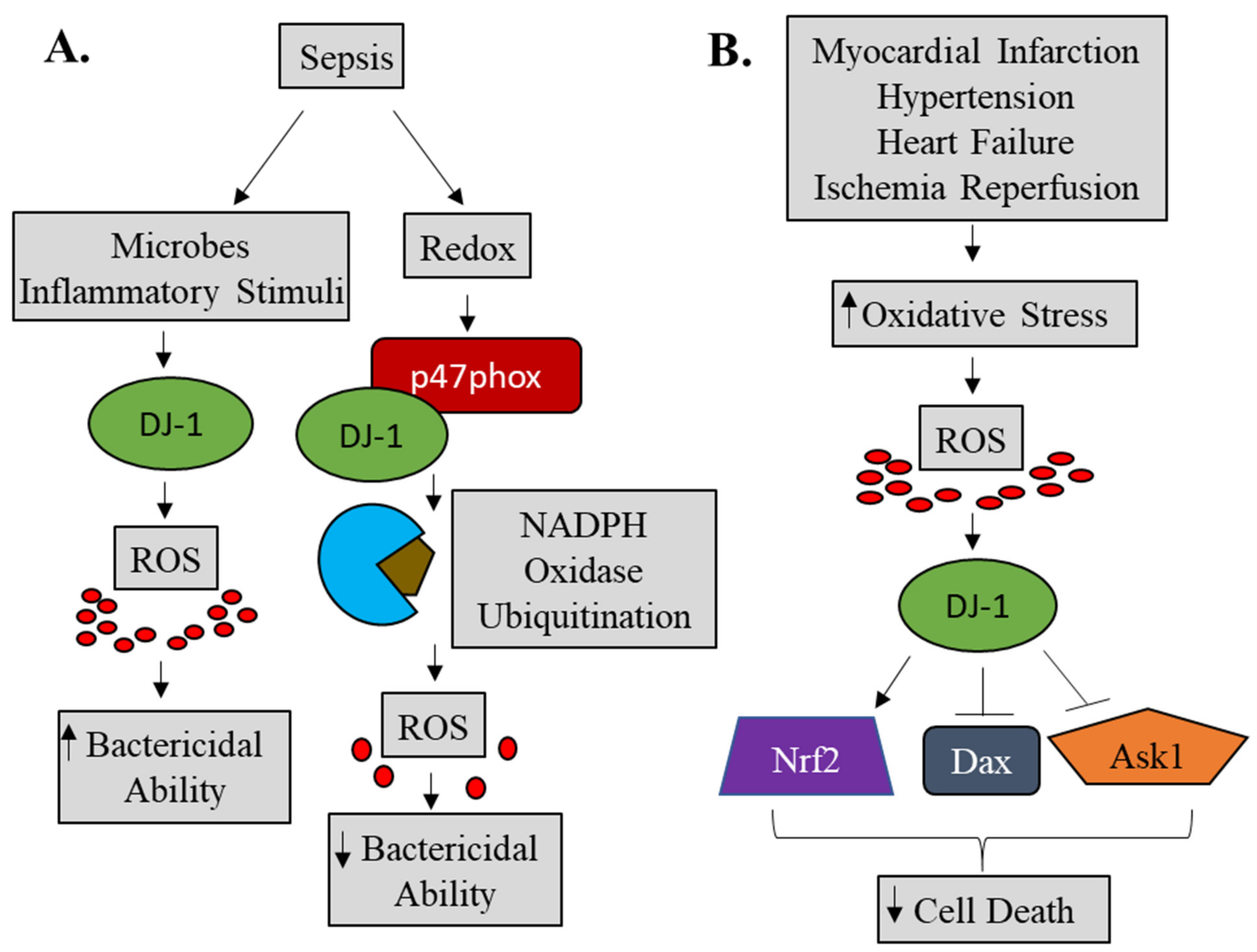
4.1. DJ-1 and Reactive Oxygen Species
4.2. DJ-1 and Cell Death
4.3. DJ-1 and Autophagy
4.4. DJ-1 and Inflammation
5. Cardioprotective Function
6. Concluding Remarks and Future Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | alanine |
| AGEs | advanced glycation end products |
| ASK1 | apoptosis signal-regulating kinase 1 |
| ATAA | ascending thoracic aortic aneurysm |
| AVS | aortic valve stenosis |
| A549 | adenocarcinomic human alveolar basal epithelial cell line 549 |
| α | alpha |
| β | beta |
| Bax | Bcl2-associated X protein |
| Bcl2 | B-cell lymphoma 2 |
| Beas2b | bronchial epithelium cell line |
| BV-2 | microglial cell line |
| C | cysteine |
| CABG | coronary artery bypass grafting |
| CD | cluster of differentiation |
| CHX | cycloheximide |
| CLP | cecal ligation and puncture |
| COS-7 | CV-1 in origin with SV40 genes cell line |
| COX4 | cytochrome oxidase subunit 4 |
| C-terminal | carboxy-terminal |
| CXCR4 | CXC chemokine receptor 4 |
| Cys | cysteine |
| D | aspartic acid |
| Daxx | death domain-associated protein |
| D2R | dopamine 2 receptor |
| DJ-1−/− | DJ-1 knockout |
| E | glutamic acid |
| ERK | extracellular signal-regulated kinase |
| Foxp3 | forkhead box P3 |
| GCLM | glutamate-cysteine ligase modifier subunit |
| Glu | glutamic acid |
| HEK293 | human embryonic kidney cell line 293 |
| HeLA | Henrietta Lacks (cancer cell line) |
| HIPK1 | homeodomain-interacting protein kinase 1 |
| HMOX1 | heme oxygenase 1 |
| H1299 | p53 deficient cell line |
| H2O2 | hydrogen peroxide |
| HL-1 | cardiac cell line |
| H9c2 | rat ventricular cell line |
| I | isoleucine |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFNγ | interferon gamma |
| IgE | immunoglobulin E |
| IKK | inhibitor of nuclear factor kappa B kinase |
| IkBα | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, |
| IL-1A | interleukin 1 alpha |
| IL-1β | interleukin 1 beta |
| IL-4 | interleukin 4 |
| IL-6 | interleukin 6 |
| IL-16 | interleukin 16 |
| IL-17 | interleukin 17 |
| iNOS | inducible nitric oxide synthase |
| INSIST | inflammation in trauma and sepsis |
| IRES | internal ribosomal entry site |
| I-TAC | interferon-inducible T-cell alpha chemoattractant |
| JNK | Janus kinase |
| K | lysine |
| Keap-1 | kelch-like ECH-associated protein 1 |
| kDa | kilodalton |
| L | leucine |
| LAT | linker for activation of T cells |
| LC3 | microtubule-associated protein light chain 3 |
| LDH | lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| LPS | lipopolysaccaride |
| LV | left ventricle |
| M | methionine |
| MAP | mitogen-activated protein |
| MEF | mouse embryonic fibroblasts |
| mmHg | millimeter of mercury |
| MN9D | mesencephalic dopaminergic neurons |
| MPP+ | 1-methyl-4-phenylpyridinium |
| mTOR | mammalian target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| mRNA | messenger RNA |
| M17 | neuroblastoma cells |
| N | asparagine |
| NAC | N-acetyl cysteine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| Neuro-2A | mouse neuroblastoma cell line |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| Nrf2 | NF-E2 related factor-2 |
| NOX | NADPH oxidase |
| NOX4 | NADPH oxidase 4 |
| P | proline |
| PARK7 | Parkinson’s disease-associated gene 7 |
| PARP | poly (ADP-ribose) polymerase |
| PD | Parkinson’s disease |
| PH | pulmonary hypertension |
| pI | isoelectric point |
| PI3K | phosphoinositide 3 kinase |
| PLCγ | phospholipase C gamma |
| POAF | post-operative atrial fibrillation |
| PTEN | phosphatase and tensin homolog |
| Q | glutamine |
| qPCR | quantitative polymerase chain reaction |
| RAA | right atrial appendage |
| RAGE | receptor for advanced glycation endproducts |
| ROS | reactive oxygen species |
| SDF-1 | stromal cell derived factor |
| SEM | standard error of the mean |
| SG2NA | S/G2 nuclear autoantigen |
