Zinc Recovery from Wulagen Sulfide Flotation Plant Tail by Applying Ether Amine Organic Collectors
Abstract
:1. Introduction
2. Experimental
2.1. Ore Sample
2.2. Reagents
2.3. Batch Flotation Tests
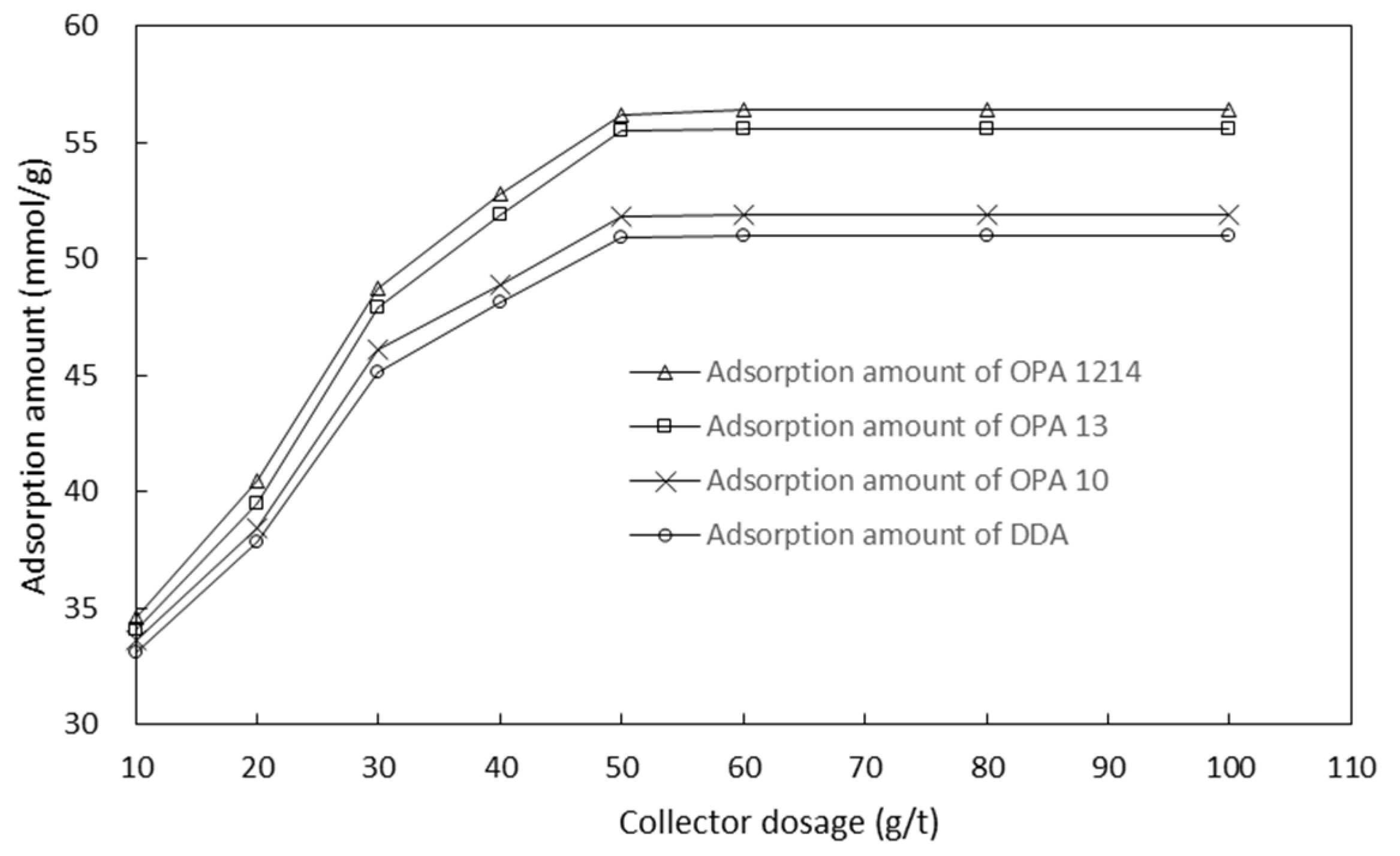
2.4. Adsorption Tests
3. Results and Discussion
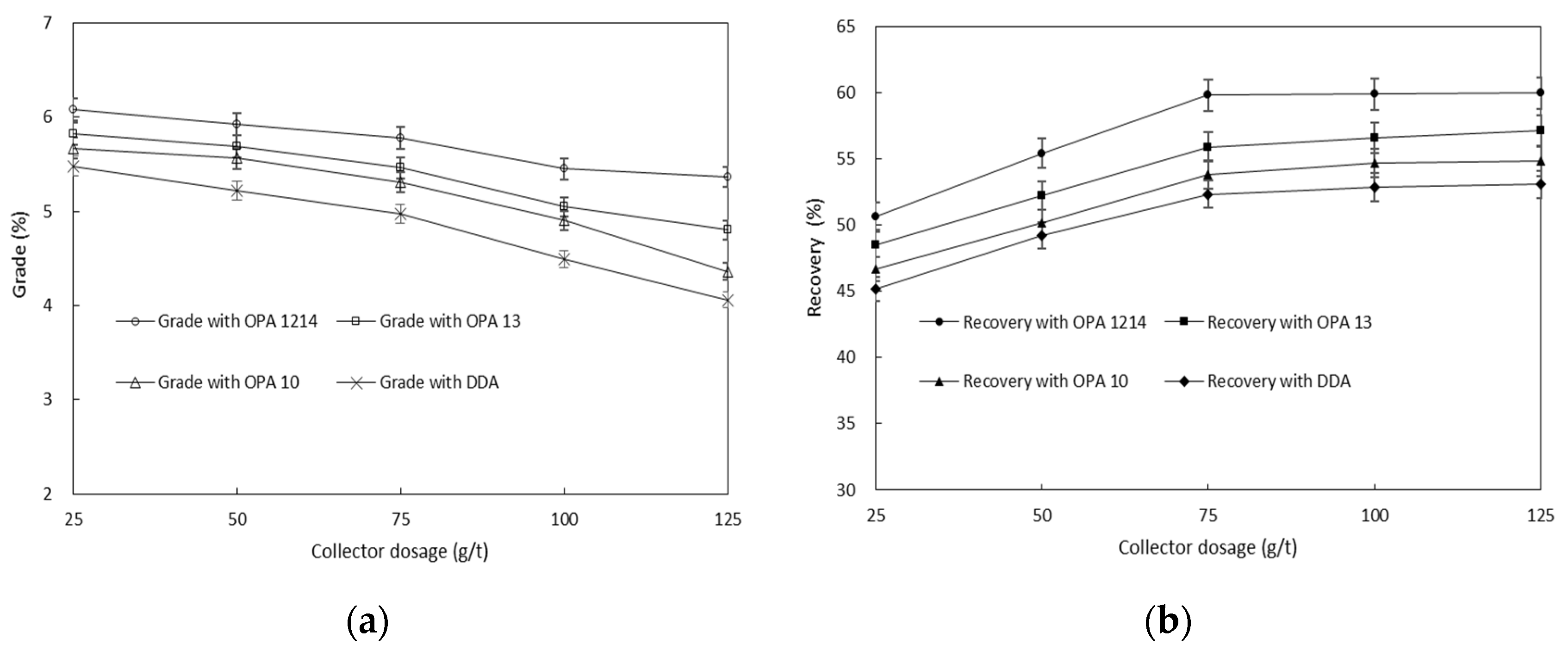
3.1. Effect of Collector
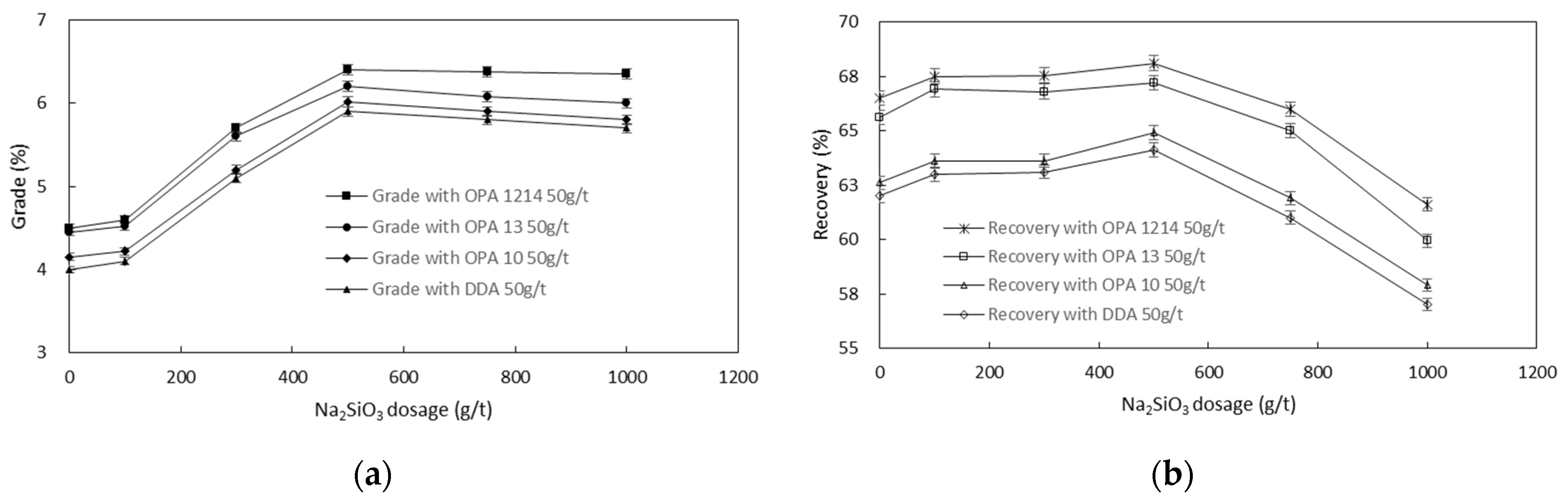
3.2. Interactions of Collector with Other Reagents
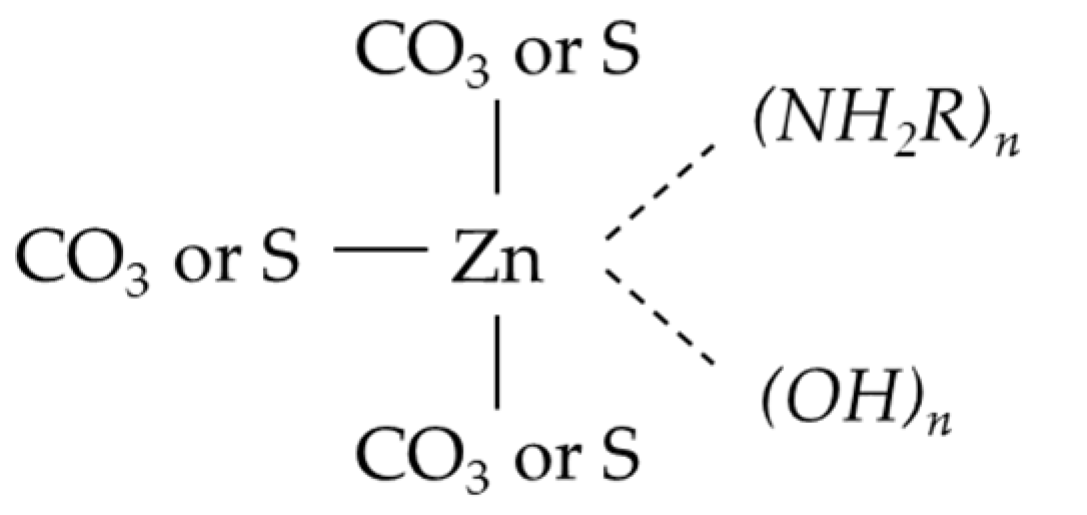
3.3. Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid. Min. Metall. Explor. 2006, 23, 87–96. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Sun, W.; Khoso, S.A.; Liu, R.; Zhang, X. Selective flotation of smithsonite from dolomite by using novel mixed collector system. Trans. Nonferrous Met. Soc. China 2019, 29, 1082–1089. [Google Scholar] [CrossRef]
- Rey, M. Memoirs of milling and process metallurgy: Flotation of oxidized ores. Trans. Inst. Min. Metall. 1979, 88, C245–C250. [Google Scholar]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Rao, S.R.; Finch, J.A. Base metal oxide flotation using long chain xanthates. Int. J. Miner. Process. 2003, 69, 251–258. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Smithsonite flotation using potassium amyl xanthate and hexylmercaptan. Miner. Process. Extr. Metall. 2006, 115, 107–112. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; da Silva, G.R.; Azizi, D.; Larachi, F.; Waters, K.E. The effect of dissolved mineral species on bastnäsite, monazite and dolomite flotation using benzohydroxamate collector. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 539, 319–334. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Zarei, H. Smithsonite flotation from zinc oxide ore using alkyl amine acetate collectors. Sep. Sci. Technol. 2014, 49, 445–457. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Li, L.; Liu, S.; Zhang, T. Selective flotation of smithsonite, quartz and calcite using alkyl diamine ether as collector. Trans. Nonferrous Met. Soc. China 2018, 28, 163–168. [Google Scholar] [CrossRef]
- Wu, D.D.; Wen, S.M.; Yang, J.; Deng, J.S.; Liu, J. The Effect of Zinc Oxide Flotation Using Dodecylamine. Adv. Mater. Res. 2013, 634, 3522–3526. [Google Scholar] [CrossRef]
- Xu, S.; Kou, J.; Sun, T.; Jong, K. A study of adsorption mechanism of dodecylamine on sphalerite. Colloids Surf. A Physicochem. Eng. Asp. 2015, 486, 145–152. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Y.; Zhao, Y.; Chen, T.; Li, H.; Chen, L.; Song, S. Regulation of coal flotation by the cations in the presence of clay. Fuel 2020, 271, 117590. [Google Scholar] [CrossRef]
- Pease, J.D.; Curry, D.C.; Young, M.F. Designing flotation circuits for high fines recovery. Minerals Eng. 2006, 19, 831–840. [Google Scholar] [CrossRef]
- Trahar, W.J. A laboratory study of the influence of sodium sulphide and oxygen on the collectorless flotation of chalcopyrite. Int. J. Miner. Process. 1983, 11, 57–74. [Google Scholar] [CrossRef]
