Vapor Phase Synthesis of SnS Facilitated by Ligand-Driven “Launch Vehicle” Effect in Tin Precursors
Abstract
:1. Introduction
2. Results and Discussion
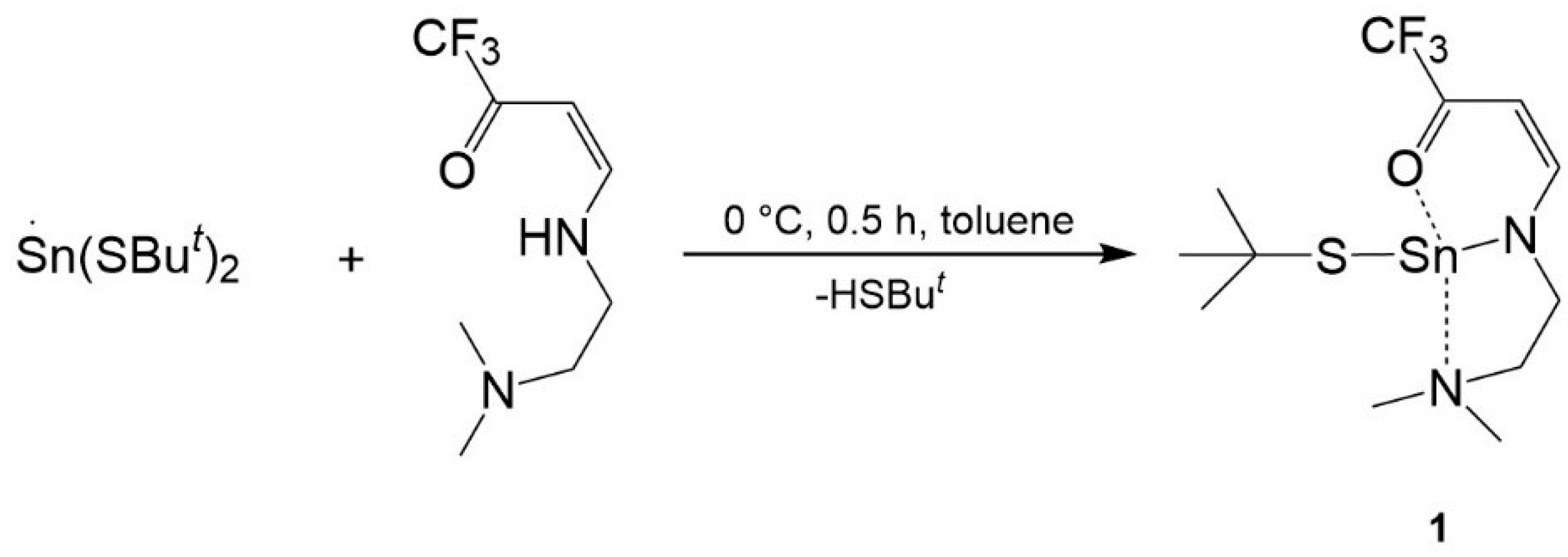
2.1. Synthesis and Characterization of New Molecular Precursor
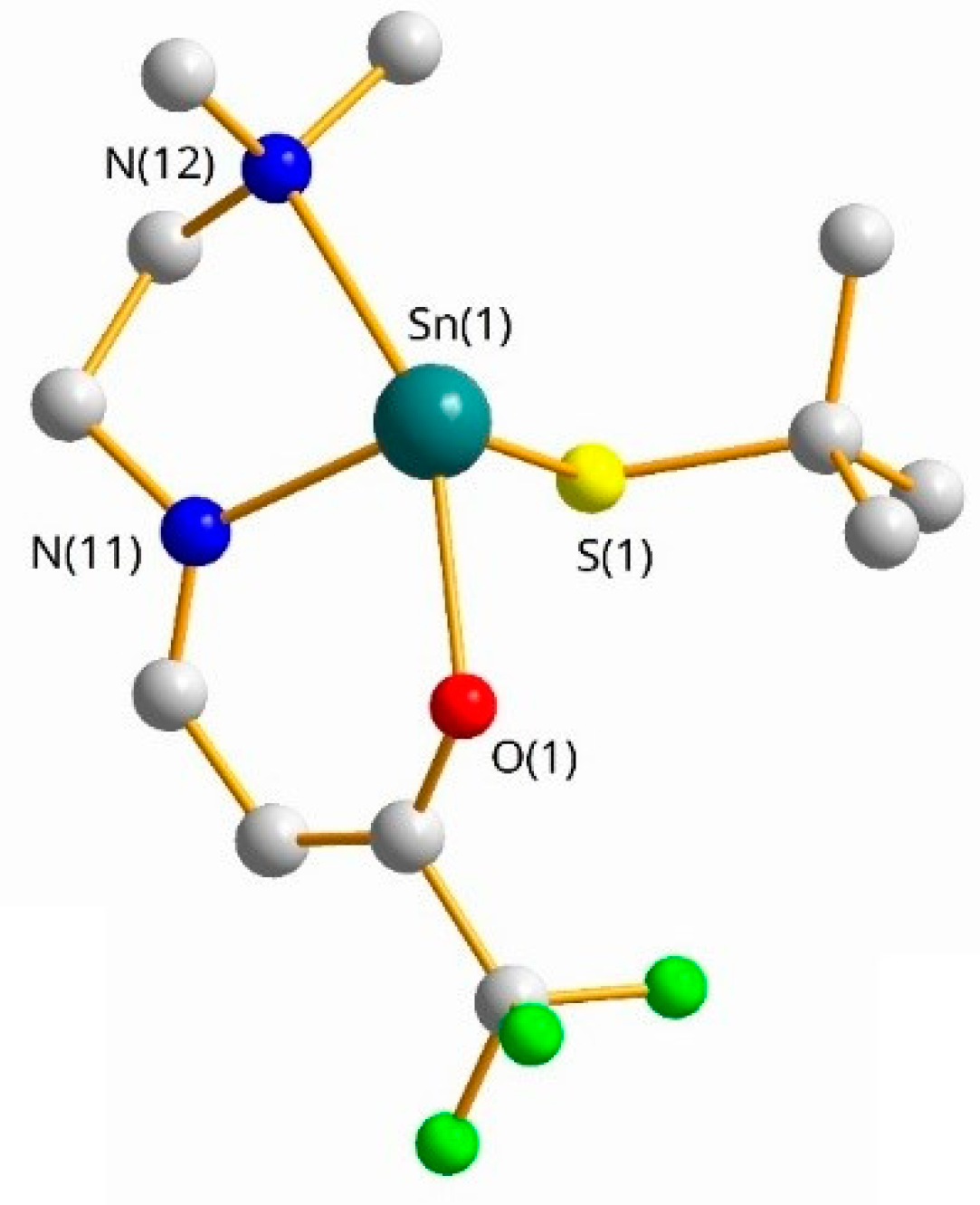
2.1.1. Single Crystal X-ray Diffraction Analysis of 1
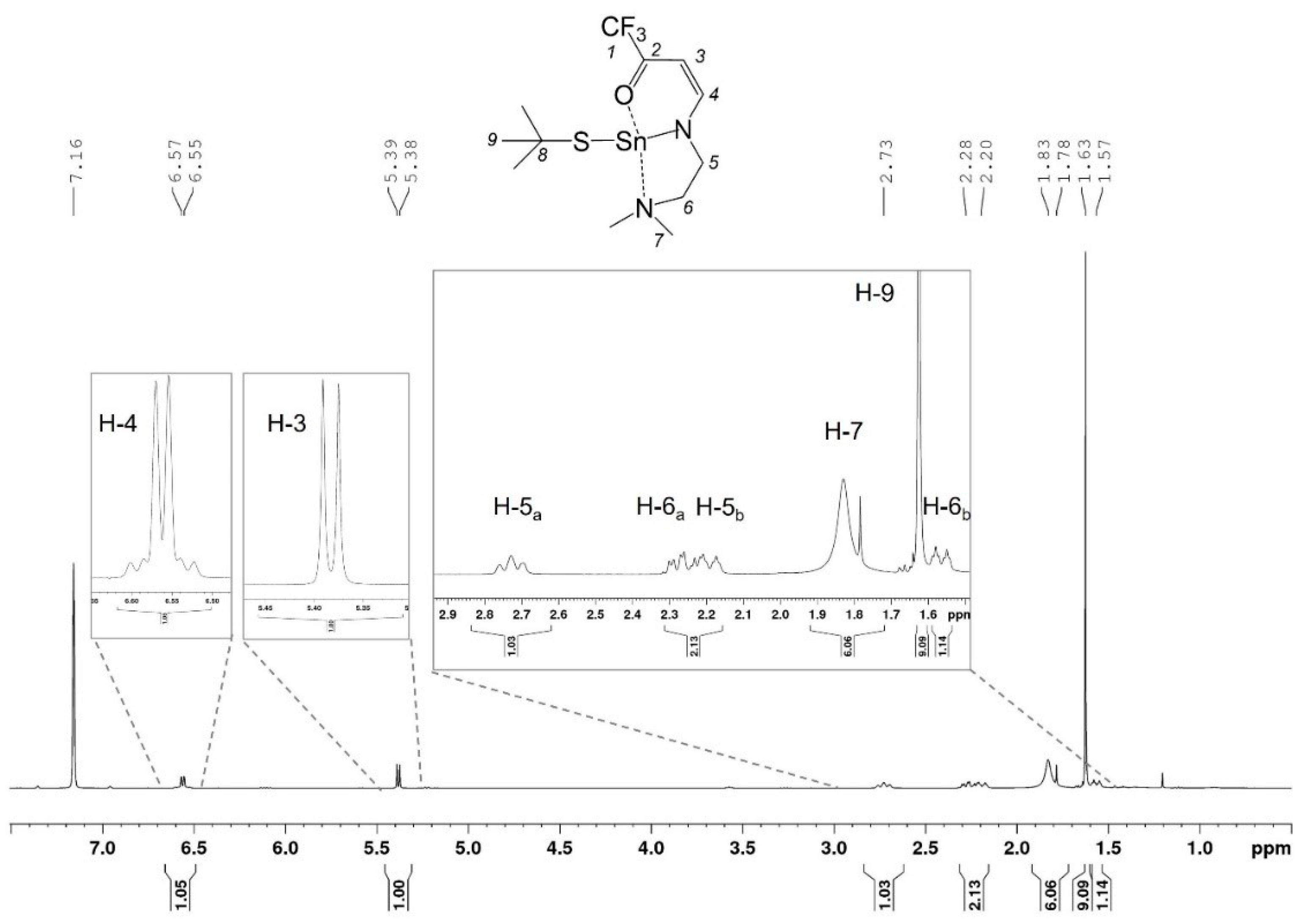
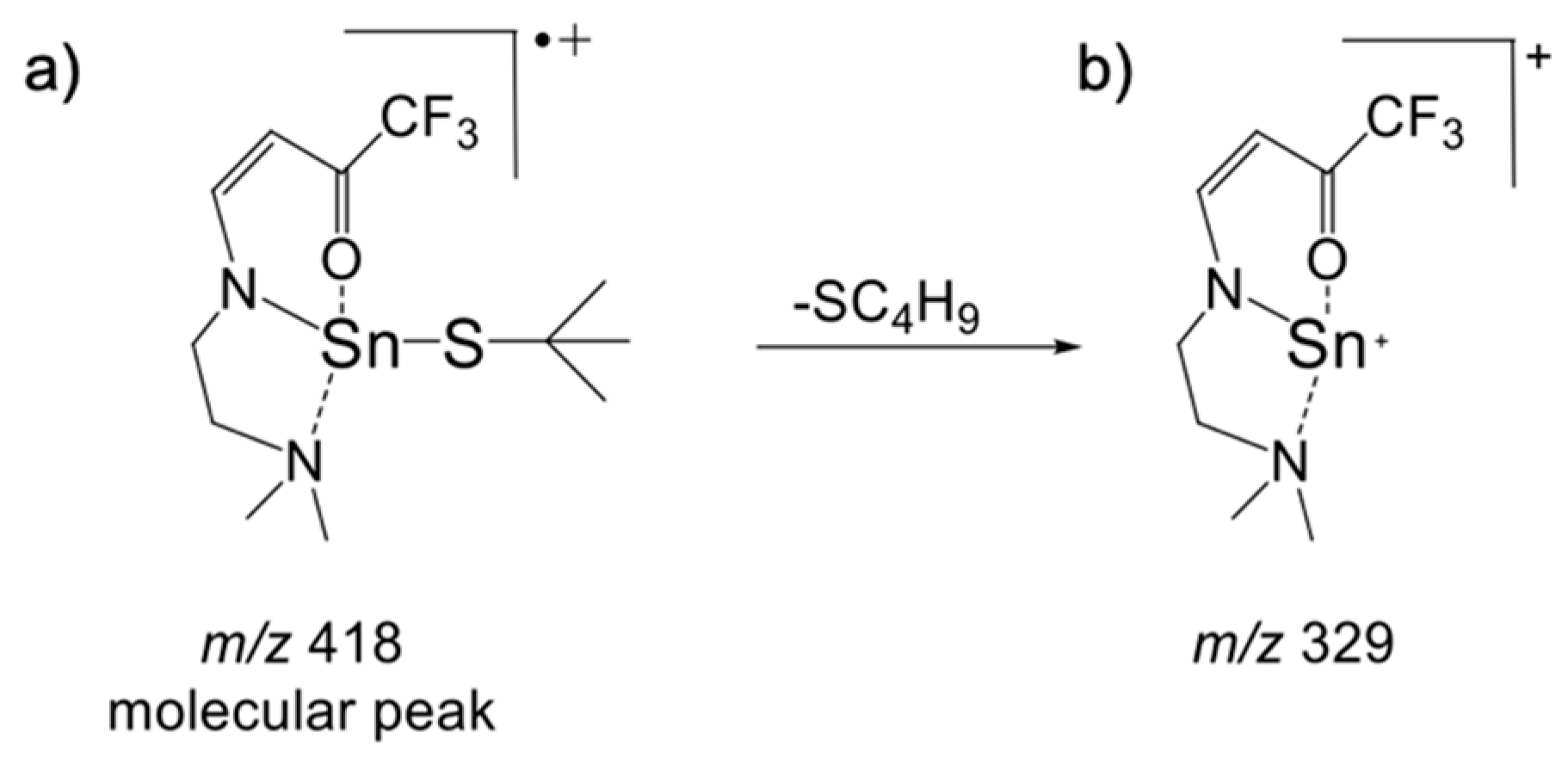
2.1.2. Spectroscopic Characterization of 1
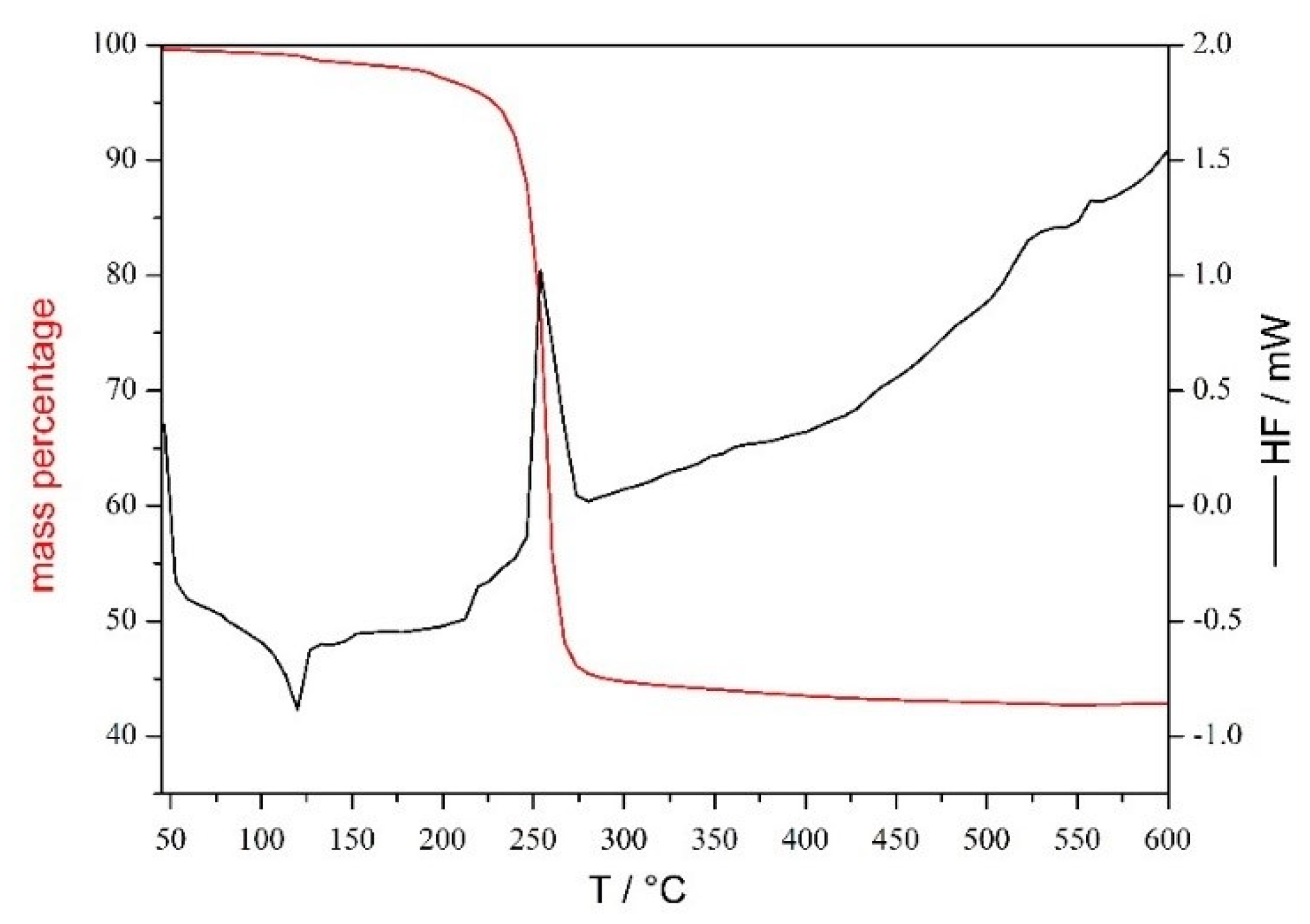
2.1.3. Thermogravimetric Analysis of 1
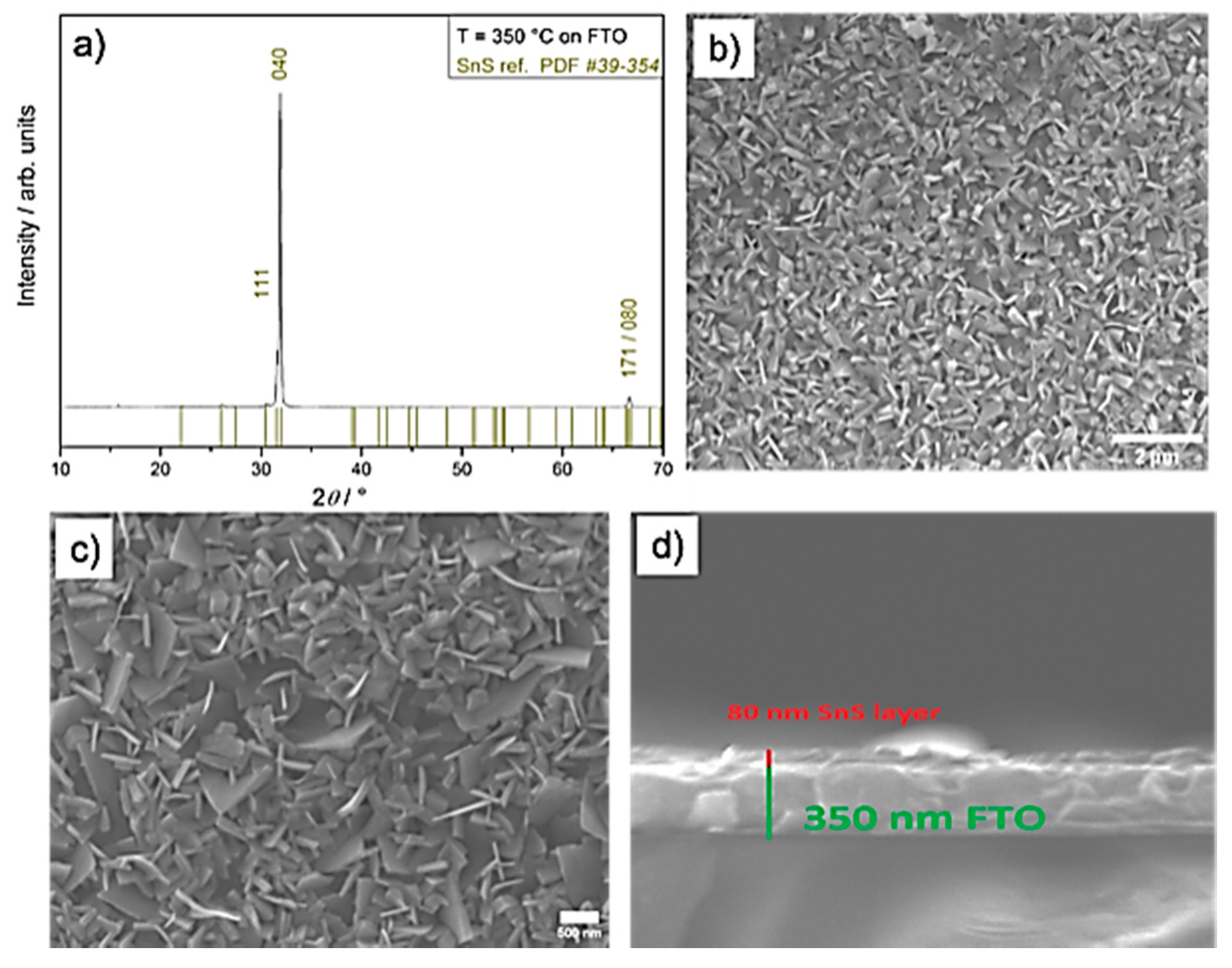
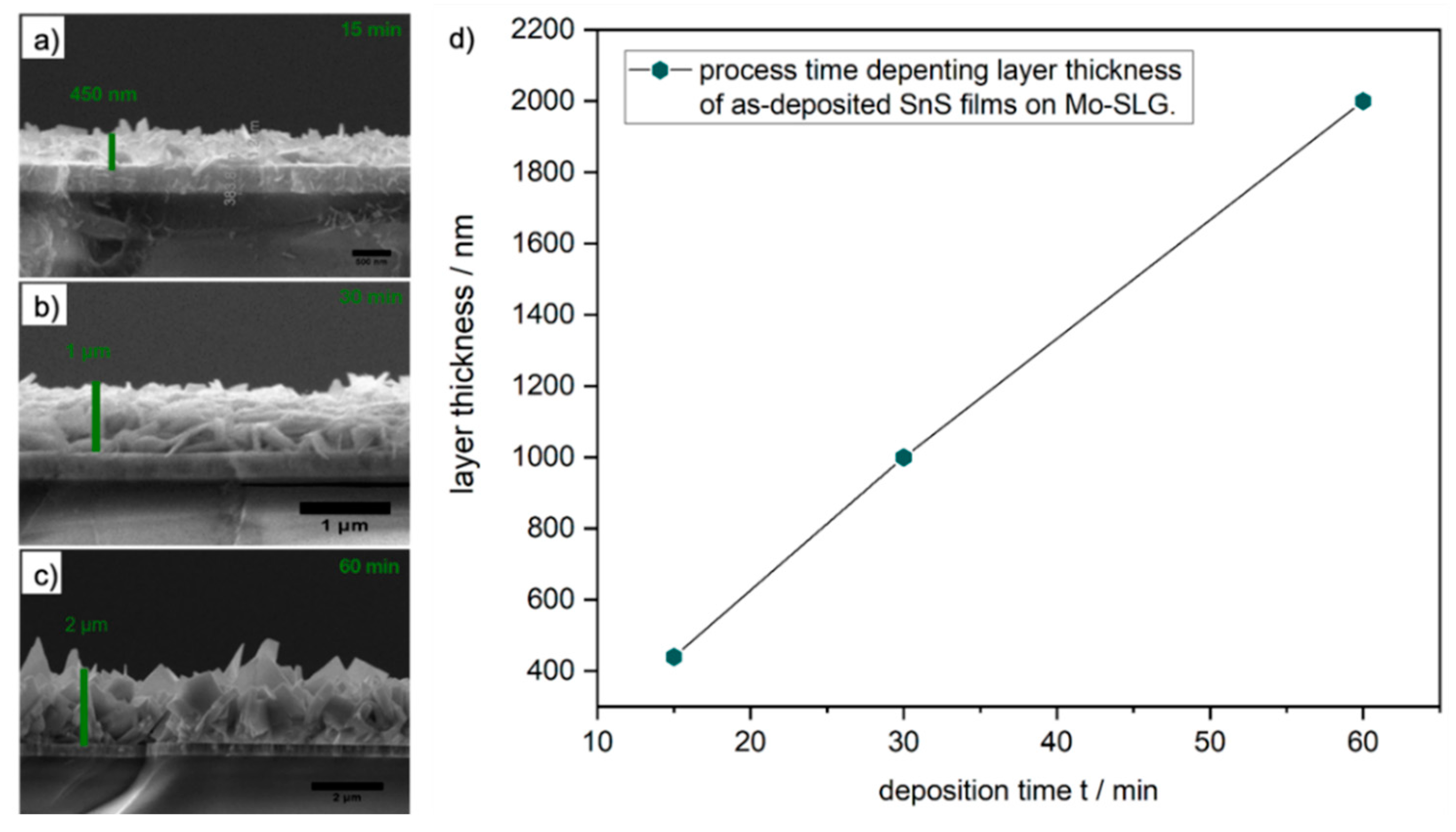
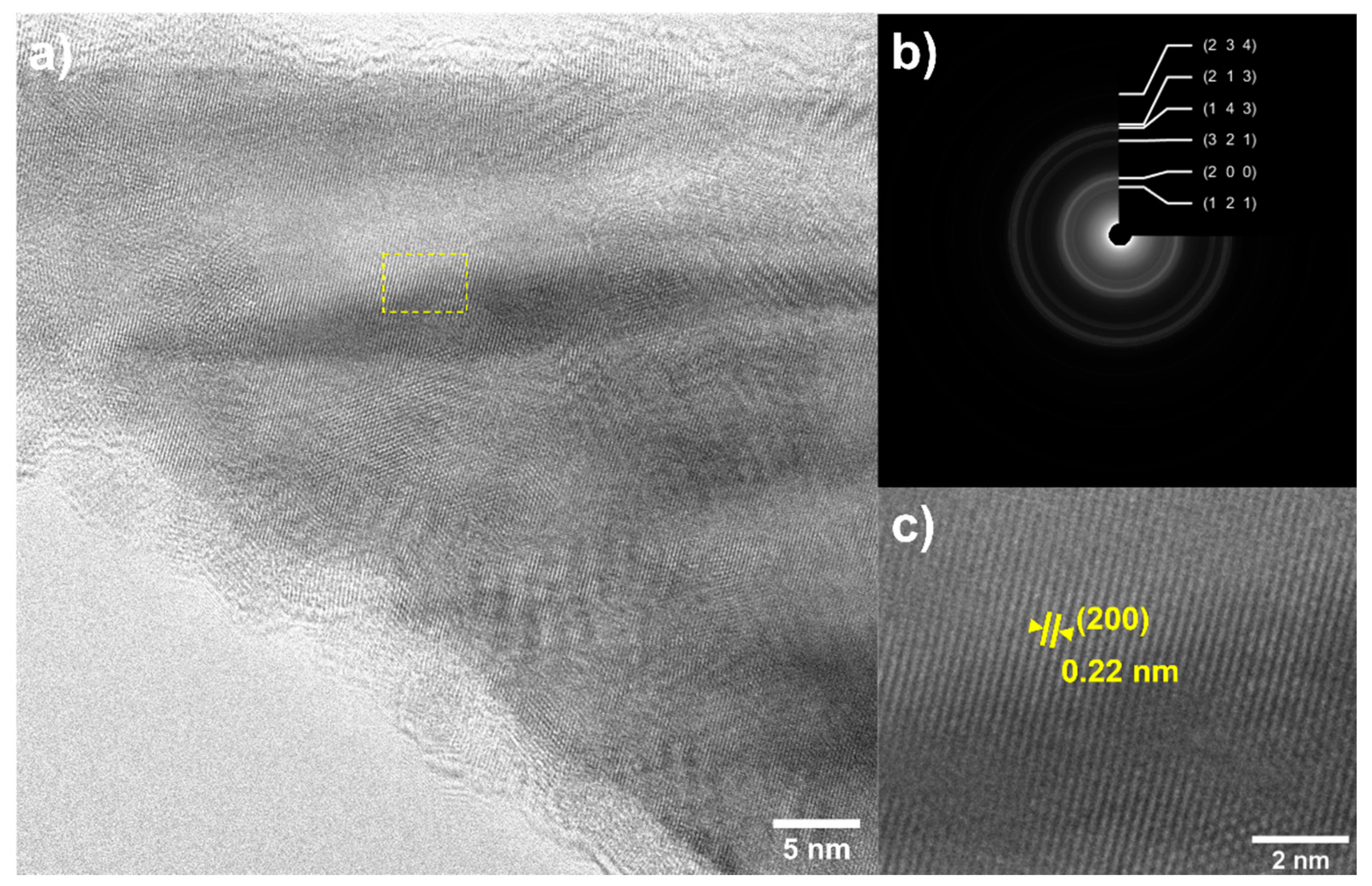
2.2. CVD Experiments
3. Materials and Methods
Synthesis of [SnII(tfb-dmeda)(SBut)] (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The Renaissance of Black Phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Woo, H.; Wu, P.; Hong, H.J.; Jung, W.G.; Kim, B.-J.; Vanel, J.-C.; Choi, J.W. Simple Eco-Friendly Synthesis of the Surfactant Free SnS Nanocrystal toward the Photoelectrochemical Cell Application. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.K.; Devika, M.; Prashantha, M.; Ramesh, K.; Gunasekhar, K.R. In Situ Structural Studies on Orthorhombic SnS Micro-Crystals. Eur. Phys. J. Appl. Phys. 2012, 60, 10102. [Google Scholar] [CrossRef]
- Ning, J.; Men, K.; Xiao, G.; Wang, L.; Dai, Q.; Zou, B.; Liu, B.; Zou, G. Facile Synthesis of IV–VI SnS Nanocrystals with Shape and Size Control: Nanoparticles, Nanoflowers and Amorphous Nanosheets. Nanoscale 2010, 2, 1699–1703. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.-Z.; Huang, X.; Mao, N.; Zhu, D.-D.; Wang, L.; Xu, H.; Meng, X.-M. Physical Vapor Deposition Synthesis of Two-Dimensional Orthorhombic SnS Flakes with Strong Angle/Temperature-Dependent Raman Responses. Nanoscale 2016, 8, 2063–2070. [Google Scholar] [CrossRef]
- Wiedemeier, H.; Georg, H.; Schnering, G. von Refinement of the Structures of GeS, GeSe, SnS and SnSe. Z. für Krist.-Cryst. Mater. 1978, 148, 295–304. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Brune, V.; Grosch, M.; Weißing, R.; Hartl, F.; Frank, M.; Mishra, S.; Mathur, S. Influence of the Choice of Precursors on the Synthesis of Two-Dimensional Transition Metal Dichalcogenides. Dalt. Trans. 2021, 6, 16087–16093. [Google Scholar]
