Self-Assembled Bimetallic Aluminum-Salen Catalyst for the Cyclic Carbonates Synthesis
Abstract
:1. Introduction
2. Results and Discussion
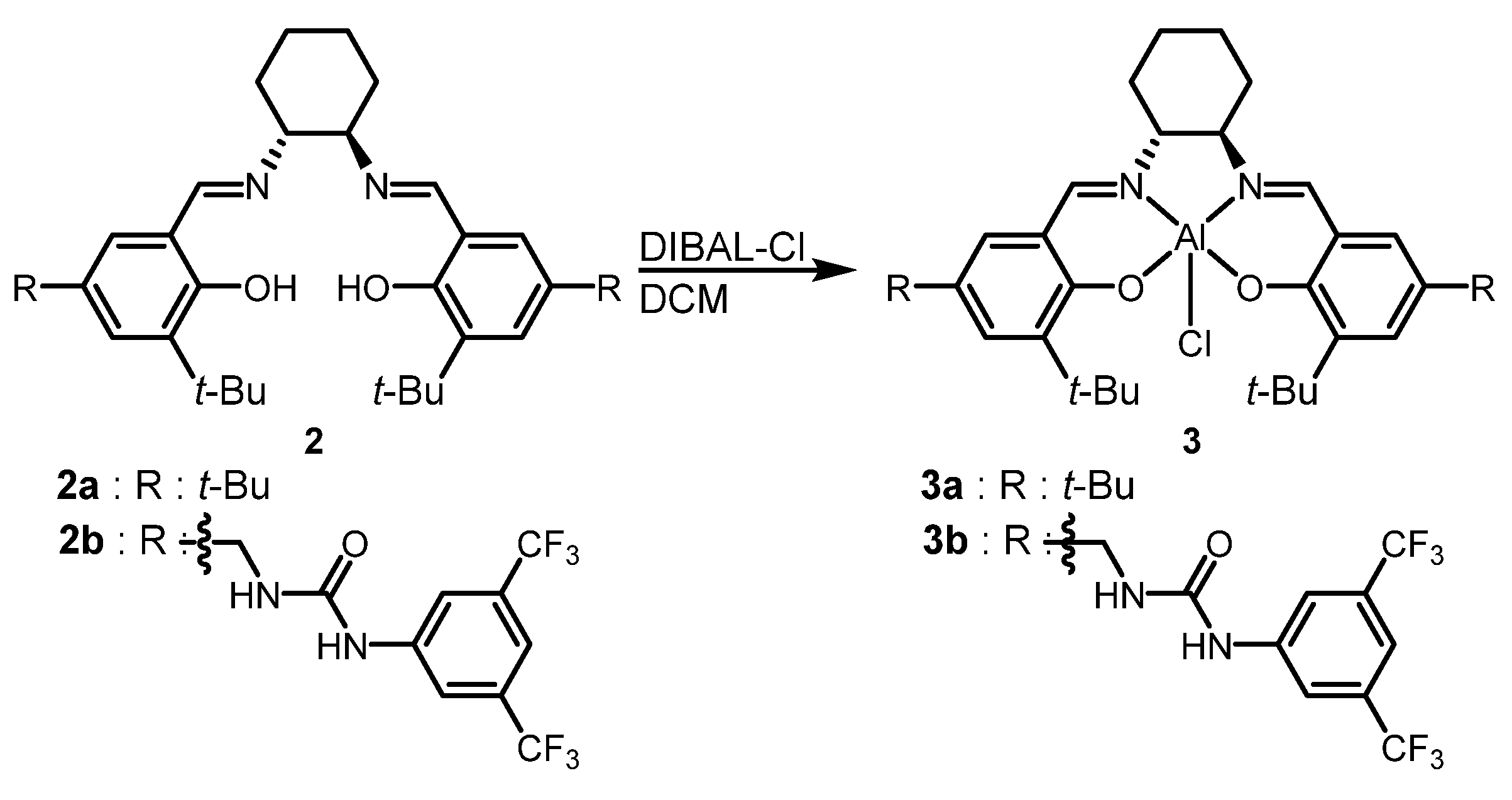
2.1. Catalyst Preparation
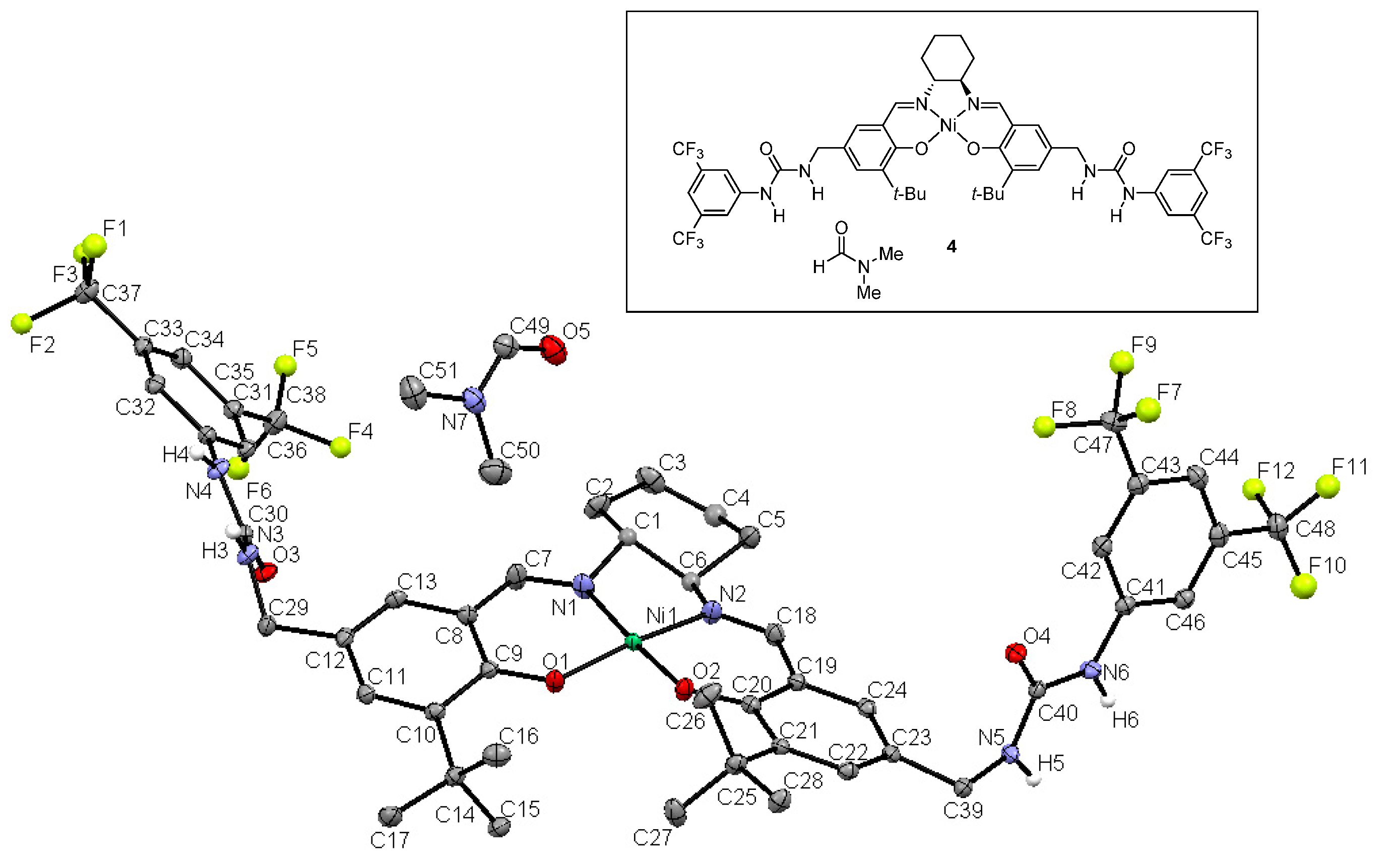
2.2. X-ray Crystallography
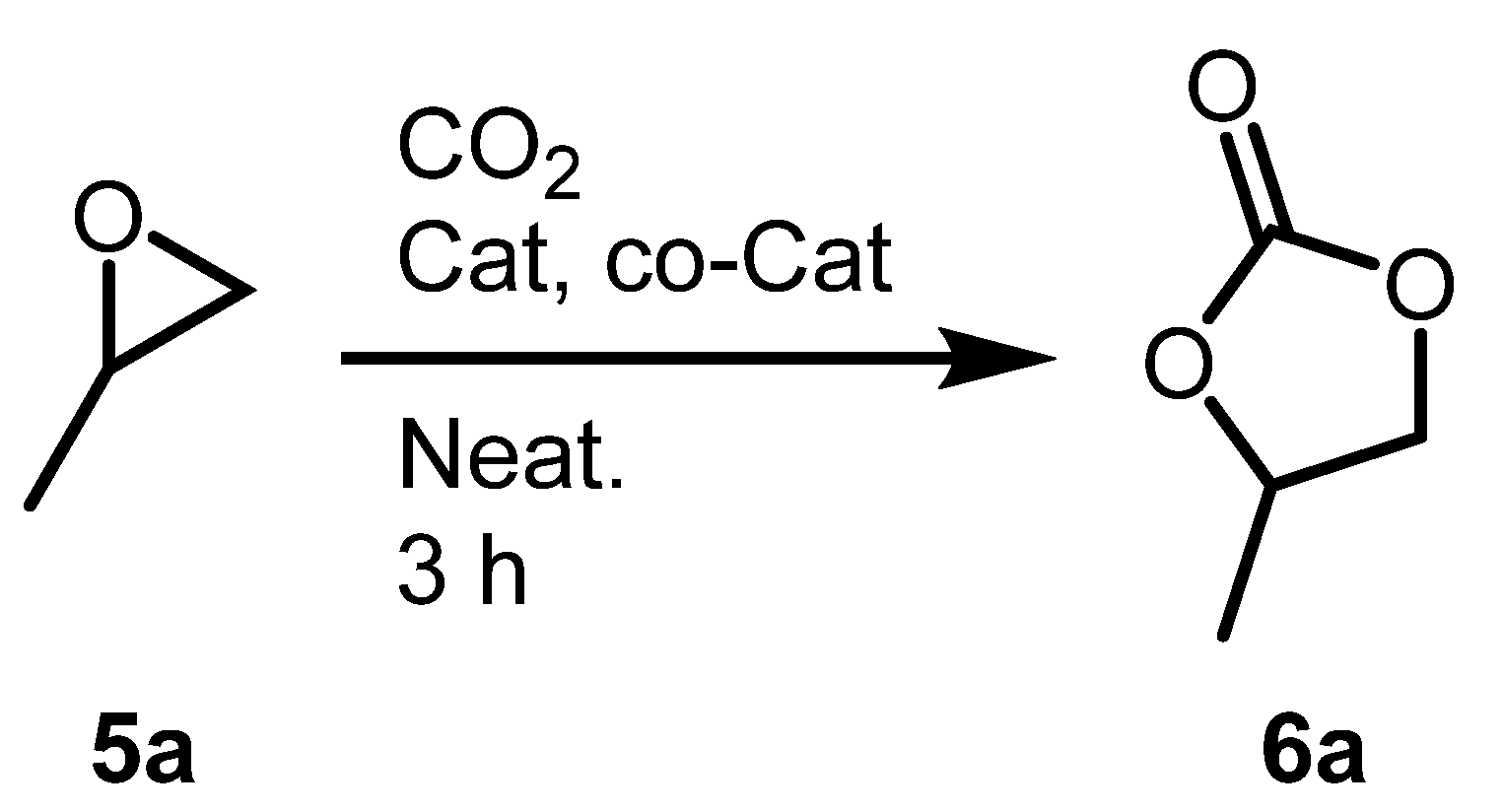
2.3. Optimization of Cyclic Carbonates Synthesis Reaciton Conditions
2.4. Cyclic Carbonate Synthesis Reactions for Various Epoxides
2.5. Origin of Beneficial Urea Effects
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Arenas, L.F.V.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Clements, J.H. Reactive Applications of Cyclic Alkylene Carbonates. Ind. Eng. Chem. Res. 2003, 42, 663–674. [Google Scholar] [CrossRef]
- Paul, S.; Romain, C.; Shaw, J.; Williams, C.K. Sequence Selective Polymerization Catalysis: A New Route to ABA Block Copoly(ester-b-carbonate-b-ester). Macromolecules 2015, 48, 6047–6056. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, H.-B.; Yue, J.-M. Organic Carbonates from Natural Sources. Chem. Rev. 2014, 114, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Zhang, L.; Qu, Q.; Liu, H.; Zhang, X.; Shen, M.; Zheng, H. A Binary Cyclic Carbonates-Based Electrolyte Containing Propylene Carbonate and Trifluoropropylene Carbonate for 5V Lithium-Ion batteries. Electrochim. Acta 2015, 167, 151–159. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalyst 2020, 10, 479. [Google Scholar] [CrossRef]