- Herrera-Urbina, R.; Sotillo, F.J.; Fuerstenau, D.W. Effect of sodium sulfide additions on the pulp potential and amyl xanthate flotation of cerussite and galena. Int. J. Miner. Process. 1999, 55, 157–170. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Mehdilo, A.; Zarei, H.; Irannajad, M.; Arjmandfar, H. Flotation of zinc oxide ores by cationic and mixed collectors. Miner. Eng. 2012, 36, 331–334. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Fa, K.-Q.; Miller, J.D.; Jiang, T.; Li, G.-H. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores. Trans. Nonferrous Met. Soc. China 2005, 5, 1138–1144. [Google Scholar]
- Marabini, A.M.; Alesse, V.; Garbassi, F. Role of sodium sulphide, xanthate and amine in flotation of lead–zinc oxidized ores. Inst. Min. Metall. 1984, 125–136. [Google Scholar]
- Liu, W.; Wang, B.; Zhao, Q.; Chen, X. Molecular-level insights into the adsorption of a hydroxy-containing tertiary amine collector on the surface of magnesite ore. Powder Technol. 2019, 355, 700–707. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Blaida, I.A.; Ponomareva, E.I.; Rozen, A.M. Effect of amine structure on gallium extraction from hydrochloric acid solutions. Hydrometallurgy 1995, 37, 393–399. [Google Scholar] [CrossRef]







| Name | Abbreviation | Chemical Formula | Total Amine Value (mg KOH/g) | Distribution of Carbon Chain (%) |
|---|---|---|---|---|
