Unambiguous NMR Structural Determination of (+)-Catechin—Laccase Dimeric Reaction Products as Potential Markers of Grape and Wine Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Dimeric Reaction Products Profiles with Three Different Oxydoreductases and (+)-Catechin
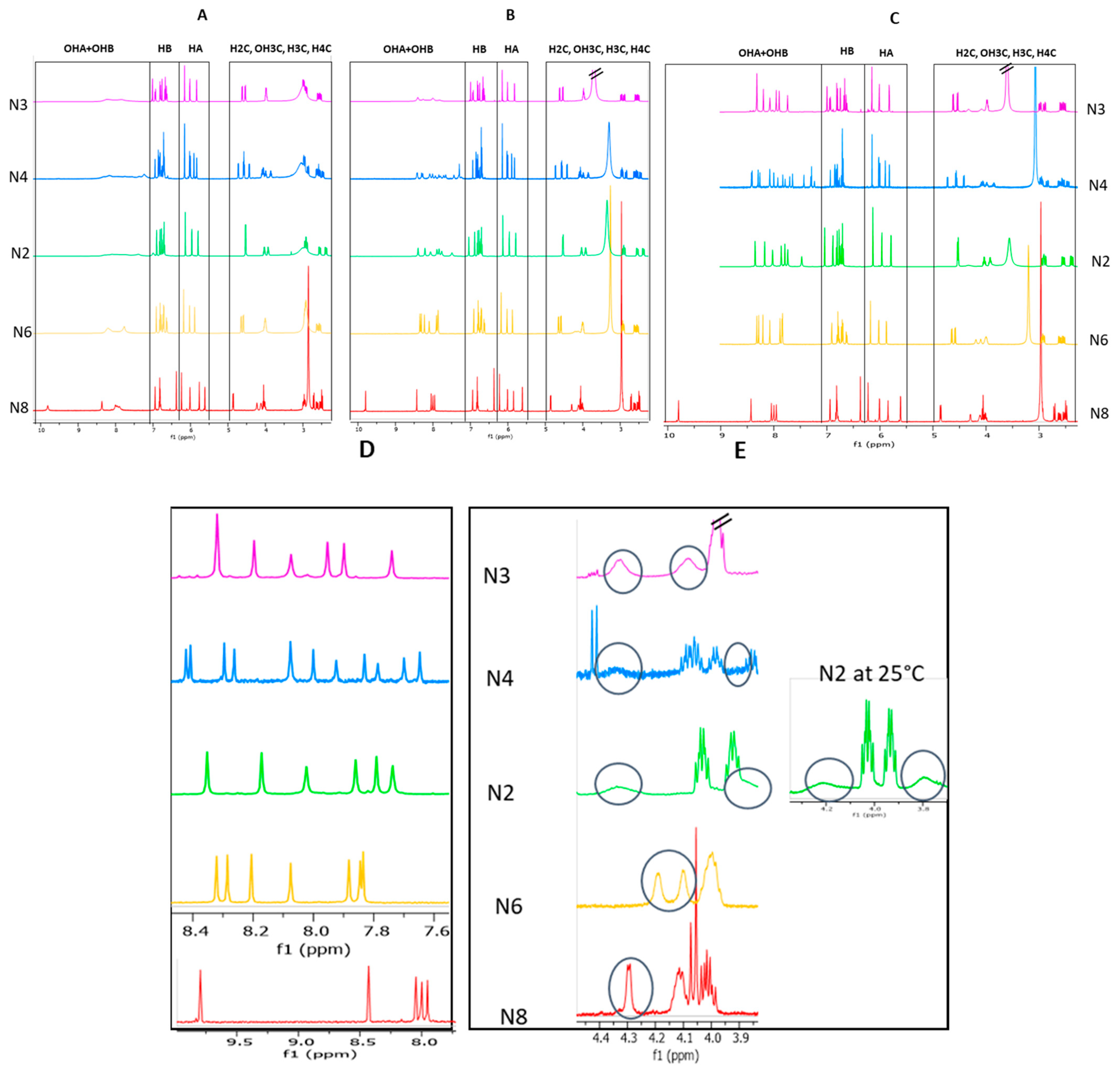
2.2. Study and Optimization of Physicochemical Parameters on 1H-NMR Phenolic and Aliphatic OH Signals
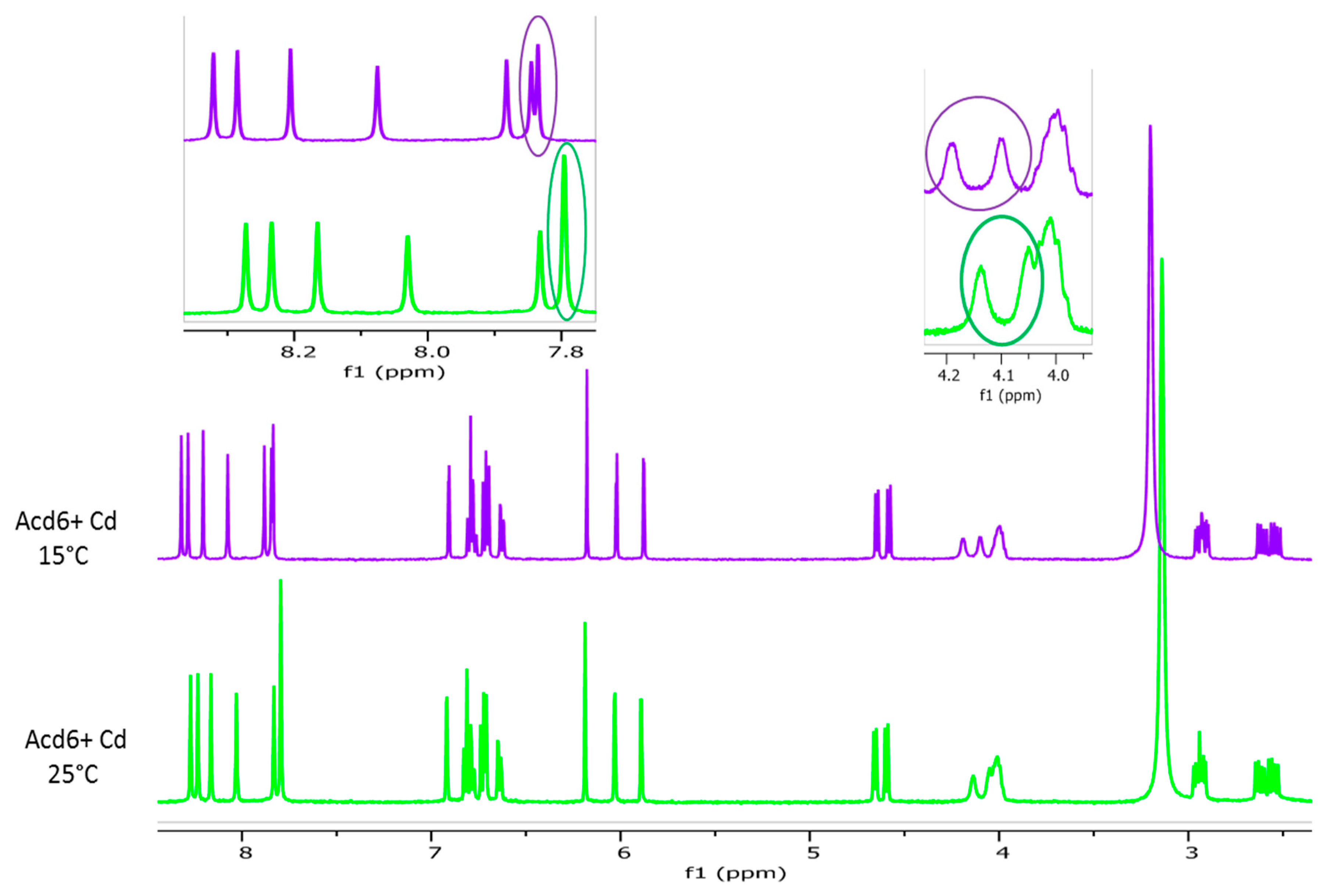
2.2.1. Effect of Cadmium Addition
2.2.2. Effect of the Temperature
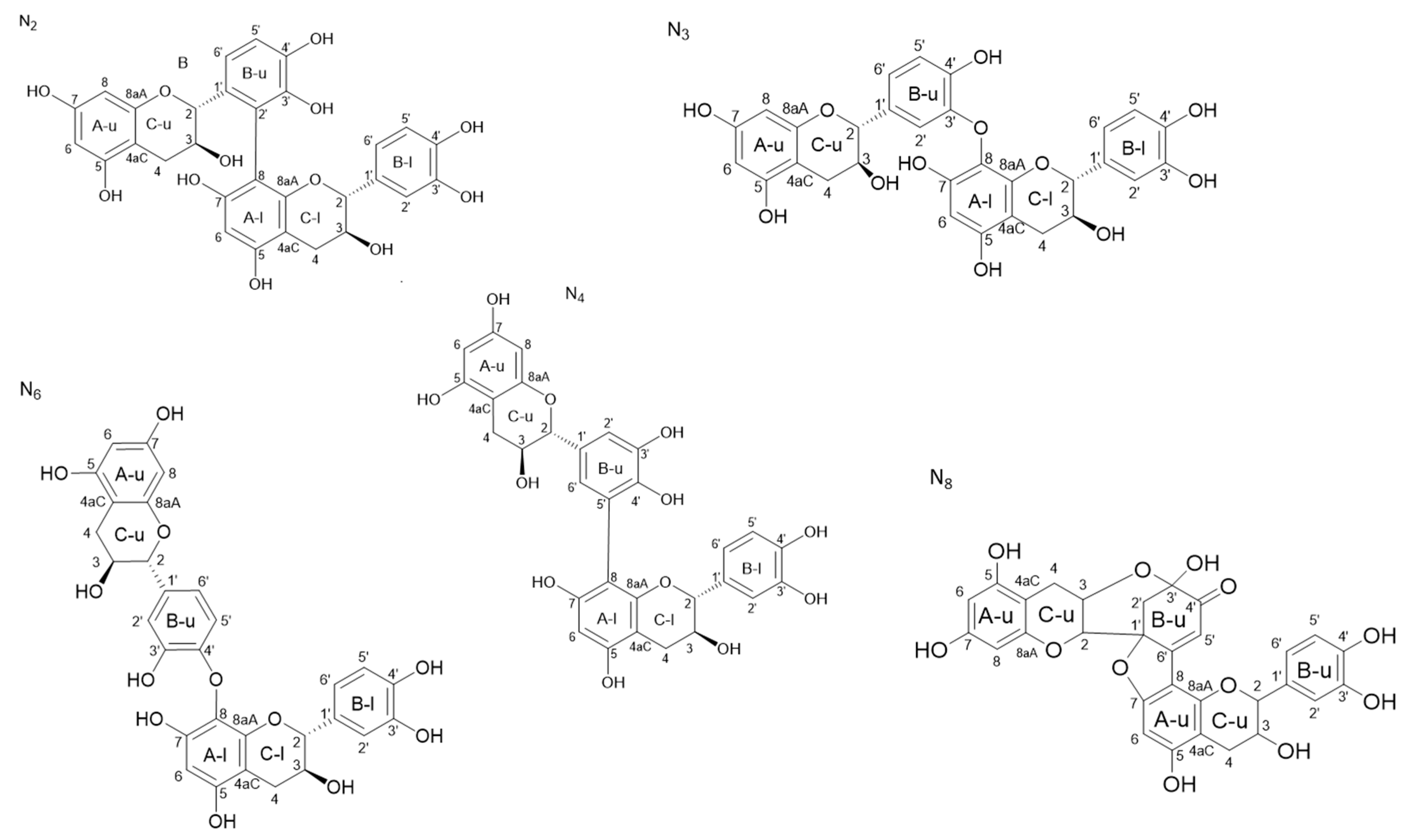
2.3. Structural Characterization of the Dimeric Standards—NMR Spectrum Analysis
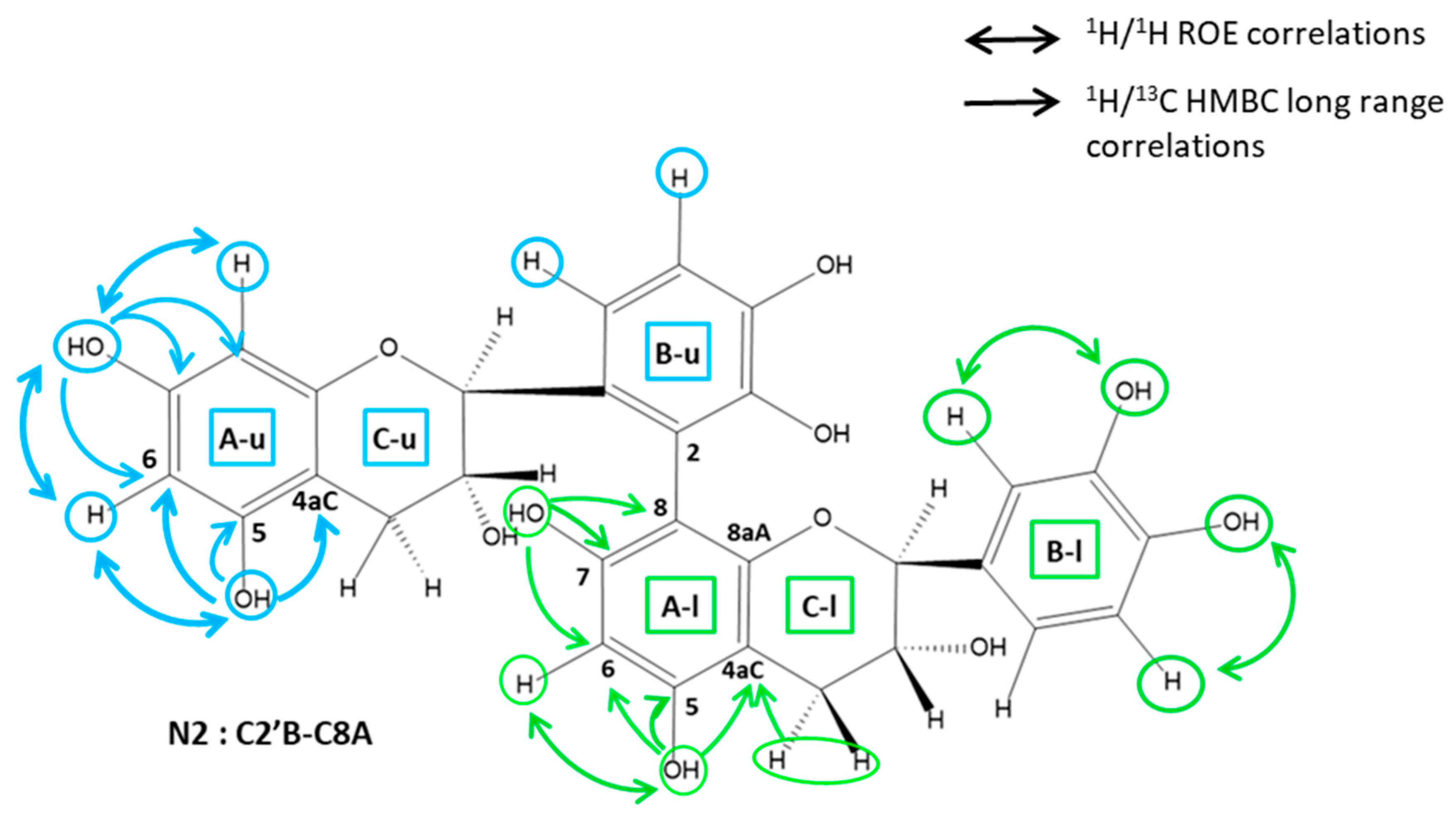
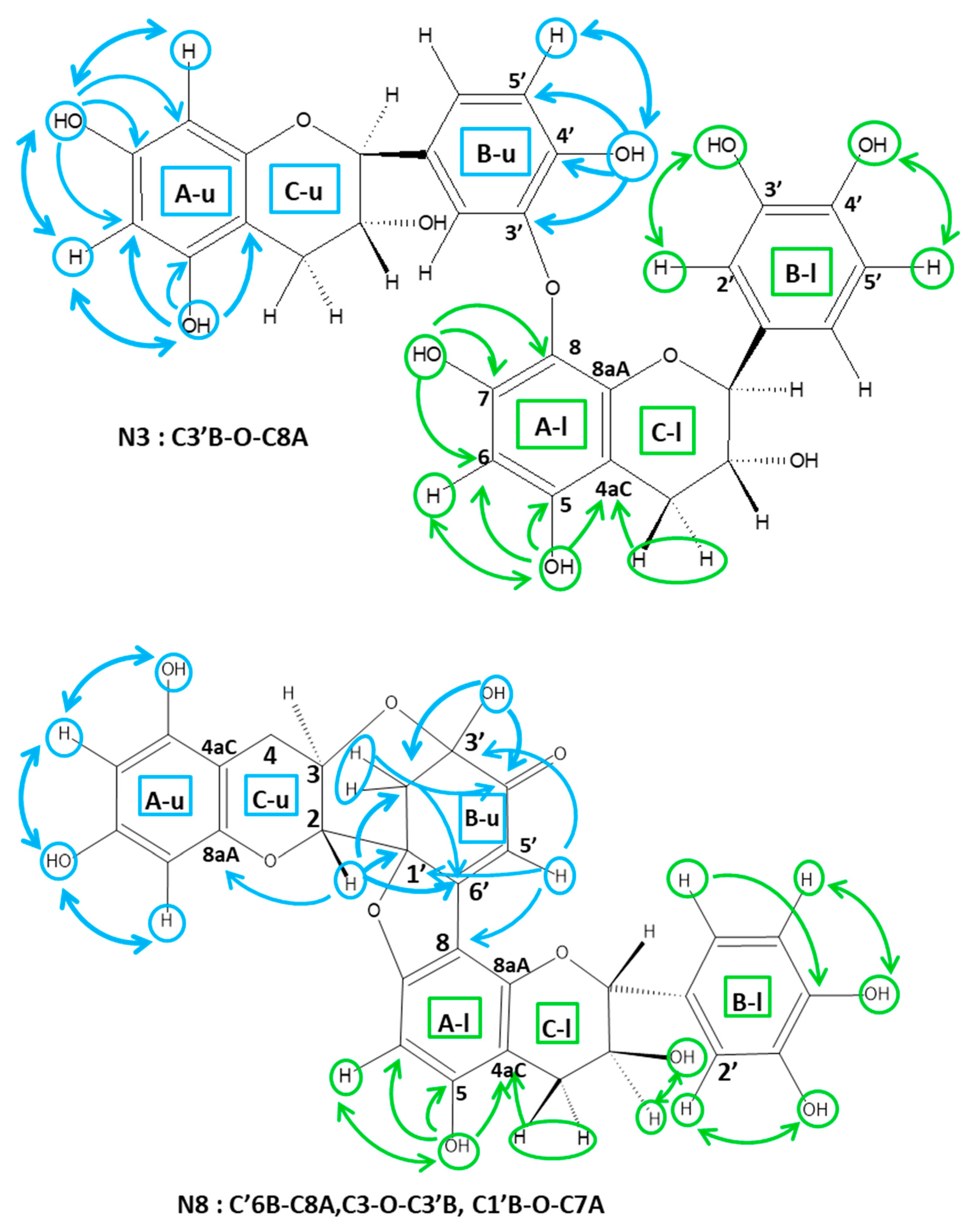
2.3.1. Determination of the A Ring Position of the IFL of the Dimers of Fractions N2, N3, N4, and N6
2.3.2. Determination of the B Ring Position of the IFL
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Model Wine Solution
3.3. Crude Grape PPO Extracts
3.4. Laccase from Botrytis Cinerea
3.5. Oxidation Procedure
3.6. Reaction Stopping on Resin Amberlite XAD7HP
3.7. Purification Procedure of the Dimeric Fraction Using Flash Chromatography
3.8. Purification Procedure of Oxidation Products from the Dimeric Fraction Using a Semi-Preparative Chromatographic System