- Shan, Y.; Li, Y.; Pang, H. Applications of Tin Sulfide-Based Materials in Lithium-Ion Batteries and Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2001298. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, W.; Fong, W.K.; Yu, Y.; Surya, C. Tin Compensation for the SnS Based Optoelectronic Devices. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.S.; Stratakis, E. Recent Advances in 2D Metal Monochalcogenides. Adv. Sci. 2020, 7, 2001655. [Google Scholar] [CrossRef]
- Choi, H.; Lee, N.; Park, H.; Choi, Y.; Kim, K.; Choi, Y.; Kim, J.; Song, S.; Yuk, H.; Jeon, H. Development of a SnS Film Process for Energy Device Applications. Appl. Sci. 2019, 9, 4606. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, M.; Takeuchi, K.; Ono, Y.; Arai, E. Electrochemical Deposition of SnS Thin Films. Thin Solid Films 2000, 361, 98–101. [Google Scholar] [CrossRef]
- Avellaneda, D.; Nair, M.T.S.; Nair, P.K. Polymorphic Tin Sulfide Thin Films of Zinc Blende and Orthorhombic Structures by Chemical Deposition. J. Electrochem. Soc. 2008, 155, D517. [Google Scholar] [CrossRef]
- Mariano, A.N.; Chopra, K.L. Polymorphism in Some IV-VI Compounds Induced by High Pressure and Thin-Film Epitaxial Growth. Appl. Phys. Lett. 1967, 10, 282–284. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Sun, L. Preparation and Properties of Zinc Blende and Orthorhombic SnS Films by Chemical Bath Deposition. Appl. Surf. Sci. 2011, 257, 6750–6755. [Google Scholar] [CrossRef]
- Polivtseva, S.; Katerski, A.; Kärber, E.; Oja Acik, I.; Mere, A.; Mikli, V.; Krunks, M. Post-Deposition Thermal Treatment of Sprayed SnS Films. Thin Solid Films 2017, 633, 179–184. [Google Scholar] [CrossRef]