| SHSY5Y | human neuroblastoma cell line |
| siRNA | small interfering ribonucleic acid |
| SIRT1 | sirtuin 1 |
| SK-N-BE-2C | human neuroblastoma cell line |
| SR | sinus rhythm |
| STAT1 | signal transducer and activator of transcription 1 |
| Syk | spleen tyrosine kinase |
| SO2 | sulfinate |
| SO3 | sulfonate |
| T | threonine |
| TNF | tumor necrosis factor |
| Tom20 | translocase of outer mitochondrial membrane 20 |
| Trx1 | thioredoxin 1 |
| Th1 | T helper 1 |
| Th17 | T helper 17 |
| μM | micromolar |
| U2O2 | human bone osteosarcoma epithelial cell line |
| WBC | white blood cell |
| WT | wild type |
References
- Nagakubo, D.; Taira, T.; Kitaura, H.; Ikeda, M.; Tamai, K.; Iguchi-Ariga, S.M.; Ariga, H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997, 231, 509–513. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.H.; Peters, M.; Jang, Y.; Shi, W.; Pintilie, M.; Fletcher, G.C.; DeLuca, C.; Liepa, J.; Zhou, L.; Snow, B.; et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005, 7, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Gehrke, S.; Haque, M.D.E.; Imai, Y.; Kosek, J.; Yang, L.; Beal, M.F.; Nishimura, I.; Wakamatsu, K.; Ito, S.; et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 13670–13675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junn, E.; Taniguchi, H.; Jeong, B.S.; Zhao, X.; Ichijo, H.; Mouradian, M.M. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl. Acad. Sci. USA 2005, 102, 9691–9696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Zhu, M.; Wilson, M.A.; Petsko, G.A.; Fink, A.L. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J. Mol. Biol. 2006, 356, 1036–1048. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, N.; Wang, H.; Elias, J.E.; Kim, C.Y.; Woldman, I.; Pifl, C.; Gygi, S.P.; Geula, C.; Yankner, B.A. The Parkinson’s disease-associated DJ-1 protein is a transcriptional coactivator that protects against neuronal apoptosis. Hum. Mol. Genet. 2005, 14, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N.; Christina, Y.; Kim, C.Y.; Rizzu, P.; Geula, C.; Porter, D.R.; Pothos, E.N.; Squitieri, F.; Heutink, P.; Xu, J. DJ-1 transcriptionally up-regulates the human tyrosine hydrox ylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J. Biol. Chem. 2006, 281, 20940–20948. [Google Scholar] [CrossRef] [Green Version]
- Richarme, G.; Marguet, E.; Forterre, P.; Ishino, S.; Ishino, Y. DJ-1 family Maillard deglycases prevent acrylamide formation. Biochem. Biophys. Res. Commun. 2016, 478, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Omans, A.D.; Leicher, R.; Osunsade, A.; Agustinus, A.S.; Finkin-Groner, E.; D’Ambrosio, H.; Liu, B.; Chandarlapaty, S.; Liu, S.; et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 2019, 10, 1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scumaci, D.; Olivo, E.; Fiumara, C.V.; La Chimia, M.; De Angelis, M.T.; Mauro, S.; Costa, G.; Ambrosio, F.A.; Alcaro, S.; Agosti, V.; et al. DJ-1 Proteoforms in Breast Cancer Cells: The Escape of Metabolic Epigenetic Misregulation. Cells 2020, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, Y.; Fan, R.; Sun, J.; Zou, D.; Yuan, Y. Knockdown of the DJ-1 (PARK7) gene sensitizes pancreatic cancer to erlotinib inhibition. Mol. Ther. Oncolytics 2021, 20, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Ma, Z.; Li, X.; Deng, Y.; Duan, G.; Zhao, L.E.; Xu, X.; Xiao, L.; Liu, H.; Zhu, Z.; et al. DJ-1 is involved in the multidrug resistance of SGC7901 gastric cancer cells through PTEN/PI3K/Akt/Nrf2 pathway. Acta Biochim. Biophys. Sin. 2020, 52, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Van der Brug, M.P.; Blackinton, J.; Chandran, J.; Hao, L.Y.; Lal, A.; Mazan-Mamczarz, K.; Martindale, J.; Xie, C.; Ahmad, R.; Thomas, K.J.; et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 10244–10249. [Google Scholar] [CrossRef] [Green Version]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef] [Green Version]