3.9. Sample Preparation for NMR Analysis
3.10. Instrument Specifications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Khan, N.; Mukhtar, H. Tea Polyphenols for Health Promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of New Flavan-3-Ol Derivatives in Fermented Cocoa Beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from Cocoa and Vascular Health—A Critical Review. Int. J. Mol. Sci. 2009, 10, 4290–4309. [Google Scholar] [CrossRef]
- Avram, A.M.; Morin, P.; Brownmiller, C.; Howard, L.R.; Sengupta, A.; Wickramasinghe, S.R. Concentrations of Polyphenols from Blueberry Pomace Extract Using Nanofiltration. Food Bioprod. Process. 2017, 106, 91–101. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of Polyphenols and Evaluation of Antioxidant Capacity in Grape Pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Saucier, C. How Do Wine Polyphenols Evolve during Wine Ageing? Cerevisia 2010, 35, 11–15. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- Mathew, A.G.; Parpia, H.A.B. Food Browning as a Polyphenol Reaction. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Stewart, G.F., Eds.; Academic Press: Cambridge, MA, USA, 1971; Volume 19, pp. 75–145. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Ugliano, M.; Moio, L. Evolution of Phenolic Compounds and Astringency during Aging of Red Wine: Effect of Oxygen Exposure before and after Bottling. J. Agric. Food Chem. 2013, 61, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.