- Beattie, C.; North, M.; Villuendas, P. Proline-Catalysed Amination Reactions in Cyclic Carbonate Solvents. Molecules 2011, 16, 3420–3432. [Google Scholar] [CrossRef]
- Laserna, V.; Fiorani, G.; Whiteoak, C.J.; Martin, E.; Escudero-Adán, E.; Kleij, A.W. Carbon dioxide as a Protecting Group: Highly Efficient and Selective Catalytic Access to Cyclic cis-Diol Scaffolds. Angew. Chem. Int. Ed. 2014, 53, 10416–10419. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J. Environ. Chem. Eng. 2021, 9, 105113–105130. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-Complex-Mediated Formation of Cyclic Carbonates by Cycloaddition of CO2 to Epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822–9837. [Google Scholar] [CrossRef] [PubMed]
- Rintjema, J.; Epping, R.; Fiorani, G.; Martin, E.; Escudero-Adán, E.; Kleij, A.W. Substrate-Controlled Product Divergence: Conversion of CO2 into Heterocyclic Products. Angew. Chem. Int. Ed. 2016, 55, 3972–3976. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hyun, K.; Ahn, D.; Kim, R.; Park, M.; Kim, Y. Efficient Aluminum Catalysts for the Chemical Conversion of CO2 into Cyclic Carbonates at Room Temperature and Atmospheric CO2 Pressure. ChemSusChem 2019, 12, 4211–4220. [Google Scholar] [CrossRef]
- Noh, J.; Kim, Y.; Park, H.; Lee, J.; Yoon, M.; Park, M.; Kim, Y.; Kim, M. Functional group effects on a metal-organic framework catalyst for CO2 cycloaddition. J. Ind. Eng. Chem. 2018, 64, 478–483. [Google Scholar] [CrossRef]