- Ariga, H.; Takahashi-Niki, K.; Kato, I.; Maita, H.; Niki, T.; Iguchi-Ariga, S.M. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 683920. [Google Scholar] [CrossRef] [Green Version]
- Blackinton, J.; Ahmad, R.; Miller, D.W.; van der Brug, M.P.; Canet-Avilés, R.M.; Hague, S.M.; Kaleem, M.; Cookson, M.R. Effects of DJ-1 mutations and polymorphisms on protein stability and subcellular localization. Brain Res. Mol. Brain Res. 2005, 134, 76–83. [Google Scholar] [CrossRef]
- Görner, K.; Holtorf, E.; Odoy, S.; Nuscher, B.; Yamamoto, A.; Regula, J.T.; Beyer, K.; Haass, C.; Kahle, P.J. Differential effects of Parkinson’s disease-associated mutations on stability and folding of DJ-1. J. Biol. Chem. 2004, 279, 6943–6951. [Google Scholar] [CrossRef] [Green Version]
- Hulleman, J.D.; Mirzaei, H.; Guigard, E.; Taylor, K.L.; Ray, S.S.; Kay, C.M.; Regnier, F.E.; Rochet, J.C. Destabilization of DJ-1 by familial substitution and oxidative modifications: Implications for Parkinson’s disease. Biochemistry 2007, 46, 5776–5789. [Google Scholar] [CrossRef]
- Im, J.Y.; Lee, K.W.; Woo, J.M.; Junn, E.; Mouradian, M.M. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 2012, 21, 3013–3024. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarasimhan, M.; Maldonado, M.T.; Zhou, W.; Fink, A.L.; Wilson, M.A. Structural impact of three Parkinsonism-associated missense mutations on human DJ-1. Biochemistry 2008, 47, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Olzmann, J.A.; Brown, K.; Wilkinson, K.D.; Rees, H.D.; Huai, Q.; Ke, H.; Levey, A.I.; Li, L.; Chin, L.S. Familial Parkinson’s disease-associated L166P mutation disrupts DJ-1 protein folding and function. J. Biol. Chem. 2004, 279, 8506–8515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004, 11, e362. [Google Scholar]
- Taira, T.; Saito, Y.; Niki, T.; Iguchi-Ariga, S.M.M.; Takahashi, K.; Ariga, H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004, 5, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meulener, M.C.; Graves, C.L.; Sampathu, D.M.; Armstrong-Gold, C.E.; Bonini, N.M.; Giasson, B.I. DJ-1 is present in a large molecular complex in human brain tissue and interacts with alpha-synuclein. J. Neurochem. 2005, 93, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Takeshi, N.; Takahashi-Niki, K.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. DJBP: A novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. 2003, 1, 247–261. [Google Scholar]
- Canet-Aviles, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef] [Green Version]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanti, G.K.; Goswami, S.K. SG2NA recruits DJ-1 and Akt into the mitochondria and membrane to protect cells from oxidative damage. Free Radic. Biol. Med. 2014, 75, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Shimoji, M.; Thomas, B.; Moore, D.J.; Yu, S.W.; Marupudi, N.I.; Torp, R.; Torgner, I.A.; Ottersen, O.P.; Dawson, T.M.; et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: Implications for pathogenesis. Hum. Mol. Genet. 2005, 14, 2063–2073. [Google Scholar] [CrossRef] [Green Version]
- Cali, T.; Calì, T.; Ottolini, D.; Soriano, M.E.; Brini, M. A new split-GFP-based probe reveals DJ-1 translocation into the mitochondrial matrix to sustain ATP synthesis upon nutrient deprivation. Hum. Mol. Genet. 2015, 24, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Irrcher, I.; Aleyasin, H.; Seifert, E.L.; Hewitt, S.J.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.C.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joselin, A.P.; Hewitt, S.J.; Callaghan, S.M.; Kim, R.H.; Chung, Y.H.; Mak, T.W.; Shen, J.; Slack, R.S.; Park, D.S. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum. Mol. Genet. 