- Jain, P.; Arun, P. Influence of Grain Size on the Band-Gap of Annealed SnS Thin Films. Thin Solid Films 2013, 548, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Balaji, Y.; Mehta, A.N.; Heyns, M.; Caymax, M.; Radu, I.; Vandervorst, W.; Delabie, A. Formation Mechanism of 2D SnS2 and SnS by Chemical Vapor Deposition Using SnCl4 and H2S. J. Mater. Chem. C 2018, 6, 6172–6178. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Hardy, A.M.E.; Clark, R.J.H.; Hibbert, T.G.; Molloy, K.C. Atmospheric Pressure Chemical Vapor Deposition of Tin Sulfides (SnS, Sn2S3, and SnS2) on Glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Barone, G.; Hibbert, T.G.; Mahon, M.F.; Molloy, K.C.; Price, L.S.; Parkin, I.P.; Hardy, A.M.E.E.; Field, M.N. Deposition of Tin Sulfide Thin Films from Tin(Iv) Thiolate Precursors. J. Mater. Chem. 2001, 11, 464–468. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.G.; Lee, Y.K.; Chung, T.M.; Park, B.K.; Kim, C.G. Tin(II) Aminothiolate and Tin(IV) Aminothiolate Selenide Compounds as Single-Source Precursors for Tin Chalcogenide Materials. Inorg. Chem. 2020, 59, 3513–3517. [Google Scholar] [CrossRef]
- Park, J.; Song, M.; Jung, W.M.; Lee, W.Y.; Lee, J.; Kim, H.; Shim, I.W. Preparation of SnS Thin Films by MOCVD Method Using Single Source Precursor, Bis(3-Mercapto-1-Propanethiolato) Sn(II). Bull. Korean Chem. Soc. 2012, 33, 3383–3386. [Google Scholar] [CrossRef] [Green Version]
- Robinson, F.; Curran, P.J.; de Groot, C.H.; Hardie, D.; Hector, A.L.; Holloway, K.; Huang, R.; Newbrook, D.; Reid, G. nBu2Sn(SnBu)2 and nBu3SnEnBu (E = S or Se) – Effective Single Source Precursors for the CVD of SnS and SnSe Thermoelectric Thin Films. Mater. Adv. 2021, 2, 4814–4823. [Google Scholar] [CrossRef]
- Catherall, A.L.; Harris, S.; Hill, M.S.; Johnson, A.L.; Mahon, M.F. Deposition of SnS Thin Films from Sn(II) Thioamidate Precursors. Cryst. Growth Des. 2017, 17, 5544–5551. [Google Scholar] [CrossRef] [Green Version]
- Kevin, P.; Lewis, D.J.; Raftery, J.; Azad Malik, M.; O’Brien, P. Thin Films of Tin(II) Sulphide (SnS) by Aerosol-Assisted Chemical Vapour Deposition (AACVD) Using Tin(II) Dithiocarbamates as Single-Source Precursors. J. Cryst. Growth 2015, 415, 93–99. [Google Scholar] [CrossRef]