- Hong, M.; Kim, Y.; Kim, H.; Cho, H.; Baik, M.; Kim, Y. Scorpionate Catalysts for Coupling CO2 and Epoxides to Cyclic Carbonates: A Rational Design Approach for Organocatalysts. J. Org. Chem. 2018, 83, 9370–9380. [Google Scholar] [CrossRef] [PubMed]
- Man, M.L.; Lam, K.C.; Sit, W.N.; Ng, S.M.; Zhou, Z.; Lin, Z.; Lau, C.P. Synthesis of Heterobimetallic Ru-Mn Complexes and the Coupling Reactions of Epoxides with Carbon Dioxide Catalyzed by these Complexes. Chem. Eur. J. 2006, 12, 1004–1015. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Tang, N. Synthesis, characterization of a new bicobalt complex [Co2L2(C2H5OH)2Cl2] and application in cyclic carbonate synthesis. Inorg. Chem. Commun. 2007, 10, 1493–1495. [Google Scholar] [CrossRef]
- Meléndez, J.; North, M.; Pasquale, R. Synthesis of Cyclic Carbonates from Atmospheric Pressure Carbon Dioxide Using Exceptionally Active Aluminium(salen) Complexes as Catalysts. Eur. J. Inorg. Chem. 2007, 3323–3326. [Google Scholar] [CrossRef]
- Clegg, W.; Harrington, R.W.; Pasquale, R.; North, M. Cyclic carbonate synthesis catalysed by bimetallic aluminium-salen complexes. Chem. Eur. J. 2010, 16, 6828–6843. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Chuang, H.-J.; Ko, B.-T. Bimetallic bis(benzotriazole iminophenolate) cobalt, nickel and zinc complexes as versatile catalysts for coupling of carbon dioxide with epoxides and copolymerization of phthalic anhydride with cyclohexene oxide. Catal. Sci. Technol. 2016, 6, 1779–1791. [Google Scholar] [CrossRef]
