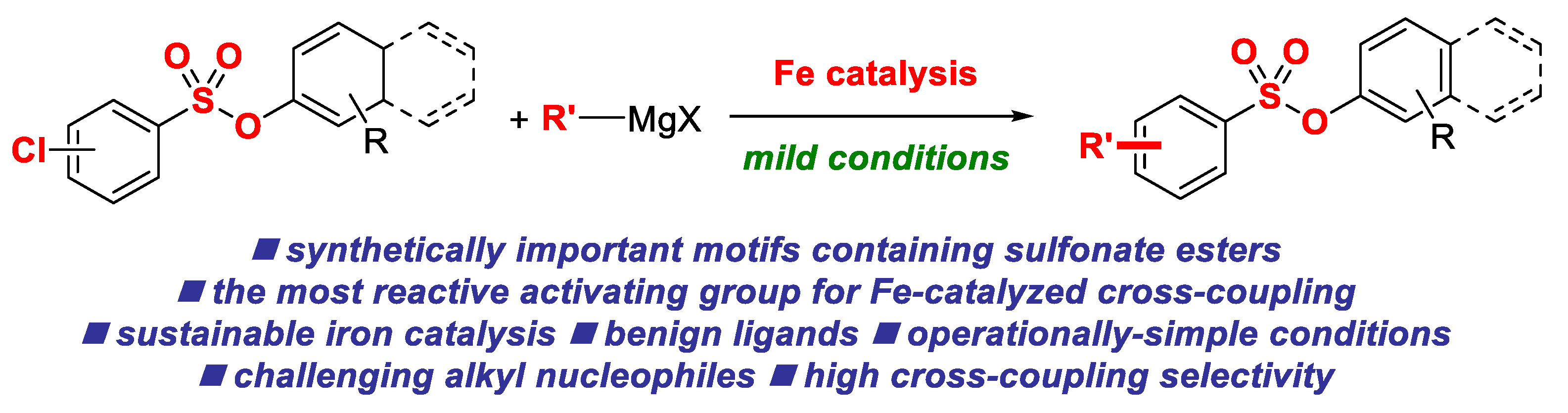
Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Chlorobenzenesulfonates
Abstract
:1. Introduction
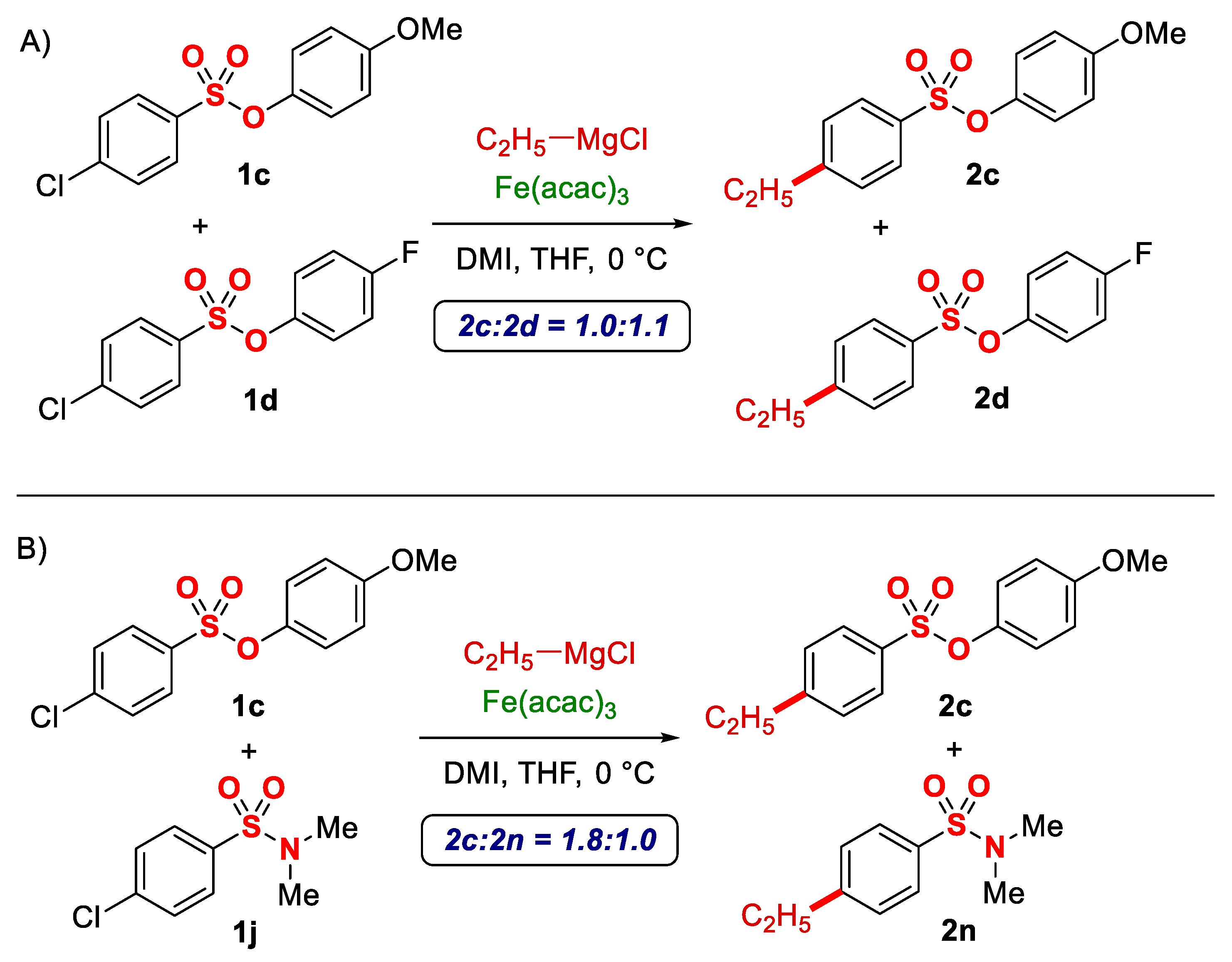
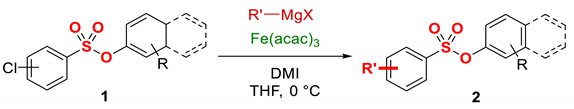
2. Results
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. General Procedure for Iron-Catalyzed C(sp2)–C(sp3) Cross-Coupling
4.3. General Procedure for Determination of Relative Reactivity
4.4. Characterization Data for Starting Materials
4.4.1. Phenyl 4-chlorobenzenesulfonate (1a)
4.4.2. 4-(tert-Butyl)phenyl 4-chlorobenzenesulfonate (1b)
4.4.3. 4-Methoxyphenyl 4-chlorobenzenesulfonate (1c)
4.4.4. 4-Fluorophenyl 4-chlorobenzenesulfonate (1d)
4.4.5. o-Tolyl 4-chlorobenzenesulfonate (1e)
4.4.6. 2,6-Dimethylphenyl 4-chlorobenzenesulfonate (1f)
4.4.7. Naphthalen-2-yl 4-chlorobenzenesulfonate (1g)
4.4.8. 4-Methoxyphenyl 3-chlorobenzenesulfonate (1h)
4.4.9. 4-Methoxyphenyl 2-chlorobenzenesulfonate (1i)
4.5. Characterization Data for Cross-Coupling Products
4.5.1. Phenyl 4-ethylbenzenesulfonate (2a)
4.5.2. 4-(tert-Butyl)phenyl 4-ethylbenzenesulfonate (2b)
4.5.3. 4-Methoxyphenyl 4-ethylbenzenesulfonate (2c)
4.5.4. 4-Fluorophenyl 4-ethylbenzenesulfonate (2d)
4.5.5. o-Tolyl 4-ethylbenzenesulfonate (2e)
4.5.6. 2,6-Dimethylphenyl 4-ethylbenzenesulfonate (2f)
4.5.7. Naphthalen-2-yl 4-ethylbenzenesulfonate (2g)
4.5.8. 4-Methoxyphenyl 3-ethylbenzenesulfonate (2h)
4.5.9. 4-Methoxyphenyl 2-ethylbenzenesulfonate (2i)
4.5.10. 4-Methoxyphenyl 4-cyclohexylbenzenesulfonate (2j)
4.5.11. 4-Methoxyphenyl 4-isopropylbenzenesulfonate (2k)
4.5.12. 4-Methoxyphenyl 4-phenethylbenzenesulfonate (2l)
4.5.13. 4-Methoxyphenyl 4-(2-(1,3-dioxan-2-yl)ethyl)benzenesulfonate (2m)
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Molander, G.A.; Wolfe, J.P.; Larhed, M. (Eds.) Science of Synthesis: Cross-Coupling and Heck-Type Reactions, 1st ed.; Thieme: Stuttgart, Germany, 2013. [Google Scholar]
- de Meijere, A.; Bräse, S.; Oestreich, M. (Eds.) Metal-Catalyzed Cross-Coupling Reactions and More, 1st ed.; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Colacot, T.J. (Ed.) New Trends in Cross-Coupling, 1st ed.; The Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Fürstner, A.; Martin, R. Advances in Iron Catalyzed Cross Coupling Reactions. Chem. Lett. 2005, 34, 624–629. [Google Scholar] [CrossRef]