| Normal dodecyl amine | DDA | CH3(CH2)11NH2 | 285–305 | C12 ≥ 98, C14 ≥ 1, C10 ≥ 1 |
| 3-(iso-decyloxy)-1-propyl amine | OPA 10 | (CH3)2CH(CH2)7-O-(CH2)3NH2 | ≥235 | C10 ≥ 98C8 ≤ 2 |
| 3-(normal dodecyloxy)-1-propyl amine | OPA 1214 | CH3(CH2)11-O-(CH2)3NH2 | ≥205 | 30 ≥ C12 ≥ 22 |
| 3-(normal tetradecyloxy)-1-propyl amine | CH3(CH2)13-O-(CH2)3NH2 | 78 ≥ C14 ≥ 68 | ||
| 3-(iso-tridecyloxy)-1-propyl amine | OPA 13 | (CH3)2CH(CH2)10-O-(CH2)3NH2 | ≥200 | C13 ≥ 98 |
| Size (μm) | Wt. (%) | Zn Grade (%) | Zn Distribution (%) |
|---|---|---|---|
| +96 | 23.14 | 0.67 | 17.56 |
| −96 + 74 | 12.11 | 0.75 | 10.29 |
| −74 + 44 | 10.80 | 0.83 | 10.15 |
| −44 + 37 | 10.49 | 1.03 | 12.24 |
| −37 + 15 | 31.61 | 1.09 | 39.02 |
| −15 | 11.85 | 0.80 | 10.74 |
| Total | 100.00 | 0.88 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, L.; Ni, X.; Liao, Y.; Liang, Z. Zinc Recovery from Wulagen Sulfide Flotation Plant Tail by Applying Ether Amine Organic Collectors. Molecules 2021, 26, 5365. https://doi.org/10.3390/molecules26175365
Ma Z, Wang L, Ni X, Liao Y, Liang Z. Zinc Recovery from Wulagen Sulfide Flotation Plant Tail by Applying Ether Amine Organic Collectors. Molecules. 2021; 26(17):5365. https://doi.org/10.3390/molecules26175365
Chicago/Turabian StyleMa, Zilong, Lei Wang, Xiao Ni, Yinfei Liao, and Zhian Liang. 2021. "Zinc Recovery from Wulagen Sulfide Flotation Plant Tail by Applying Ether Amine Organic Collectors" Molecules 26, no. 17: 5365. https://doi.org/10.3390/molecules26175365