- Cozzolino, M.; Press, K.; Mazzeo, M.; Lamberti, M. Carbon dioxide/epoxide reaction catalyzed by bimetallic salalen aluminum complexes. ChemCatChem 2016, 8, 455–460. [Google Scholar] [CrossRef]
- Cozzolino, M.; Melchionno, F.; Santulli, F.; Mazzeo, M.; Lamberti, M. Aldimine-thioether-phenolate based mono- and bimetallic zinc complexes as catalysts for the reaction of CO2 with cyclohexene oxide. Eur. J. Inorg. Chem. 2020, 2020, 1645–1653. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Martin, E.; Escudero-Adán, E.; Kleij, A.W. Stereochemical Divergence in the Formation of Organic Carbonates Derived from Internal Epoxides. Adv. Synth. Catal. 2013, 355, 2233–2239. [Google Scholar] [CrossRef]
- Buonerba, A.; Nisi, A.D.; Grassi, A.; Milione, S.; Vagin, S.; Rieger, B.; Capacchione, C. Novel iron(III) catalyst for the efficient and selective coupling of carbon dioxide and epoxides to form cyclic carbonates. Catal. Sci. Technol. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Della Monica, F.; Vummaleti, S.V.C.; Buonerba, A.; De Nisi, A.; Monari, M.; Milione, S.; Grassi, A.; Cavallo, L.; Capacchione, C. Coupling of Carbon Dioxide with Epoxides Efficiently Catalyzed by Thioether-Triphenolate Bimetallic Iron(III) Complexes: Catalyst Structure–Reactivity Relationship and Mechanistic DFT Study. Adv. Synth. Catal. 2016, 358, 3231–3243. [Google Scholar] [CrossRef]