- Sherry, B.D.; Fürstner, A. The Promise and Challenge of Iron-Catalyzed Cross Coupling. Acc. Chem. Res. 2008, 41, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Czaplik, W.M.; Mayer, M.; Cvengros, J.; Jacobi von Wangelin, A. Coming of Age: Sustainable Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2009, 2, 396–417. [Google Scholar] [CrossRef] [PubMed]
- Plietker, B. Topic in Organometallic Chemistry Also Available Electronically. In Iron Catalysis–Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 33. [Google Scholar]
- Bauer, E.B. Iron Catalysis II. Top. Organomet. Chem.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 50. [Google Scholar]
- Marek, I.; Rappoport, Z. The Chemistry of Organoiron Compounds; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Bauer, I.; Knölker, H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef]
- Bakas, N.J.; Neidig, M.L. Additive and Counterion Effects in Iron-Catalyzed Reactions Relevant to C-C Bond Formation. ACS Catal. 2021, 11, 8493–8503. [Google Scholar] [CrossRef]
- Fürstner, A. Base-Metal Catalysis Marries Utilitarian Aspects with Academic Fascination. Adv. Synth. Catal. 2016, 358, 2362. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Giri, R.; Thapa, S.; Kafle, A. Palladium- Catalysed, Directed C-H Coupling with Organometallics. Adv. Synth. Catal. 2014, 356, 1395–1411. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef] [Green Version]
- Piontek, A.; Bisz, E.; Szostak, M. Iron-Catalyzed Cross-Coupling in the Synthesis of Pharmaceuticals: In Pursuit of Sustainability. Angew. Chem. Int. Ed. 2018, 57, 11116–11128. [Google Scholar] [CrossRef]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Bostrçm, J. Analysis of Past and Present Synthetic Methodologies in Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2016, 59, 4443–4458. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.; Lowe, D.M.; Sayle, R.A.; Tarselli, M.A.; Landrum, G.A. Big Data from Pharmaceuticals Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter. J. Med. Chem. 2016, 59, 4385–4402. [Google Scholar] [CrossRef]
- Cahiez, G.; Avedissian, H. Highly Stereo- and Chemoselective Iron-Catalyzed Alkenylation of Organomagnesium Compounds. Synthesis 1998, 1199–1205. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. Cyclic Ureas (DMI, DMPU) as Efficient, Sustainable Ligands in Iron-Catalyzed C(sp2)–C(sp3) Coupling of Aryl Chlorides and Tosylates. Green Chem. 2017, 19, 5361–5366. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. 