-B.; Vidal, S.; Cheynier, V. Sensory Characteristics Changes of Red Grenache Wines Submitted to Different Oxygen Exposures Pre and Post Bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef]
- Dubernet, M.; Ribereau-Gayon, P.; Lerner, H.R.; Harel, E.; Mayer, A.M. Purification and Properties of Laccase from Botrytis Cinerea. Phytochemistry 1977, 16, 191–193. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Ployon, S.; Attina, A.; Vialaret, J.; Walker, A.S.; Hirtz, C.; Saucier, C. Laccases 2 & 3 as Biomarkers of Botrytis Cinerea Infection in Sweet White Wines. Food Chem. 2020, 315, 126233. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A Comprehensive Review of Its Mechanism. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Du Toit, W.J.; Marais, J.; Pretorius, I.S.; du Toit, M. Oxygen in Must and Wine: A Review. S. Afr. J. Enol. Vitic. 2006, 27, 76–94. [Google Scholar] [CrossRef] [Green Version]
- Kutyrev, A.A.; Moskva, V.V. Nucleophilic Reactions of Quinones. Russ. Chem. Rev. 1991, 60, 72. [Google Scholar] [CrossRef]
- Guyot, S.; Cheynier, V.; Souquet, J.M.; Moutounet, M. Influence of PH on the Enzymic Oxidation of (+)-Catechin in Model Systems. J. Agric. Food Chem. 1995, 43, 2458–2462. [Google Scholar] [CrossRef]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Structural Determination of Colourless and Yellow Dimers Resulting from (+)-Catechin Coupling Catalysed by Grape Polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Sun, W.; Miller, J.M. Tandem Mass Spectrometry of the B-Type Procyanidins in Wine and B-Type Dehydrodicatechins in an Autoxidation Mixture of (+)-Catechin and (−)-Epicatechin. J. Mass Spectrom. 2003, 38, 438–446. [Google Scholar] [CrossRef]
- Jiménez-Atiénzar, M.; Cabanes, J.; Gandía-Herrero, F.; García-Carmona, F. Kinetic Analysis of Catechin Oxidation by Polyphenol Oxidase at Neutral PH. Biochem. Biophys. Res. Commun. 2004, 319, 902–910. [Google Scholar] [CrossRef]
- Cheynier, V.; Basire, N.; Rigaud, J. Mechanism of Trans-Caffeoyltartaric Acid and Catechin Oxidation in Model Solutions Containing Grape Polyphenoloxidase. J. Agric. Food Chem. 1989, 37, 1069–1071. [Google Scholar] [CrossRef]
- López-Serrano, M.; Ros Barceló, A. Reversed-Phase and Size-Exclusion Chromatography as Useful Tools in the Resolution of Peroxidase-Mediated (+)-Catechin Oxidation Products. J. Chromatogr. A 2001, 919, 267–273. [Google Scholar] [CrossRef]