- Ramasamy, K.; Kuznetsov, V.L.; Gopal, K.; Malik, M.A.; Raftery, J.; Edwards, P.P.; O’Brien, P. Organotin Dithiocarbamates: Single-Source Precursors for Tin Sulfide Thin Films by Aerosol-Assisted Chemical Vapor Deposition (AACVD). Chem. Mater. 2013, 25, 266–276. [Google Scholar] [CrossRef]
- Bade, B.P.; Garje, S.S.; Niwate, Y.S.; Afzaal, M.; O’Brien, P. Tribenzyltin(IV)Chloride Thiosemicarbazones: Novel Single Source Precursors for Growth of SnS Thin Films. Chem. Vap. Depos. 2008, 14, 292–295. [Google Scholar] [CrossRef]
- Ahmet, I.Y.; Guc, M.; Sánchez, Y.; Neuschitzer, M.; Izquierdo-Roca, V.; Saucedo, E.; Johnson, A.L. Evaluation of AA-CVD Deposited Phase Pure Polymorphs of SnS for Thin Films Solar Cells. RSC Adv. 2019, 9, 14899–14909. [Google Scholar] [CrossRef] [Green Version]
- Ahmet, I.Y.; Hill, M.S.; Johnson, A.L.; Peter, L.M. Polymorph-Selective Deposition of High Purity SnS Thin Films from a Single Source Precursor. Chem. Mater. 2015, 27, 7680–7688. [Google Scholar] [CrossRef] [Green Version]
- Kana, A.T.; Hibbert, T.G.; Mahon, M.F.; Molloy, K.C.; Parkin, I.P.; Price, L.S. Organotin Unsymmetric Dithiocarbamates: Synthesis, Formation and Characterisation of Tin(II) Sulfide Films by Atmospheric Pressure Chemical Vapour Deposition. Polyhedron 2001, 20, 2989–2995. [Google Scholar] [CrossRef]
- Hibbert, T.G.; Mahon, M.F.; Molloy, K.C.; Price, L.S.; Parkin, I.P. Deposition of Tin Sulfide Thin Films from Novel, Volatile (Fluoroalkythiolato)Tin(IV) Precursors. J. Mater. Chem. 2001, 11, 469–473. [Google Scholar] [CrossRef]
- Parkin, I.P.; Price, L.S.; Hibbert, T.G.; Molloy, K.C. The First Single Source Deposition of Tin Sulfide Coatings on Glass: Aerosol-Assisted Chemical Vapour Deposition Using [Sn(SCH2CH2S)2]. J. Mater. Chem. 2001, 11, 1486–1490. [Google Scholar] [CrossRef]
- Frank, M.; Jürgensen, L.; Leduc, J.; Stadler, D.; Graf, D.; Gessner, I.; Zajusch, F.; Fischer, T.; Rose, M.-A.; Mueller, D.N.; et al. Volatile Rhenium(I) Compounds with Re–N Bonds and Their Conversion into Oriented Rhenium Nitride Films by Magnetic Field-Assisted Vapor Phase Deposition. Inorg. Chem. 2019, 58, 10408–10416. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.H.; Lappert, M.F. Monomeric, Volatile Bivalent Amides of Group IVB Elements, M(NR12)2 and M(NR1R2)2 (M = Ge, Sn, or Pb; R1 = Me3Si, R2 = Me3C). J. Chem. Soc. Chem. Commun. 1974, 895–896. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van Der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Pyykkö, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1–118. Chem. Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Veith, M.; Hobein, P.; Rösler, R. Cyclische Diazastannylene, XXX [1] Symmetrisch Und Asymmetrisch Substituierte German- Und Stannandiyle Mit Amid-, Alkoholat- Und Thiolat-Liganden, Teil I [2]. Z. fur Naturforsch.-Sect. B J. Chem. Sci. 1989, 44, 1067–1081. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Lappert, M.F.; Samways, B.J.; Weinberg, E.L. Metal (Li, GeII, GeIII, SnII, and PbII) 2,6-Dialkylbenzenethiolates; X-Ray Crystal Structures of Sn(SAr)2 (Ar = C6H2But3-2,4,6) and [M(SAr)2]3 (M = Sn or Pb, Ar = C6H3Pri2-2,6). J. Chem. Soc. - Chem. Commun. 1983, 1492–1494. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Portnyagin, I.A.; Zemlyansky, N.N.; Borisova, I.V.; Nechaev, M.S.; Ustynyuk, Y.A.; Antipin, M.Y.; Lunin, V. New Stable Germylenes, Stannylenes, and Related Compounds: 6. Heteroleptic Germanium(II) and Tin(II) Compounds [(SiMe3)2N-E14-OCH2CH2NMe2]n (E14 = Ge, n = 1; Sn, n = 2): Synthesis and Structure. J. Organomet. Chem. 2005, 690, 1172–1177. [Google Scholar] [CrossRef]
- Zemlyanskii, N.N.; Borisova, I.V.; Kuznetsova, M.G.; Khrustalev, E.N.; Antipin, M.Y.; Ustynyuk, Y.A.; Lunin, E.E.; Eaborn, C.; Hill, M.S.; Smith, J.D. New Stable Germylenes, Stannylenes, and Related Compounds II. Bis(Butylthio)Tin(II) and Ate-Complexes [(Me3Si)3CE(μ-SBu)2Li(THF)2] (E = Ge, Sn). Synthesis and Structure. Russ. J. Org. Chem. 2003, 39, 491–500. [Google Scholar] [CrossRef]
- Eichhöfer, A.; Jiang, J.J.; Sommer, H.; Weigend, F.; Fuhr, O.; Fenske, D.; Su, C.Y.; Buth, G. 1-D-Tin(II) Phenylchalcogenolato Complexes ∞1[Sn(EPh)2] (E = S, Se, Te) - Synthesis, Structures, Quantum Chemical Studies and Thermal Behaviour. Eur. J. Inorg. Chem. 2010, 2010, 410–418. [Google Scholar] [CrossRef]