2-Methyltetrahydrofuran: A Green Solvent for Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2018, 11, 1290–1294. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. Iron-Catalyzed C(sp2)–C(sp3) Cross-Coupling of Chlorobenzenesulfonamides with Alkyl Grignard Reagents: Entry to Alkylated Aromatics. J. Org. Chem. 2018, 84, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Bisz, E.; Szostak, M. Iron-Catalyzed C(sp2)–C(sp3) Cross-Coupling of Chlorobenzamides with Alkyl Grignard Reagents: Development of Catalyst System, Synthetic Scope and Application. Adv. Synth. Catal. 2019, 361, 85–95. [Google Scholar] [CrossRef]
- Yan, L.; Müller, C.E. Preparation, Properties, Reactions, and Adenosine Receptor Affinities of Sulfophenylxanthine Nitrophenyl Esters: Toward the Development of Sulfonic Acid Prodrugs with Peroral Bioavailability. J. Med. Chem. 2004, 47, 1031–1043. [Google Scholar] [CrossRef]
- Zuse, A.; Schmidt, P.; Baasner, S.; Böhm, K.J.; Müller, K.; Gerlach, M.; Günther, E.G.; Unger, E.; Prinz, H. Sulfonate Derivatives of Naphtho[2,3-b]thiophen-4(9H)-one and 9(10H)-anthracenone as Highly Active Antimicrotubule Agents. Synthesis, Antiproliferative Activity, and Inhibition of Tubulin Polymerization. J. Med. Chem. 2007, 50, 6059–6066. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, G.-E.; Lee, S.-D.; Hien, T.T.; Kim, S.; Yang, J.W.; Cho, J.-H.; Ko, H.; Lim, S.-C.; Kim, Y.-G.; et al. Discovery of Novel 2,5-dioxoimidazolidine-based P2X(7) Receptor Antagonists as Constrained Analogues of KN62. J. Med. Chem. 2015, 58, 2114–2134. [Google Scholar] [CrossRef]
- Pisani, L.; Barletta, M.; Soto- Otero, R.; Nicolotti, O.; Mendez-Alvarez, E.; Catto, M.; Introcaso, A.; Stefanachi, A.; Cellamare, S.; Altomare, C.; et al. Discovery, Biological Evaluation, and Structure-Activity and -Selectivity Relationships of 6′-Substituted (E)-2-(Benzofuran3(2H)-ylidene)-N-methylacetamides, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors. J. Med. Chem. 2013, 56, 2651–2664. [Google Scholar] [CrossRef]
- Betts, L.M.; Tam, N.C.; Kabir, S.M.; Langer, R.F.; Crandall, I. Ether Aryl Sylfonic acid Esters with Improved Anticancer/Antimalarial Activities. Aust. J. Chem. 2006, 59, 277–282. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Alam, M.S.; Koo, S. Deprotection of Durable Benzenesulfonyl Protection for Phenols-Efficient Synthesis of Polyphenols. Synth. Commun. 2018, 48, 247–254. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. Iron-Catalyzed C-O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. [Google Scholar] [CrossRef]
