Reductive Amination Reaction for the Functionalization of Cellulose Nanocrystals
Abstract
:1. Introduction
2. Results
2.1. Synthesis of the Pyrene Dye
2.2. Synthesis of Sulfated and Neutral CNCs
2.3. Synthesis of Pyrene Derivatives of CNCs
2.4. Photophysical Characterization
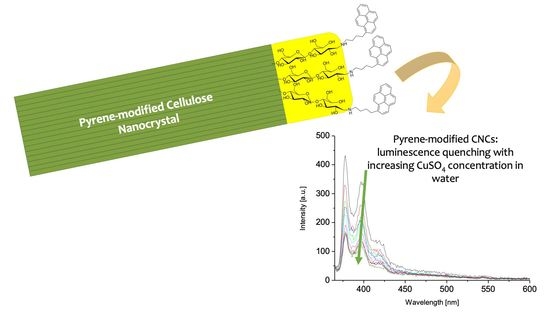
2.5. Luminescence Quenching Experiments
3. Discussion
4. Materials and Methods
4.1. Synthesis of 4-(1-pyrenyl)Butanamine 4
4.2. Hydrolysis of Cellulose, Nanocrystals Isolation and Functionalization
4.3. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dufresne, A. Nanocellulose; Walter de Gruyter GmbH: Berlin/Boston, Germany, 2018. [Google Scholar]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017. [Google Scholar]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic Modulus of Single Cellulose Microfibrils from Tunicate Measured by Atomic Force Microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef]
- Nishino, T.; Takano, K.; Nakamae, K. Elastic modulus of the crystalline regions of cellulose polymorphs. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1647–1651. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, S.; Jia, Z.; Parvinian, S.; Li, Y.; Vaaland, O.; Hu, L.; Li, T. Anomalous scaling law of strength and toughness of cellulose nanopaper. Proc. Natl. Acad. Sci. USA 2015, 112, 8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Fu, S.; Lavoine, N.; Lucia, L.A. Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials—A review. Carbohydr. Polym. 2020, 247, 116722. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from UntreatedBiomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Colombo, L.; Zoia, L.; Violatto, M.B.; Previdi, S.; Talamini, L.; Sitia, L.; Nicotra, F.; Orlandi, M.; Salmona, M.; Recordati, C.; et al. Organ Distribution and Bone Tropism of Cellulose Nanocrystals in Living Mice. Biomacromolecules 2015, 16, 2862–2871. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market projections of cellulose nanomaterial-enabled products—Part 1: Applications. TAPPI J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Liang, H.-W.; Chen, L.-F.; Hu, B.-C.; Yu, S.-H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Tang, C.; Spinney, S.; Shi, Z.; Tang, J.; Peng, B.; Luo, J.; Tam, K.C. Amphiphilic Cellulose Nanocrystals for Enhanced Pickering Emulsion Stabilization. Langmuir 2018, 34, 12897–12905. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef]
- Zhan, C.; Sharma, P.R.; He, H.; Sharma, S.K.; McCauley-Pearl, A.; Wang, R.; Hsiao, B.S. Rice husk based nanocellulose scaffolds for highly efficient removal of heavy metal ions from contaminated water. Environ. Sci. Water Res. Technol. 2020, 6, 3080–3090. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic (III) Removal by Nanostructured DialdehydeCellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A study of TiO (2) nanocrystal growth and environmental remediation capability of TiO (2)/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef] [Green Version]
- Operamolla, A. Recent Advances on Renewable and Biodegradable Cellulose Nanopaper Substrates for Transparent Light-Harvesting Devices: Interaction with Humid Environment. Int. J. Photoenergy 2019, 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, R.; Babudri, F.; Operamolla, A. Chapter 3—Nanocellulose-Based Functional Paper. In Nanocellulose Based Composites for Electronics; Thomas, S., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–72. [Google Scholar]
- Sawalha, S.; Milano, F.; Guascito, M.R.; Bettini, S.; Giotta, L.; Operamolla, A.; Da Ros, T.; Prato, M.; Valli, L. Improving 2D organization of fullerene Langmuir-Schäfer thin films by interaction with cellulose nanocrystals. Carbon 2020, 167, 906–917. [Google Scholar] [CrossRef]
- Milano, F.; Guascito, M.R.; Semeraro, P.; Sawalha, S.; Da Ros, T.; Operamolla, A.; Giotta, L.; Prato, M.; Valli, L. Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis. Polymers 2021, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Battista, O.A. Hydrolysis and Crystallization of Cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Zafeiropoulous, N.E. Engineering the fibre—Matrix interface in natural-fibre composites. In Properties and Performance of Natural Fibre Composites; Pickering, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 127–162. [Google Scholar]
- Yalpani, M. Polysaccharides. Syntheses, Modifications and Structure/Property Relations, 1st ed.; Studies in Organic Synthesis Series; Elsevier: Amsterdam, The Netherlands, 1988; Volume 36. [Google Scholar]