2012, 21, 4888–4903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebiehl, G.; Ruckerbauer, S.; Burbulla, L.F.; Kieper, N.; Maurer, B.; Waak, J.; Wolburg, J.H.; Gizatullina, Z.; Gellerich, F.N.; Woitalla, D.; et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS ONE 2010, 5, e9367. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.Y.; Park, J.H.; Kim, S.J.; Seo, K.S.; Han, J.S.; Lee, S.H.; Kim, J.M.; Park, J.; Park, S.K.; Lim, K.; et al. DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: Involvement of mitochondrial complex I assembly. PLoS ONE 2012, 7, e32629. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.Y.; Lardelli, M.; Collins-Praino, L.; Barthelson, K. Loss of park7 activity has differential effects on expression of iron responsive element (IRE) gene sets in the brain transcriptome in a zebrafish model of Parkinson’s disease. Mol. Brain 2021, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 2011, 15, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–10037. [Google Scholar] [CrossRef] [Green Version]
- Repici, M.; Hassanjani, M.; Maddison, D.C.; Garção, P.; Cimini, S.; Patel, B.; Szegö, É.M.; Straatman, K.R.; Lilley, K.S.; Borsello, T.; et al. The Parkinson’s Disease-Linked Protein DJ-1 Associates with Cytoplasmic mRNP Granules During Stress and Neurodegeneration. Mol. Neurobiol. 2019, 56, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Kinumi, T.; Kimata, J.; Taira, T.; Ariga, H.; Niki, E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2004, 317, 722–728. [Google Scholar] [CrossRef]
- Mitsumoto, A.; Nakagawa, Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic. Res. 2001, 35, 885–893. [Google Scholar] [CrossRef]
- Mitsumoto, A.; Nakagawa, Y.; Takeuchi, A.; Okawa, K.; Iwamatsu, A.; Takanezawa, Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic. Res. 2001, 35, 301–310. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Cookson, M.R. Evolutionary and functional relationships within the DJ1 superfamily. BMC Evol. Biol. 2004, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Chen, H.; Zhou, Y.; Föller, M.; Mak, T.W.; Madhuri, S.M.S.; Lang, F. Differential effect of DJ-1/PARK7 on development of natural and induced regulatory T cells. Sci. Rep. 2015, 5, 17723. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Kelsen, S.G.; Merali, S. Proteomic analysis of oxidative stress-responsive proteins in human pneumocytes: Insight into the regulation of DJ-1 expression. J. Proteome Res. 2008, 7, 4955–4961. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.S.; Park, J.Y.; Suh, Y.H.; Jou, I.; Joe, F.H.; Park, S.M. DJ-1 Associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum. Mol. Genet. 2013, 22, 4805–4817. [Google Scholar] [CrossRef] [Green Version]
- Andres-Mateos, E.; Perier, C.; Zhang, L.; Blanchard-Fillion, B.; Greco, T.M.; Thomas, B.; Ko, H.S.; Sasaki, M.; Ischiropoulos, H.; Przedborski, S.; et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA 2007, 104, 14807–14812. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Kitaura, H.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 2009, 35, 1331–1341. [Google Scholar] [PubMed]
- Martinat, C.; Shendelman, S.; Jonason, A.J.; Leete, T.; Bea, M.F.; Lichuan Yang, L.; Floss, T.; Abeliovich, A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: An ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004, 2, e327. [Google Scholar] [CrossRef] [PubMed]
- Waak, J.; Weber, S.S.; Waldenmaier, A.; Görner, K.; Alunni-Fabbroni, M.; Schell, H.; Vogt-Weisenhorn, D.; Pham, T.; Reumers, V.; Baekelandt, V.; et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009, 23, 2478–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billia, F.; Hauck, L.; Grothe, D.; Konecny, F.; Rao, V.; Kim, R.H.; Mak, T.W. Parkinson-susceptibility gene DJ-1/PARK7 protects the murine heart from oxidative damage in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 6085–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inden, M.; Taira, T.; Kitamura, Y.; Yanagida, T.; Tsuchiya, D.; Takata, K.; Yanagisawa, D.; Nishimura, K.; Taniguchi, T.; Kiso, Y.; et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol. Dis. 2006, 24, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Niki, K.; Niki, T.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson’s disease patients. Biochem. Biophys. Res. Commun. 2004, 320, 389–397. [Google Scholar] [CrossRef]