- Montoya, C.A.; Gómez, C.F.; Paninho, A.B.; Najdanovic-Visak, V.; Martins, L.M.D.R.S.; da Silva, M.F.C.G.; Pombeiro, A.J.L.; Ponte, M.N.; Nunes, A.V.M.; Mahmudov, K.T. Cyclic carbonate synthesis from CO2 and epoxides using zinc(II) complexes of arylhydrazones of β-diketones. J. Catal. 2016, 335, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An Efficient Iron Catalyst for the Synthesis of Five- and Six- Membered Organic Carbonates under Mild Conditions. Adv. Synth. Catal. 2012, 354, 469–476. [Google Scholar] [CrossRef]
- Arunachalam, R.; Chinnaraja, E.; Subramanian, S.; Suresh, E.; Subramanian, P.S. Catalytic Conversion of Carbon Dioxide Using Binuclear Double-Stranded Helicates: Cyclic Carbonate from Epoxides and Diol. ACS Omega 2020, 5, 14890–14899. [Google Scholar] [CrossRef]
- Maeda, C.; Taniguchi, T.; Ogawa, K.; Ema, T. Bifunctional Catalysts Based on m-Phenylene-Bridged Porphyrin Dimer and Trimer Platforms: Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides. Angew. Chem. Int. Ed. 2015, 54, 134–138. [Google Scholar] [CrossRef]
- He, S.; Wang, F.; Tong, W.-L.; Yiu, S.-M.; Chan, M.C.W. Topologically diverse shape-persistent bis-(Zn–salphen) catalysts: Efficient cyclic carbonate formation under mild conditions. Chem. Commun. 2016, 52, 1017–1020. [Google Scholar] [CrossRef]