- Zhou, T.; Szostak, M. Palladium-Catalyzed Cross-Couplings by C–O Bond Activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, Z.; Di, X.; Huang, Q.; Wang, Y.; Zhu, J. Pd(NHC)(cinnamyl)Cl-Catalyzed Suzuki Cross-Coupling Reaction of Aryl Sulfonates with Arylboronic Acids. Mol. Divers. 2020, 24, 903–911. [Google Scholar] [CrossRef]
- Piontek, A.; Ochędzan-Siodłak, W.; Bisz, E.; Szostak, M. Nickel-Catalyzed C(sp2)−C(sp3) Kumada Cross-Coupling of Aryl Tosylates with Alkyl Grignard Reagents. Adv. Synth. Catal. 2019, 361, 2329–2336. [Google Scholar] [CrossRef]
- Piontek, A.; Ochędzan-Siodłak, W.; Bisz, E.; Szostak, M. Cobalt-NHC Catalyzed C(sp2)−C(sp3) and C(sp2)−C(sp2) Kumada Cross-Coupling of Aryl Tosylates with Alkyl and Aryl Grignard Reagents. ChemCatChem 2021, 13, 202–206. [Google Scholar] [CrossRef]
- Ahmed, N.; Dubuc, C.; Rousseau, J.; Benard, F.; van Lier, J. Synthesis, Characterization, and Estrogen Receptor Binding Affinity of Flavone-, Indole-, and Furan-estradiol Conjugates. Bioorg. Med. Chem. Lett. 2007, 17, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- Bensulong, S.; Boonsombat, J.; Ruchirawat, S. DBU-mediated Regioselective Intramolecular Cyclization/Dehydration of Ortho Diketo Phenoxyethers: A Synthesis of 2,3-substituted g-benzopyranones. Tetrahedron 2013, 69, 9335–9348. [Google Scholar] [CrossRef]
- Avitabile, B.G.; Smith, C.A.; Judd, D.B. Pentafluorophenyl Sulfonate Ester as a Protecting Group for the Preparation of Biaryl- and Heterobiaryl Sulfonate Esters. Org. Lett. 2005, 7, 843–846. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. Iron- Catalyzed C(sp2)–C(sp3) Cross-Coupling of Aryl Chlorobenzoates with Alkyl Grignard Reagents. Molecules 2020, 25, 230. [Google Scholar] [CrossRef] [Green Version]
- Jesso, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A 2015, 471, 502–527. [Google Scholar] [CrossRef] [Green Version]
- Fürstner, A.; Leitner, A. Iron-Catalyzed Cross-Coupling Reactions of Alkyl-Grignard Reagents with Aryl Chlorides, Tosylates, and Triflates. Angew. Chem. Int. Ed. 2002, 41, 609–612. [Google Scholar] [CrossRef]
- Fürstner, A.; Leitner, A.; Mendez, M.; Krause, H. Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863. [Google Scholar] [CrossRef] [PubMed]
- Czaplik, W.M.; Mayer, M.; Jacobi von Wangelin, A. Domino Iron Catalysis: Direct Aryl-Alkyl Cross-Coupling. Angew. Chem. Int. Ed. 2009, 48, 607–610. [Google Scholar] [CrossRef]