- Mouls, L.; Fulcrand, H. UPLC-ESI-MS Study of the Oxidation Markers Released from Tannin Depolymerization: Toward a Better Characterization of the Tannin Evolution over Food and Beverage Processing: Oxidation Markers Released from Tannin Depolymerization. J. Mass Spectrom. 2012, 47, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Mouls, L.; Fulcrand, H. Identification of New Oxidation Markers of Grape-Condensed Tannins by UPLC-MS Analysis after Chemical Depolymerization. Tetrahedron 2015, 71, 3012–3019. [Google Scholar] [CrossRef]
- López-Serrano, M.; Ros Barceló, A. Comparative Study of the Products of the Peroxidase-Catalyzed and the Polyphenoloxidase-Catalyzed (+)-Catechin Oxidation. Their Possible Implications in Strawberry (Fragaria × Ananassa) Browning Reactions. J. Agric. Food Chem. 2002, 50, 1218–1224. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.P.; Cheynier, V.; Le Guernevé, C.; Hollman, P.C.H.; Gruppen, H. Efficient Isolation of Major Procyanidin A-Type Dimers from Peanut Skins and B-Type Dimers from Grape Seeds. Food Chem. 2009, 117, 713–720. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Guernevé, C.L.; Ducrot, P.H. Application of NMR Spectroscopy and Mass Spectrometry to the Structural Elucidation of Modified Flavan-3-ols and Their Coupling Reaction Products*. Spectrosc. Lett. 2008, 41, 41–56. [Google Scholar] [CrossRef]