- Ioelovich, M. Recent Findings and the Energetic Potential of Plant Biomass as a Renewable Source of Biofuels—A Review. Bioresources 2015, 10, 1879. [Google Scholar]
- Delmer, D.P.; Amor, Y. Cellulose biosynthesis. Plant Cell 1995, 7, 987–1000. [Google Scholar]
- Gardner, K.H.; Blackwell, J. The structure of native cellulose. Biopolymers 1974, 13, 1975–2001. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Afonso, C.A.M.; Farinha, J.P.S. Synthesis of 4-aryl-butylamine fluorescent probes. J. Chem. Res. 2002, 11, 584–586. [Google Scholar] [CrossRef]
- Battistini, G.; Cozzi, P.G.; Jalkanen, J.-P.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Zerbetto, F.; Battistini, G.; Cozzi, P.G.; Jalkanen, J.-P.; et al. The Erratic Emission of Pyrene on Gold Nanoparticles. ACS Nano 2008, 2, 77–84. [Google Scholar] [CrossRef]
- Lu, P.; Lo Hsieh, Y. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Haafiza, M.K.M.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oilpalm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, U.P. Raman Spectroscopy in the Analysis of Cellulose Nanomaterials. In Nanocelluloses: Their Preparation, Properties, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; pp. 75–90. [Google Scholar]
- Castellan, A.; Ruggiero, R.; Frollini, E.; Ramos, L.A.; Chirat, C. Studies on fluorescence of cellulosics. Holzforschung 2007, 61, 504–508. [Google Scholar] [CrossRef]
- Kalita, E.; Nath, B.K.; Agan, F.; More, V.; Deb, P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crop. Prod. 2015, 65, 550–555. [Google Scholar] [CrossRef]
- Ding, Q.; Han, W.; Li, X.; Jiang, Y.; Zhao, C. New insights into the autofluorescence properties of cellulose/nanocellulose. Sci. Rep. 2020, 10, 21387. [Google Scholar] [CrossRef] [PubMed]
- Arndt, E.R.; Stevens, E.S. Vacuum ultraviolet circular dichroism of simple saccharides. J. Am. Chem. Soc. 1993, 115, 7849–7853. [Google Scholar] [CrossRef]
- Gan, L.; Feng, N.; Liu, S.; Zheng, S.; Li, Z.; Huang, J. Assembly-Induced Emission of Cellulose Nanocrystals for Hiding Information. Part. Part. Syst. Charact. 2019, 36, 1800412. [Google Scholar] [CrossRef]
- Yan, D.; Popp, J.; Pletz, M.W.; Frosch, T. Highly sensitive broadband Raman sensing of antibiotics in step-index hollow-core photonic crystal fibers. ACS Photonics 2017, 4, 138. [Google Scholar] [CrossRef]
- Belli, F.; Abdolvand, A.; Travers, J.C.; Russell, P.S.J. Control of ultrafast pulses in a hydrogen-filled hollow-core photonic-crystal fiber by Raman coherence. Phys. Rev. A 2018, 97, 013814. [Google Scholar] [CrossRef] [Green Version]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid crystal systems from fibrillar polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Zhou, J.; Zhang, L. Synthesis and Photophysical Behavior of Pyrene-Bearing Cellulose Nanocrystals for Fe3+ Sensing. Macromol. Chem. Phys. 2012, 213, 1612–1617. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, J.; Huang, Y.; Theato, P.; Huang, Q.; Chen, T. Self-diffusion driven ultrafast detection of ppm-level nitroaromatic pollutants in aqueous media using a hydrophilic fluorescent paper sensor. ACS Appl. Mater. Interfaces 2017, 9, 23884. [Google Scholar] [CrossRef] [PubMed]
- Operamolla, A.; Casalini, S.; Console, D.; Capodieci, L.; Di Benedetto, F.; Bianco, G.V.; Babudri, F. Tailoring water stability of cellulose nanopaper by surface functionalization. Soft Matter 2018, 14, 7390–7400. [Google Scholar] [CrossRef]







| Sample | λem,DMSO (nm) |
|---|---|
| 4 | 378, 399, 420, 475 1 |
| S_CNC | 378, 400 |
| N_CNC | 383 |
| Commercial sulfated nanocrystals | - |
| Pyrene-modified S_CNC 7a | 378, 398, 419, 470 |
| Pyrene-modified N_CNC 7b | 378, 398, 419, 470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan Omar, O.; Giannelli, R.; Colaprico, E.; Capodieci, L.; Babudri, F.; Operamolla, A. Reductive Amination Reaction for the Functionalization of Cellulose Nanocrystals. Molecules 2021, 26, 5032. https://doi.org/10.3390/molecules26165032
Hassan Omar O, Giannelli R, Colaprico E, Capodieci L, Babudri F, Operamolla A. Reductive Amination Reaction for the Functionalization of Cellulose Nanocrystals. Molecules. 2021; 26(16):5032. https://doi.org/10.3390/molecules26165032
Chicago/Turabian StyleHassan Omar, Omar, Rosa Giannelli, Erica Colaprico, Laura Capodieci, Francesco Babudri, and Alessandra Operamolla. 2021. "Reductive Amination Reaction for the Functionalization of Cellulose Nanocrystals" Molecules 26, no. 16: 5032. https://doi.org/10.3390/molecules26165032