- Park, J.; Hong, S. Cooperative bimetallic catalysis in asymmetric transformations. Chem. Soc. Rev. 2012, 41, 6931–6943. [Google Scholar] [CrossRef]
- Beattie, C.; Villuendas, P.; Young, C.; North, M. Influence of Temperature and Pressure on Cyclic Carbonate Synthesis Catalyzed by Bimetallic Aluminum Complexes and Application to Overall syn-Bis-hydroxylation of Alkenes. J. Org. Chem. 2013, 78, 419–426. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Leitner, W.; Muller, T.E.; North, M.; Offermans, W.K. Unprecedented Carbonato Intermediates in Cyclic Carbonate Synthesis Catalysed by Bimetallic Aluminium(Salen) Complexes. ChemSusChem 2016, 9, 791–794. [Google Scholar] [CrossRef]
- Wu, X.; North, M. A Bimetallic Aluminium(Salphen) Complex for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide. ChemSusChem 2017, 10, 74–78. [Google Scholar] [CrossRef]
- Park, J.; Lang, K.; Abboud, K.A.; Hong, S. Self-Assembly Approach toward Chiral Bimetallic Catalysts: Bis-Urea-Functionalized (Salen)Cobalt Complexes for the Hydrolytic Kinetic Resolution of Epoxides. Chem. Eur. J. 2011, 17, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Broghammer, F.; Brodbeck, D.; Junge, T.; Peters, R. Cooperative Lewis acid−onium salt catalysis as tool for the desymmetrization of meso-epoxides. Chem. Commun. 