- De Bruyne, T.; Pieters, L.A.C.; Dommisse, R.A.; Kolodziej, H.; Wray, V.; Domke, T.; Vlietinck, A.J. Unambiguous Assignments for Free Dimeric Proanthocyanidin Phenols from 2D NMR. Phytochemistry 1996, 43, 265–272. [Google Scholar] [CrossRef]
- Charisiadis, P.; Primikyri, A.; Exarchou, V.; Tzakos, A.; Gerothanassis, I.P. Unprecedented Ultra-High-Resolution Hydroxy Group 1H NMR Spectroscopic Analysis of Plant Extracts. J. Nat. Prod. 2011, 74, 2462–2466. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Jaschok-Kentner, B.; Wray, V.; Winterhalter, P. Structure Elucidation of Procyanidin Oligomers by Low-Temperature 1H NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Weinges, K.; Ebert, W.; Huthwelker, D.; Mattauch, H.; Perner, J. Oxydative Kupplung von Phenolen, II1) Konstitution und Bildungsmechanismus des Dehydro-dicatechins A. Justus Liebigs Ann. Chem. 1969, 726, 114–124. [Google Scholar] [CrossRef]
- Zou, H.; Kilmartin, P.A.; Inglis, M.J.; Frost, A. Extraction of Phenolic Compounds during Vinification of Pinot Noir Wine Examined by HPLC and Cyclic Voltammetry. Aust. J. Grape Wine Res. 2002, 8, 163–174. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar]
- Quijada-Morin, N.; Garcia, F.; Lambert, K.; Walker, A.S.; Tiers, L.; Viaud, M.; Sauvage, F.X.; Hirtz, C.; Saucier, C. Strain Effect on Extracellular Laccase Activities from Botrytis Cinerea. Aust. J. Grape Wine Res. 2018, 24, 241–251. [Google Scholar] [CrossRef]