- Gülak, S.; Jacobi von Wangelin, A. Chlorostyrenes in Iron-Catalyzed Biaryl Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, O.M.; Steib, A.K.; Markiewicz, J.T.; Flubacher, D.; Knochel, P. Ligand-Accelerated Iron- and Cobalt-Catalyzed Cross-Coupling Reactions between N-Heteroaryl Halides and Aryl Magnesium Reagents. Angew. Chem. Int. Ed. 2013, 52, 4945–4949. [Google Scholar] [CrossRef]
- Fürstner, A.; Martin, R.; Krause, H.; Seidel, G.; Goddard, R.; Lehmann, C.W. Preparation, Structure, and Reactivity of Nonstabilized Organoiron Compounds. Implications for Iron-Catalyzed Cross Coupling Reactions. J. Am. Chem. Soc. 2008, 130, 8773–8787. [Google Scholar] [CrossRef] [Green Version]
- Cassani, C.; Bergonzini, G.; Wallentin, C.J. Active Species and Mechanistic Pathways in Iron-Catalyzed C–C Bond-Forming Cross-Coupling Reactions. ACS Catal. 2016, 6, 1640–1648. [Google Scholar] [CrossRef]
- Gilman, H.; Beaber, N.J.; Meyers, C.H. The Reaction Between Aryl Sulfonates and Organomagnesium Halides. J. Am. Chem. Soc. 1925, 47, 2047–2052. [Google Scholar] [CrossRef]
- Liebman, J.; Greenberg, A. The Origin of Rotational Barriers in Amides and Esters. Biophys. Chem. 1974, 1, 222–226. [Google Scholar] [CrossRef]
- Piontek, A.; Szostak, M. Iron-Catalyzed C(sp2)-C(sp3) Cross-Coupling of Alkyl Grignard Reagents with Polyaromatic Tosylates. Eur. J. Org. Chem. 2017, 48, 7271–7276. [Google Scholar] [CrossRef]
- Agrawal, T.; Cook, S.P. Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Sulfamates and Tosylates. Org. Lett. 2013, 15, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Bisz, E.; Kardela, M.; Szostak, M. Ligand Effect on Iron-Catalyzed Cross-Coupling Reactions: Evaluation of Amides as O-coordinating Ligands. ChemCatChem 2019, 11, 5733–5737. [Google Scholar] [CrossRef]
- Ratushnyy, M.; Kamenova, M.; Gevorgyan, V. A Mild Light-Induced Cleavage of S–O Bond of Aryl Sulfonate Esters Enables Efficient Sulfonylation of Vinylarenes. Chem. Sci. 2018, 9, 7193–7197. [Google Scholar] [CrossRef] [Green Version]
- Perez, K.A.; Rogers, C.R.; Weiss, E.A. Quantum Dot-Catalyzed Photoreductive Removal of Sulfonyl-Based Protecting Groups. Angew. Chem. Int. Ed. 2020, 59, 14091–14095. [Google Scholar] [CrossRef]



| Entry | Fe(acac)3 (mol%) | Ligand | mol% | Time | Yield (%) 2 |
|---|---|---|---|---|---|
| 1 | - | - | - | 10 min | 0 |
| 2 | 5 | - | - | 10 min | 73 |