2017, 53, 1156–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacy, A.C.; Moreby, E.; Phanopoulos, A.; Williams, C.K. Co(III)/Alkali-Metal(I) Heterodinuclear Catalysts for the Ring-Opening Copolymerization of CO2 and Propylene Oxide. J. Am. Chem. Soc. 2020, 142, 19150–19160. [Google Scholar] [CrossRef] [PubMed]
- Deacy, A.C.; Durr, C.B.; Kerr, R.W.F.; Williams, C.K. Heterodinuclear catalysts Zn(II)/M and Mg(II)/M, where M = Na(I), Ca(II) or Cd(II), for phthalic anhydride/cyclohexene oxide ring opening copolymerisation. Catal. Sci. Technol. 2021, 11, 3109–3118. [Google Scholar] [CrossRef]
- Noh, E.; Na, S.; Sujith, S.; Kim, S.-W.; Lee, B. Two Components in a Molecule: Highly Efficient and Thermally Robust Catalytic System for CO2/Epoxide Copolymerization. J. Am. Chem. Soc. 2007, 129, 8082–8083. [Google Scholar] [CrossRef]
- Lidston, C.A.L.; Abel, B.A.; Coates, G.W. Bifunctional Catalysis Prevents Inhibition in Reversible-Deactivation Ring-Opening Copolymerizations of Epoxides and Cyclic Anhydrides. J. Am. Chem. Soc. 2020, 142, 20161–20169. [Google Scholar] [CrossRef]
- Hubbell, A.K.; Coates, G.W. Nucleophilic Transformations of Lewis Acid-Activated Disubstituted Epoxides with Catalyst-Controlled Regioselectivity. J. Org. Chem. 2020, 85, 13391–13414. [Google Scholar] [CrossRef] [PubMed]
- Quek, S.C.Z.; Pridmore, N.E.; Whitwood, A.C.; Wu, X.; North, M. Aluminum(salen) Complexes as Catalysts for the Kinetic Resolution of Terminal Epoxides via CO2 Coupling. ACS Catal. 2015, 5, 3398–3402. [Google Scholar]