- Yokota, T.; Sugawara, K.; Ito, K.; Takahashi, R.; Ariga, H.; Mizusawa, H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem. Biophys. Res. Commun. 2003, 312, 1342–1348. [Google Scholar] [CrossRef]
- Zhou, W.; Freed, C.R. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J. Biol. Chem. 2005, 280, 43150–43158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Pol, A.; Van Gilst, W.H.; Voors, A.A.; Van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Blackinton, J.; Lakshminarasimhan, M.; Thomas, K.J.; Ahmad, R.; Greggio, E.; Raza, A.S.; Cookson, M.R.; Wilson, M.A. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J. Biol. Chem. 2009, 284, 6476–6485. [Google Scholar] [CrossRef] [Green Version]
- Zucchelli, S.; Vilotti, S.; Calligaris, R.; Lavina, Z.S.; Biagioli, M.; Foti, R.; De Maso, L.; Pinto, M.; Gorza, M.; Speretta, E.; et al. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 2009, 16, 428–438. [Google Scholar] [CrossRef]
- Choi, J.; Sullards, M.C.; Olzmann, J.A.; Rees, H.D.; Weintraub, S.T.; Bostwick, D.E.; Gearing, M.; Levey, A.; Chin, L.S.; Li, L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006, 281, 10816–10824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, D.; Thimmulappa, R.; Navas-Acien, A.; Sandford, A.; Elliott, M.; Singh, A.; Chen, L.; Zhuang, X.; Hogg, J.; Pare, P.; et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008, 178, 592–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P.Y. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, Y.; Kochevar, I.E.; Jurkunas, U.V. Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant defense and increased UV-A-induced apoptosis in corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5551–5560. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, S.; Zhang, Y.; Yang, Y.; Escano, C.; Asico, L.; Jones, J.E.; Armando, I.; Jose, P.A. Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 2012, 59, 446–452. [Google Scholar] [CrossRef]
- Cuevas, S.; Yang, Y.; Konkalmatt, P.; Asico, L.D.; Feranil, J.; Jones, J.; Villar, V.A.; Armando, I.; Jose, P.A. Role of Nrf2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension 2015, 65, 1251–1257. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, B.; Zhang, C.; Su, Z.; Guo, B.; Zhao, Y.; Zheng, R. The DJ1-Nrf2-STING axis mediates the neuroprotective effects of Withaferin A in Parkinson’s disease. Cell Death Differ. 2021. online ahead of print. [Google Scholar]
- Ren, H.; Fu, K.; Mu, C.; Li, B.; Wang, D.; Wang, G. DJ-1, a cancer and Parkinson’s disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett. 2010, 297, 101–108. [Google Scholar] [CrossRef]
- Liu, F.; Nguyen, J.L.; Hulleman, J.D.; Li, L.; Rochet, J.C. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson’s disease. J. Neurochem. 2008, 105, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Aleyasin, H.; Rousseaux, M.W.C.; Phillips, M.; Kim, R.H.; Bland, R.J.; Callaghan, S.; Slack, R.S.; During, M.J.; Mak, T.W.; Park, D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA 2007, 104, 18748–18753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.H.; Lee, M.J.; Liou, H.C.; Liou, H.H.; Fu, W.M. Microglia-Derived Cytokines/Chemokines Are Involved in the Enhancement of LPS-Induced Loss of Nigrostriatal Dopaminergic Neurons in DJ-1 Knockout Mice. PLoS ONE 2016, 11, e0151569. [Google Scholar]