- Rekken, B.D.; Brown, T.M.; Fettinger, J.C.; Lips, F.; Tuononen, H.M.; Herber, R.H.; Power, P.P. Dispersion Forces and Counterintuitive Steric Effects in Main Group Molecules: Heavier Group 14 (Si–Pb) Dichalcogenolate Carbene Analogues with Sub-90° Interligand Bond Angles. J. Am. Chem. Soc. 2013, 135, 10134–10148. [Google Scholar] [CrossRef]
- Dean, P.A.W.; Vittal, J.J.; Payne, N.C. Syntheses and X-Ray Structural Analyses of [(C6H5)4As][Sn(EC6H5)3], E = S and Se. Can. J. Chem. 1985, 63, 394–400. [Google Scholar] [CrossRef]
- Barone, G.; Hibbert, T.G.; Mahon, M.F.; Molloy, K.C.; Parkin, I.P.; Price, L.S.; Silaghi-Dumitrescu, I. Structural Distortions in Homoleptic (RE)4A (E = O, S, Se; A = C, Si, Ge, Sn): Implications for the CVD of Tin Sulfides. J. Chem. Soc. Dalt. Trans. 2001, 3435–3445. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Power, P.P. Structural Studies of Tin(II) and Lead(II) Dimethylamides: X-Ray Crystal Structure of [Sn(NMe2)2]2 and Isolation of Its Lead Analogue. Inorg. Chem. 1984, 23, 413–415. [Google Scholar] [CrossRef]
- Zemlyansky, N.N.; Borisova, I.V.; Kuznetsova, M.G.; Khrustalev, V.N.; Ustynyuk, Y.A.; Nechaev, M.S.; Lunin, V.V.; Barrau, J.; Rima, G. New Stable Germylenes, Stannylenes, and Related Compounds. 1. Stable Germanium(II) and Tin(II) Compounds M(OCH2CH2NMe2)2 (M = Ge, Sn) with Intramolecular Coordination Metal-Nitrogen Bonds. Synthesis and Structure. Organometallics 2003, 22, 1675–1681. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Tin Coordination Compounds: Classification and Analysis of Crystallographic and Structural Data. Main Gr. Met. Chem. 1998, 21, 371–488. [Google Scholar] [CrossRef]
- Persson, I.; D’Angelo, P.; Lundberg, D. Hydrated and Solvated Tin(II) Ions in Solution and the Solid State, and a Coordination Chemistry Overview of the D10s2 Metal Ions. Chem. Eur. J. 2016, 22, 18583–18592. [Google Scholar] [PubMed] [Green Version]
- Wang, L.; Kefalidis, C.E.; Roisnel, T.; Sinbandhit, S.; Maron, L.; Carpentier, J.-F.; Sarazin, Y. Structure vs 119Sn NMR Chemical Shift in Three-Coordinated Tin(II) Complexes: Experimental Data and Predictive DFT Computations. Organometallics 2014, 34, 2139–2150. [Google Scholar] [CrossRef]