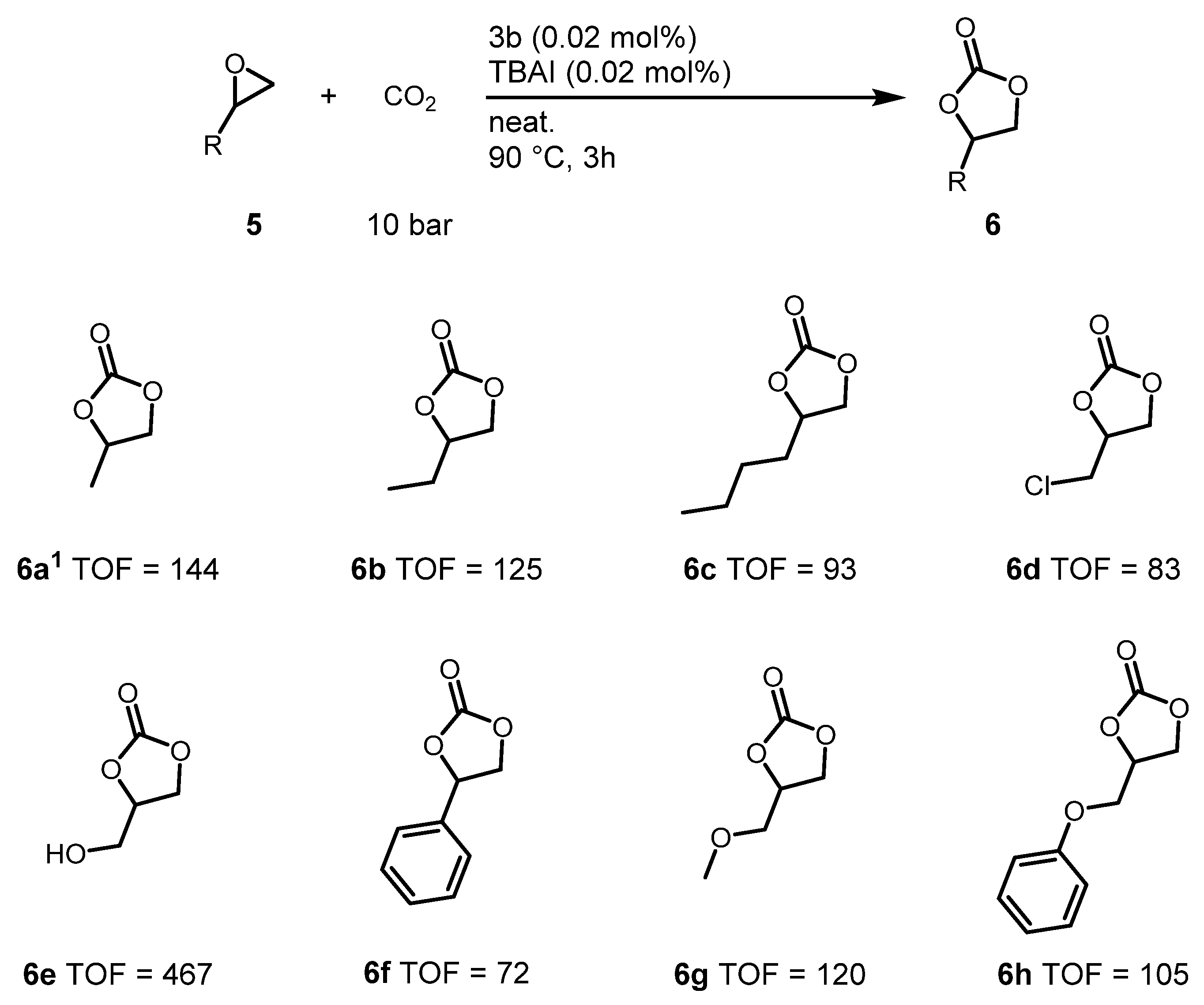
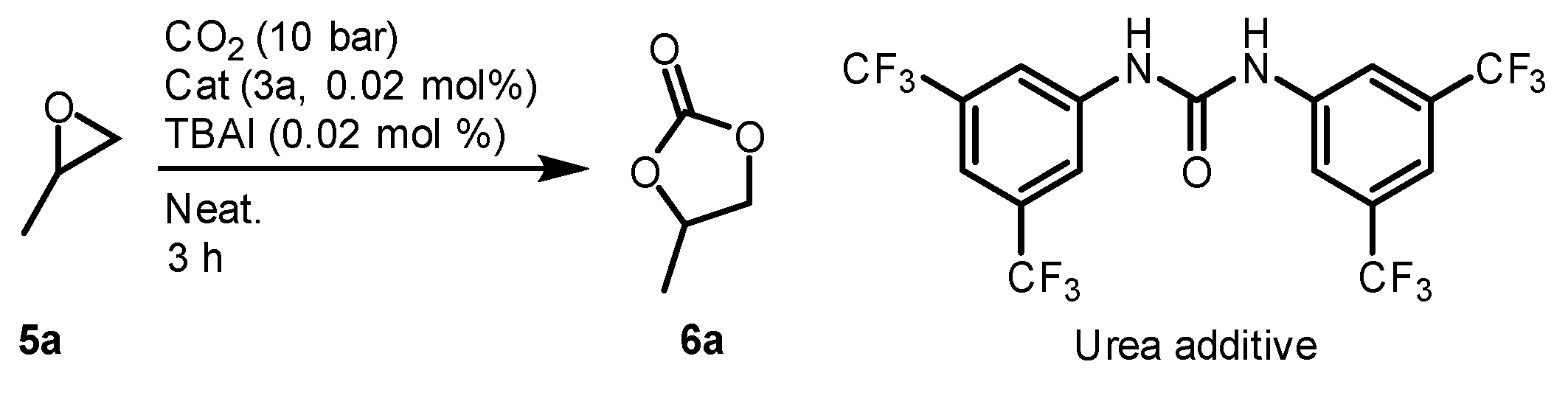


| Entry | Catalyst (mol%) | Cocatalyst (mol%) | CO2 (Bar) | T (°C) | TOF (h−1) 1 |
|---|---|---|---|---|---|
| 1 2 | 3a (1) | (n-Bu)4N+Br− (1) | 1 | 45 | 1 |
| 2 | 3b (0.02) | (n-Bu)4N+Br− (0.02) | 1 | 45 | 13 |
| 3 | 3b (0.02) | (n-Bu)4N+I− (0.02) | 1 | 45 | 17 |
| 4 | 3a (0.02) | (n-Bu)4N+I− (0.02) | 10 | 90 | 65 |
| 5 | 3b (0.02) | (n-Bu)4N+I− (0.02) | 10 | 90 | 144 |
| 6 | 1 (0.02) | (n-Bu)4N+I− (0.02) | 10 | 90 | 47 3 |
| Entry | Urea Additive (mol%) | TOF (h−1) |
|---|---|---|
| 1 | - | 64 |
| 2 | 0.08 mol% | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, W.; Hahm, H.; Kim, S.; Park, J.; Abboud, K.A.; Hong, S. Self-Assembled Bimetallic Aluminum-Salen Catalyst for the Cyclic Carbonates Synthesis. Molecules 2021, 26, 4097. https://doi.org/10.3390/molecules26134097
Seong W, Hahm H, Kim S, Park J, Abboud KA, Hong S. Self-Assembled Bimetallic Aluminum-Salen Catalyst for the Cyclic Carbonates Synthesis. Molecules. 2021; 26(13):4097. https://doi.org/10.3390/molecules26134097
Chicago/Turabian StyleSeong, Wooyong, Hyungwoo Hahm, Seyong Kim, Jongwoo Park, Khalil A. Abboud, and Sukwon Hong. 2021. "Self-Assembled Bimetallic Aluminum-Salen Catalyst for the Cyclic Carbonates Synthesis" Molecules 26, no. 13: 4097. https://doi.org/10.3390/molecules26134097