- Spinelli, D.; Fatarella, E.; Di Michele, A.; Pogni, R. Immobilization of Fungal (Trametes Versicolor) Laccase onto Amberlite IR-120 H Beads: Optimization and Characterization. Process Biochem. 2013, 48, 218–223. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Guernevé, C.; Hallé, H.; Meudec, E.; Véran, F.; Williams, P.; Robillard, B.; Garcia, F.; Poncet-Legrand, C.; Cheynier, V. Multimethod Approach for Extensive Characterization of Gallnut Tannin Extracts. J. Agric. Food Chem. 2020, 68, 13426–13438. [Google Scholar] [CrossRef]


| Compound | Rt (min) | λmax (nm) | m/z (Th) | Molar Yield (%) |
|---|---|---|---|---|
| N1 | 6.14 ± 0.02 | 280 | 579 | 0.4 |
| N2 | 6.91 ± 0.02 | 280 | 579 | 0.1 |
| N3 | 10.34 ± 0.03 | 280 | 579 | 0.7 |
| N4 | 14.11 ± 0.07 | 280 | 579 | 0.3 |
| N5 | 17.13 ± 0.02 | 280 | 579 | 0.2 |
| N6 | 19.03 ± 0.01 | 280 | 579 | 0.7 |
| N7 | 20.63 ± 0.03 | 263, 318 | 577 | 0.1 |
| N8 | 25.23 ± 0.01 | 256, 280, 286 | 577 | 0.2 |
| Rt (min) | |||
|---|---|---|---|
| Compound | Laccase from Trametes versicolor | Laccase from Botrytis cinerea | Polyphenoloxidase Extracted from Grapes |
| N1 | 6.14 ± 0.02 | 6.14 ± 0.02 | 6.12 ± 0.01 |
| N2 | 6.91 ± 0.02 | 6.86 ± 0.01 | 6.80 ± 0.01 |