- Kim, R.H.; Smith, P.D.; Aleyasin, H.; Hayley, S.; Mount, M.P.; Pownall, S.; Wakeham, A.; You-Ten, A.J.; Kalia, S.K.; Horne, P.; et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. USA 2005, 102, 5215–5220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseur, S.; Afzal, S.; Tardivel-Lacombe, J.; David, S.; Park, D.S.; Iovanna, J.L.; Mak, T.W. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc. Natl. Acad. Sci. USA 2009, 106, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Yang, W.; Qi, Z.; Lu, L.; Duan, C.; Zhao, C.; Yang, H. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J. Mol. Biol. 2012, 423, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Waak, J.; Weber, S.S.; Görner, K.; Schall, C.; Ichijo, H.; Stehle, T.; Kahle, P.J. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J. Biol. Chem. 2009, 284, 14245–14257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Choi, D.G.; Jeong, H.K.; Kim, J.; Kim, D.W.; Choi, S.Y.; Park, S.M.; Suh, Y.H.; Jou, I.; Joe, E.H. DJ-1 facilitates the interaction between STAT1 and its phosphatase SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 2013, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paterna, J.C.; Leng, A.; Weber, E.; Feldon, J.; Büeler, H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol. Ther. 2007, 15, 698–704. [Google Scholar] [CrossRef]
- Yanagida, T.; Takata, K.; Inden, M.; Kitamura, Y.; Taniguchi, T.; Yoshimoto, K.; Taira, T.; Ariga, H. Distribution of DJ-1, Parkinson’s disease-related protein PARK7, and its alteration in 6-hydroxydopamine-treated hemi-parkinsonian rat brain. J. Pharmacol. Sci. 2006, 102, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullett, S.J.; Maio, R.D.; Greenamyre, J.T.; Hinkle, D.A. DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress. J. Mol. Neurosci. 2013, 49, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Cui, T.; Fan, C.; Zhao, H.; Zhao, C.; Lu, L.; Yang, H. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem. Biophys. Res. Commun. 2009, 383, 469–474. [Google Scholar] [CrossRef]
- Saeed, U.; Ray, A.; Valli, R.K.; Kumar, A.M.R.; Ravindranath, V. DJ-1 loss by glutaredoxin but not glutathione depletion triggers Daxx translocation and cell death. Antioxid. Redox Signal. 2010, 13, 127–144. [Google Scholar] [CrossRef]
- Sekito, A.; Yoshida, S.; Niki, T.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. DJ-1 interacts with HIPK1 and affects H2O2-induced cell death. Free Radic. Res. 2006, 40, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ren, H.; Jia, N.; Fei, H.; Zhou, T.; Jiang, P.; Wu, M.; Wang, G. DJ-1 Decreases Bax Expression through repressing p53 transcriptional activity. J. Biol. Chem. 2008, 283, 4022–4030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretaud, S.; Allen, C.; Ingham, P.W.; Bandmann, O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson’s disease. J. Neurochem. 2007, 100, 1626–1635. [Google Scholar] [CrossRef]
- Kato, I.; Maita, H.; Takahashi-Niki, K.; Saito, Y.; Noguchi, N.; Iguchi-Ariga, S.M.; Ariga, H. Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol. Cell Biol. 2013, 33, 340–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseur, S.; Afzal, S.; Tomasini, R.; Guillaumond, F.; Tardivel-Lacombe, J.; Mak, T.W.; Iovanna, J.L. Consequences of DJ-1 upregulation following p53 loss and cell transformation. Oncogene 2012, 31, 664–6670. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Niki, K.; Ganaha, Y.; Niki, T.; Nakagawa, S.; Kato-Ose, I.; Iguchi-Ariga, S.M.M.; Ariga, H. DJ-1 activates SIRT1 through its direct binding to SIRT1. Biochem. Biophys. Res. Commun. 2016, 474, 131–136. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Zhou, W.; Li, D.; Zhang, H.; Chen, Y.; Ning, L.; Zhang, Y.; Li, S.; Yu, M.; et al. Deficiency in the anti-apoptotic protein DJ-1 promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via p53. J. Biol. Chem. 2020, 295, 4237–4251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Luo, C.; Lin, K.; Bu, F.; Ye, F.; Huang, C.; Luo, H.; Huang, J.; Zhu, Z. Overexpression of DJ-1 enhances colorectal cancer cell proliferation through the cyclin-D1/MDM2-p53 signaling pathway. Biosci. Trends 2020, 14, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Kim, H.S.; Kim, A.R.; Kim, J.H.; Kim, B.; Noh, G.; Kim, H.S.; Beaven, M.A.; Kim, Y.M.; Choi, W.S. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J. Allergy Clin. Immunol. 2012, 131, 1653–1662. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Won, K.J.; Lee, K.P.; Lee, D.H.; Yu, S.; Lee, D.Y.; Seo, F.H.; Kang, H.; Park, F.S.; Kim, H.J.; et al. DJ-1 protein regulates CD3+ T cell migration via overexpression of CXCR4 receptor. Atherosclerosis 2014, 235, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.E.M.; Waak, J.; Weber, S.S.; Fiesel, F.C.; Oberhettinger, P.; Schütz, M.S.; Autenrieth, I.B.; Springer, W.S.; Kahle, P.J. Parkinson’s disease-associated DJ-1 modulates innate immunity signaling in Caenorhabditis elegans. J. Neural. Transm. 2010, 117, 599–604. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.; Xie, C.; Wang, W.N. LvDJ-1 plays an important role in resistance against Vibrio alginolyticus in Litopenaeus vannamei. Fish Shellfish Immunol. 2015, 44, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Amatullah, H.; Maron-Gutierrez, T.; Shan, Y.; Gupta, S.; Tsoporis, J.N.; Varkouhi, A.K.; Teixeira, A.P.; Monteiro He, X.; Yin, J.; Marshall, J.C.; et al. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2020, 38, 101796. [Google Scholar] [CrossRef]
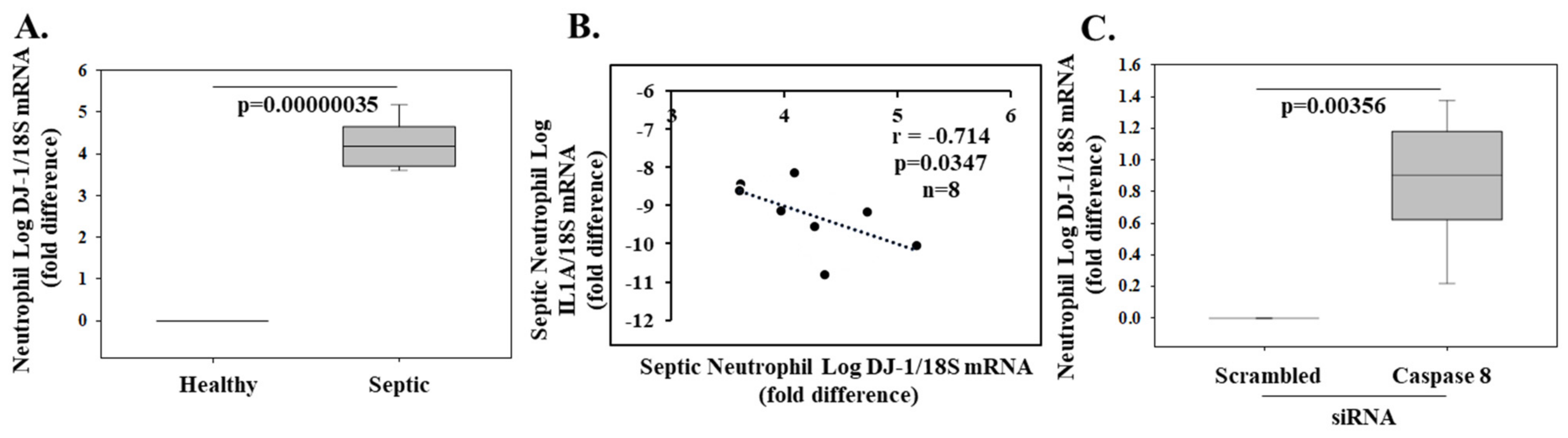
- Amatullah, H.; Shan, Y.; Beauchamp, B.L.; Gali, P.L.; Sahil Gupta, S.; Maron-Gutierrez, T.; Speck, E.R.; Fox-Robichaud, A.E.; Tsang, J.L.Y.; Mei, S.H.J.; et al. DJ-1/PARK7 impairs bacterial clearance in sepsis. Am. J. Crit. Care Med. 2017, 195, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lee, C.M.; Wang, J.F.; Parodo, J.; Jia, S.H.; Hu, J.; Marshall, J.C. Heat-shock protein-90 prolongs septic neutrophil survival by protecting c-Src kinase and caspase-8 from proteasomal degradation. J. Leukoc. Biol. 2018, 103, 933–944. [Google Scholar] [CrossRef]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef]
- Lu, H.S.; Chen, H.P.; Wang, S.; Yu, H.H.; Huang, X.S.; Huang, Q.R.; He, M. Hypoxic preconditioning up-regulates DJ-1 protein expression in rat heart-derived H9c2 cells through the activation of extracellular-regulated kinase 1/2 pathway. Mol. Cell Biochem. 2012, 370, 231–240. [Google Scholar] [CrossRef]
- Yan, Y.F.; Chen, H.P.; Huang, X.S.; Qiu, L.Y.; Liao, Z.P.; Huang, Q.R. DJ-1 Mediates the Delayed Cardioprotection of Hypoxic Preconditioning Through Activation of Nrf2 and Subsequent Upregulation of Antioxidative Enzymes. J. Cardiovasc. Pharmacol. 2015, 66, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.H.; Liu, W.J.; Song, T.; Zhang, L. Overexpression of DJ-1 expression protects cardiomyocyte apoptosis induced by ischemia reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1722–1729. [Google Scholar]
- Wang, H.; Li, Y.Y.; Qiu, L.Y.; Yan, Y.F.; Liao, Z.P.; Chen, H.P. Involvement of DJ-1 in ischemic preconditioning-induced delayed cardioprotection in vivo. Mol. Med. Rep. 2017, 15, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Dongworth, R.K.; Mukherjee, U.A.; Hall, A.R.; Astin, R.; Ong, S.B.; Yao, Z.; Dyson, A.; Szabadkai, G.; Davidson, S.M.; Yellon, D.M.; et al. DJ-1 protects against cell death following acute cardiac ischemia-reperfusion injury. Cell Death Dis. 2014, 5, e1082. [Google Scholar] [CrossRef] [Green Version]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The Future Challenge of Reactive Oxygen Species (ROS) in Hypertension: From Bench to Bed Side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef] [Green Version]