- Du Mont, W.-W.; Grenz, M. Dimere Phospha- Und Thiastannylene: Ylidartige Diphospha- Und Dithiadistannetane. Chem. Ber. 1985, 118, 1045–1049. [Google Scholar] [CrossRef]
- Pieper, N.; Klaus-Mrestani, C.; Schürmann, M.; Jurkschat, K.; Biesemans, M.; Verbruggen, I.; Martins, J.C.; Willem, R. Synthesis, Molecular Structure, and Stereochemical Nonrigidity of Bis(3-(Dimethylamino)Propyl)Difluorostannane Dihydrate, {[Me2N(CH2)3]2 SnF2 ·2H2O}, and Enhanced Reactivity of Its Fluoride Adduct {[Me2N(CH2)3]2SnF3}-Bu4N+ Toward Dichloromethane. Organometallics 1997, 16, 1043–1052. [Google Scholar] [CrossRef]
- Meurice, J.C.; Duboudin, J.G.; Ratier, M.; Pétraud, M.; Willem, R.; Biesemans, M. Conformational Investigations of Ester-Functionalized Gem-Distannyl Derivatives by 1H-119Sn Correlation NMR. Organometallics 1999, 18, 1699–1704. [Google Scholar] [CrossRef]
- Martins, J.C.; Biesemans, M.; Willem, R. Tin NMR Based Methodologies and Their Use in Structural Tin Chemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2000, 36, 271–322. [Google Scholar] [CrossRef]
- Janzen, A.F.; Vaidya, O.C.; Willis, C.J. Reaction of Arsenic, Antimony, Bismuth and Sulfur Fluorides with (t-Butylthio) Trimethylsilane. J. Inorg. Nucl. Chem. 1981, 43, 1469–1471. [Google Scholar] [CrossRef]
- Steinmann, V.; Chakraborty, R.; Rekemeyer, P.H.; Hartman, K.; Brandt, R.E.; Polizzotti, A.; Yang, C.; Moriarty, T.; Gradečak, S.; Gordon, R.G.; et al. A Two-Step Absorber Deposition Approach To Overcome Shunt Losses in Thin-Film Solar Cells: Using Tin Sulfide as a Proof-of-Concept Material System. ACS Appl. Mater. Interfaces 2016, 8, 22664–22670. [Google Scholar] [CrossRef] [PubMed]
- Revathi, N.; Bereznev, S.; Iljina, J.; Safonova, M.; Mellikov, E.; Volobujeva, O. PVD Grown SnS Thin Films onto Different Substrate Surfaces. J. Mater. Sci. Mater. Electron. 2013, 24, 4739–4744. [Google Scholar] [CrossRef]
- Sutter, P.; Sutter, E. Growth Mechanisms of Anisotropic Layered Group IV Chalcogenides on van Der Waals Substrates for Energy Conversion Applications. ACS Appl. Nano Mater. 2018, 1, 3026–3034. [Google Scholar] [CrossRef]