| N3 | 10.34 ± 0.03 | 10.29 ± 0.02 | 10.29 ± 0.01 |
| N4 | 14.11 ± 0.07 | 14.10 ± 0.01 | 14.04 ± 0.05 |
| N4′ | / | 15.67 ± 0.01 | 15.66 ± 0.05 |
| N5 | 17.13 ± 0.02 | 17.12 ± 0.04 | 17.11 ± 0.06 |
| N6 | 19.03 ± 0.01 | 19.00 ± 0.003 | 19.00 ± 0.03 |
| N7 | 20.63 ± 0.03 | 20.59 ± 0.01 | 20.60 ± 0.03 |
| N8 | 25.23 ± 0.01 | 25.21 ± 0.02 | 25.22 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshaies, S.; le Guernevé, C.; Suc, L.; Mouls, L.; Garcia, F.; Saucier, C. Unambiguous NMR Structural Determination of (+)-Catechin—Laccase Dimeric Reaction Products as Potential Markers of Grape and Wine Oxidation. Molecules 2021, 26, 6165. https://doi.org/10.3390/molecules26206165
Deshaies S, le Guernevé C, Suc L, Mouls L, Garcia F, Saucier C. Unambiguous NMR Structural Determination of (+)-Catechin—Laccase Dimeric Reaction Products as Potential Markers of Grape and Wine Oxidation. Molecules. 2021; 26(20):6165. https://doi.org/10.3390/molecules26206165
Chicago/Turabian StyleDeshaies, Stacy, Christine le Guernevé, Lucas Suc, Laetitia Mouls, François Garcia, and Cédric Saucier. 2021. "Unambiguous NMR Structural Determination of (+)-Catechin—Laccase Dimeric Reaction Products as Potential Markers of Grape and Wine Oxidation" Molecules 26, no. 20: 6165. https://doi.org/10.3390/molecules26206165