- Won, K.J.; Jung, S.H.; Jung, S.H.; Lee, K.P.; Lee, H.M.; Lee, D.Y.; Park, E.S.; Kim, J.; Kim, B. DJ-1/park7 modulates vasorelaxation and blood pressure via epigenetic modification of endothelial nitric oxide synthase. Cardiovasc. Res. 2014, 101, 473–481. [Google Scholar] [CrossRef] [Green Version]
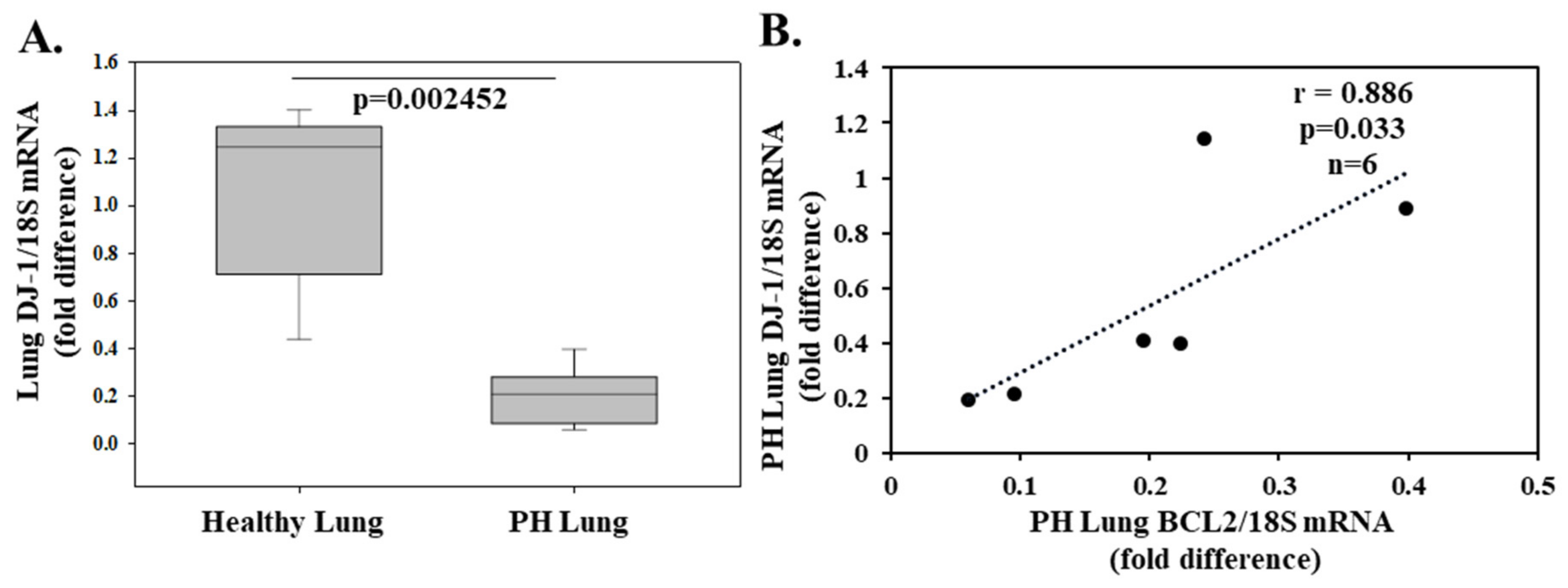
- Gao, W.; Shao, R.; Zhang, X.; Liu, D.; Liu, Y.; Fa, X. Up-regulation of caveolin-1 by DJ-1 attenuates rat pulmonary arterial hypertension by inhibiting TGFβ/Smad signaling pathway. Exp. Cell Res. 2017, 361, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Murao, S.; Kozuka, M.; Shimizu, M.; Suzuki, J.; Kubo, C.; Yamaguchi, A.; Musashi, M.; Minegishi, Y.; Momose, I.; et al. Serum DJ-1 level is positively associated with improvements in some aspects of metabolic syndrome in Japanese women through lifestyle intervention. Nutr. Res. 2014, 34, 851–855. [Google Scholar] [CrossRef]
- Rizos, I.K.; Tsoporis, J.N.; Toumpoulis, I.K.; Salpeas, V.; Izhar, S.; Rigopoulos, A.G.; Sakadakis, E.A.; Parker, T.G. Antiapoptotic Effect of beta1 Blockers in Ascending Thoracic Aortic Smooth Muscle Cells: The Role of HSP70 Expression. J. Cardiovasc. Pharmacol. 2018, 72, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Jiang, J.; Dong, B.; Tan, W.; Sun, Y.; Zhao, J.; Chen, Y.; Dong, Y.; Liu, C. DJ-1 activates autophagy in the repression of cardiac hypertrophy. Arch. Biochem. Biophys. 2017, 633, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nicholson, C.K.; Polavarapu, R.; Pantner, Y.; Husain, A.; Naqvi, N.; Chin, L.S.; Li, L.; Calvert, J.W. Role of DJ-1 in modulating glycative stress in heart failure. J. Am. Heart Assoc. 2020, 9, e014691. [Google Scholar] [CrossRef] [PubMed]
- Tsirebolos, G.; Tsoporis, J.N.; Rigopoulos, A.; Sakadakis, E.; Simoudis, A.; Izhar, S.; Parker, T.G.; Rizos, I.K. Decreased level of the soluble form of the receptor for advanced glycation end products is associated with increased risk for coronary artery disease in non-diabetic patients. Circulation 2019, 140, A16247. [Google Scholar]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Rao, S.P.; Kalivendi, S.V. The deglycase activity of DJ-1 mitigates α-synuclein glycation and aggregation in dopaminergic cells: Role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radic. Biol. Med. 2019, 135, 28–37. [Google Scholar] [CrossRef]
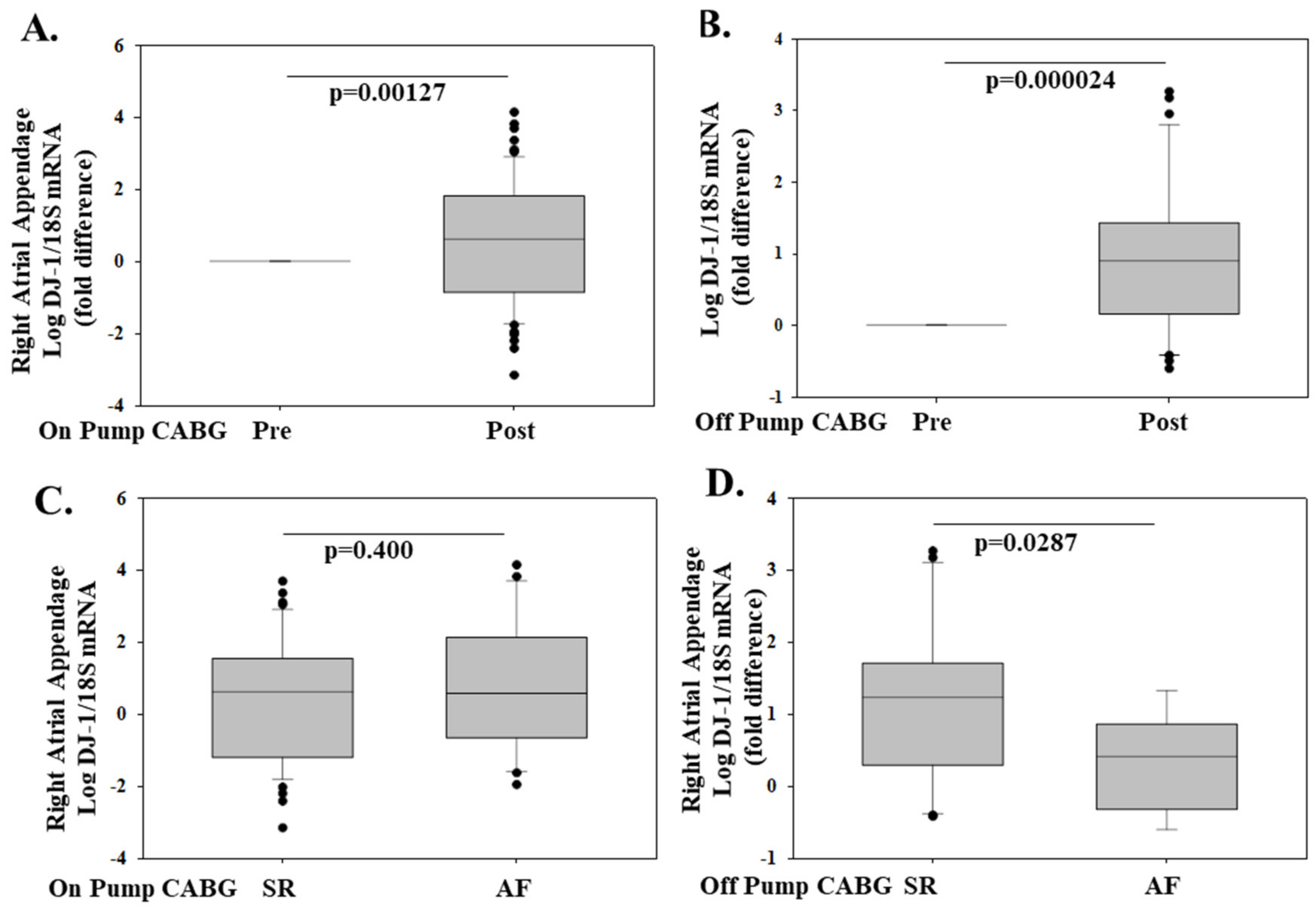
- Tsoporis, J.N.; Fazio, A.; Rizos, I.K.; Izhar, S.; Proteau, G.; Salpeas, V.; Rigopoulos, A.; Sakadakis, E.; Toumpoulis, I.K.; Parker, T.G. Increased right atrial appendage apoptosis is associated with differential regulation of candidate MicroRNAs 1 and 133A in patients who developed atrial fibrillation after cardiac surgery. J. Mol. Cell. Cardiol. 2018, 121, 25–32. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoporis, J.N.; Drosatos, I.-A.; Gupta, S.; Amatullah, H.; Izhar, S.; dos Santos, C.C.; Salpeas, V.; Rigopoulos, A.G.; Toumpoulis, I.K.; Triantafyllis, A.S.; et al. Cytoprotective Mechanisms of DJ-1: Implications in Cardiac Pathophysiology. Molecules 2021, 26, 3795. https://doi.org/10.3390/molecules26133795
Tsoporis JN, Drosatos I-A, Gupta S, Amatullah H, Izhar S, dos Santos CC, Salpeas V, Rigopoulos AG, Toumpoulis IK, Triantafyllis AS, et al. Cytoprotective Mechanisms of DJ-1: Implications in Cardiac Pathophysiology. Molecules. 2021; 26(13):3795. https://doi.org/10.3390/molecules26133795
Chicago/Turabian StyleTsoporis, James N., Ioannis-Alexandros Drosatos, Sahil Gupta, Hajera Amatullah, Shehla Izhar, Claudia C. dos Santos, Vasileos Salpeas, Angelos G. Rigopoulos, Ioannis K. Toumpoulis, Andreas S. Triantafyllis, and et al. 2021. "Cytoprotective Mechanisms of DJ-1: Implications in Cardiac Pathophysiology" Molecules 26, no. 13: 3795. https://doi.org/10.3390/molecules26133795