- Lee, H.; Yang, W.; Tan, J.; Park, J.; Shim, S.G.; Park, Y.S.; Yun, J.W.; Kim, K.M.; Moon, J. High-Performance Phase-Pure SnS Photocathodes for Photoelectrochemical Water Splitting Obtained via Molecular Ink-Derived Seed-Assisted Growth of Nanoplates. ACS Appl. Mater. Interfaces 2020, 12, 15155–15166. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schläfer, J.; Graf, D.; Fornalczyk, G.; Mettenbörger, A.; Mathur, S. Fluorinated Cerium(IV) Enaminolates: Alternative Precursors for Chemical Vapor Deposition of CeO2 Thin Films. Inorg. Chem. 2016, 55, 5422–5429. [Google Scholar] [CrossRef]
- Müller, R.; Hernandez-Ramirez, F.; Shen, H.; Du, H.; Mader, W.; Mathur, S. Influence of Precursor Chemistry on Morphology and Composition of CVD-Grown SnO2 Nanowires. Chem. Mater. 2012, 24, 4028–4035. [Google Scholar] [CrossRef]
- Graf, D.; Schläfer, J.; Garbe, S.; Klein, A.; Mathur, S. Interdependence of Structure, Morphology, and Phase Transitions in CVD Grown VO2 and V2O3 Nanostructures. Chem. Mater. 2017, 29, 5877–5885. [Google Scholar] [CrossRef] [Green Version]
- Gahlot, S.; Purohit, B.; Jeanneau, E.; Mishra, S. Coinage Metal Complexes with Di-Tertiary-Butyl Sulfide as Precursors with Ultra-Low Decomposition Temperature. Chem. Eur. J. 2021, 27, 10826–10832. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atamtürk, U.; Brune, V.; Mishra, S.; Mathur, S. Vapor Phase Synthesis of SnS Facilitated by Ligand-Driven “Launch Vehicle” Effect in Tin Precursors. Molecules 2021, 26, 5367. https://doi.org/10.3390/molecules26175367
Atamtürk U, Brune V, Mishra S, Mathur S. Vapor Phase Synthesis of SnS Facilitated by Ligand-Driven “Launch Vehicle” Effect in Tin Precursors. Molecules. 2021; 26(17):5367. https://doi.org/10.3390/molecules26175367
Chicago/Turabian StyleAtamtürk, Ufuk, Veronika Brune, Shashank Mishra, and Sanjay Mathur. 2021. "Vapor Phase Synthesis of SnS Facilitated by Ligand-Driven “Launch Vehicle” Effect in Tin Precursors" Molecules 26, no. 17: 5367. https://doi.org/10.3390/molecules26175367