| 3 | 5 | DMI | 20 | 10 min | 81 |
| 4 | 5 | DMI | 50 | 10 min | 87 |
| 5 | 5 | DMI | 200 | 10 min | 98 |
| 6 3 | 5 | DMI | 600 | 10 min | >98 |
| Entry | Substrate | 2 | Product | Yield (%) |
|---|---|---|---|---|
| 1 |  | 2a | 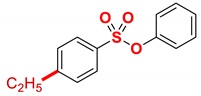 | 98 |
| 2 | 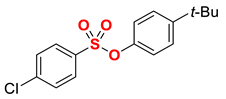 | 2b | 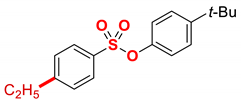 | 98 |
| 3 | 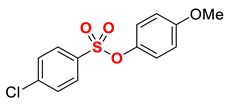 | 2c |  | 98 |
| 4 | 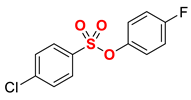 | 2d | 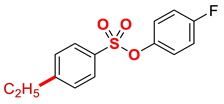 | 95 |
| 5 | 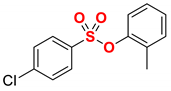 | 2e | 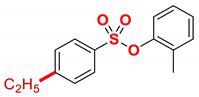 | 98 |
| 6 | 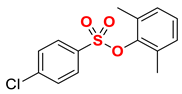 | 2f | 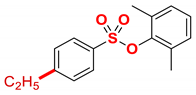 | 97 |
| 7 2 | 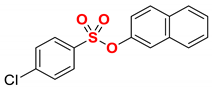 | 2g |  | 73 |
| 8 |  | 2h | 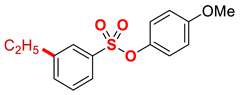 | 98 |
| 9 3 | 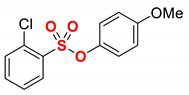 | 2i | 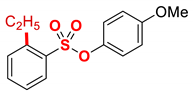 | 59 |
| 10 3 |  | 2j | 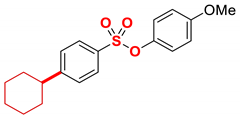 | 98 |
| 11 3 |  | 2k | 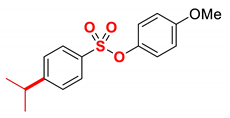 | 98 |
| 12 4 | 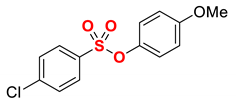 | 2l | 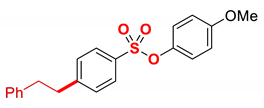 | 98 |
| 13 5 | 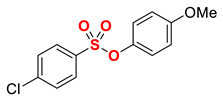 | 2m | 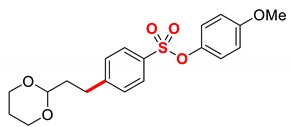 | 98 |
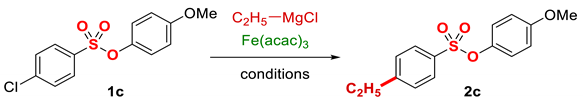
| Entry | Fe(acac)3 (mol%) | Ligand | mol% | Time (min) | Yield (%) 2 |
|---|---|---|---|---|---|
| 1 | 5 |  | 600 | 10 | >98 |
| 2 | 5 | 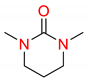 | 600 | 10 | >98 |
| 3 | 5 | 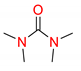 | 600 | 10 | 94 |
| 4 | 5 |  | 600 | 10 | >98 |
| 5 | 5 | 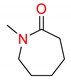 | 600 | 10 | 97 |
| 6 | 5 | 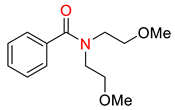 | 600 | 10 | 78 |
| 7 | 5 | 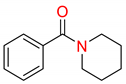 | 600 | 10 | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisz, E. Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Chlorobenzenesulfonates. Molecules 2021, 26, 5895. https://doi.org/10.3390/molecules26195895
Bisz E. Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Chlorobenzenesulfonates. Molecules. 2021; 26(19):5895. https://doi.org/10.3390/molecules26195895
Chicago/Turabian StyleBisz, Elwira. 2021. "Iron-Catalyzed Cross-Coupling Reactions of Alkyl Grignards with Aryl Chlorobenzenesulfonates" Molecules 26, no. 19: 5895. https://doi.org/10.3390/molecules26195895






