Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies
Abstract
:1. Introduction
2. Results and Discussion
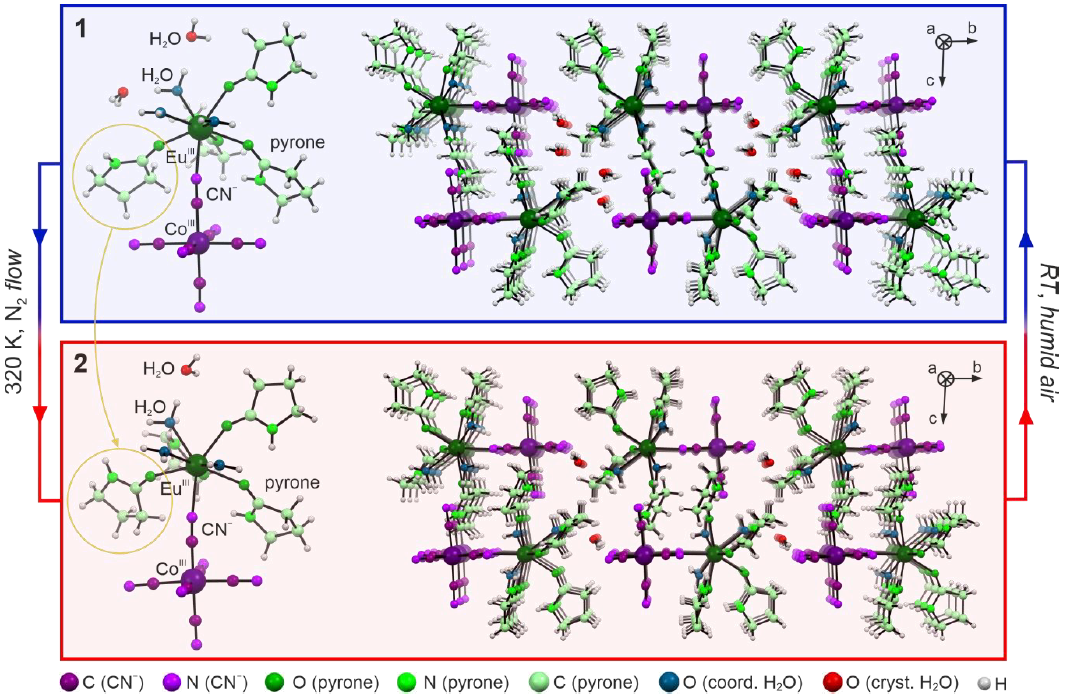
2.1. Basic Characterization and Structural Studies
2.2. Sorption Properties
2.3. Photoluminescent Properties
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis and Basic Characterization of 1
4.3. Preparation and Basic Characterization of 2
4.4. Crystallographic Studies
4.5. Physical Techniques
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, W.-Q.; Fu, D.-W.; Ye, H.-Y.; Liu, C.-M.; Chen, Z.-N.; Xiong, R.-G. The first organic-inorganic hybrid luminescent multiferroic: (Pyrrolidinium)MnBr3. Adv. Mater. 2015, 27, 3942–3946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Wen, T.; Zhou, Y.; Li, N.; Han, F.; Xiao, Y.; Chow, P.; Sun, J.; Pravica, M.; et al. Pressure-driven cooperative spin-crossover, large-volume collapse, and semiconductor-to-metal transition in manganese(II) honeycomb lattices. J. Am. Chem. Soc. 2016, 138, 15751–15757. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Tsunobuchi, Y.; Matsuda, T.; Hashimoto, K.; Namai, A.; Hakoe, F.; Tokoro, H. Synthesis of a metal oxide with a room-temperature photoreversible phase transition. Nat. Chem. 2010, 2, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Fittipaldi, M.; Cini, A.; Annino, G.; Vindigni, A.; Caneschi, A.; Sessoli, R. Electric field modulation of magnetic exchange in molecular helices. Nat. Mater. 2019, 18, 329–334. [Google Scholar] [CrossRef]
- Kimura, T.; Goto, T.; Shintani, H.; Ishizaka, K.; Arima, T.; Tokura, Y. Magnetic control of ferroelectric polarization. Nature 2003, 426, 55–58. [Google Scholar] [CrossRef]
- Vogelsberg, C.S.; Garcia-Garibay, M.A. Crystalline molecular machines: Function, phase order, dimentionality, and composition. Chem. Soc. Rev. 2012, 41, 1892–1910. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C.T.; Morsali, A.; Zhou, H.-C. Switching of metal-organic frameworks. Angew. Chem. Int. Ed. 2020, 59, 4652–4669. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide organic framework luminescent thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-W.; Kitagawa, H. Proton transport in metal-organic frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Decker, G.E.; Lorzing, G.R.; Deegan, M.M.; Bloch, E.D. MOF-mimetic molecules: Carboxylate-based supramolecular complexes as molecular metal-organic framework analogues. J. Mater. Chem. A 2020, 8, 4217–4229. [Google Scholar] [CrossRef]
- Hang, T.; Zhang, W.; Ye, H.-Y.; Xiong, R.-G. Metal-organic complex ferroelectrics. Chem. Soc. Rev. 2011, 40, 3577–3598. [Google Scholar] [CrossRef]
- Létard, J.-F. Photomagnetism of iron(II) spin crossover complexes–the T(LIESST) approach. J. Mater. Chem. 2006, 16, 2550–2559. [Google Scholar] [CrossRef]
- Chorazy, S.; Stanek, J.J.; Nogaś, W.; Majcher, A.M.; Rams, M.; Kozieł, M.; Juszyńska-Gałązka, E.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B.; et al. Tuning of charge transfer assisted phase transition and slow magnetic relaxation functionalities in {Fe9-xCox[W(CN)8]6} (x = 0–9) molecular solid solution. J. Am. Chem. Soc. 2016, 138, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Flores Gonzalez, J.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334. [Google Scholar] [CrossRef]
- Otto, S.; Harris, J.P.; Heinze, K.; Reber, C. Molecular ruby under pressure. Angew. Chem. Int. Ed. 2018, 57, 11069–11973. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3228. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pillet, S.; de Graaf, C.; Magott, M.; Bendeif, E.-E.; Guionneau, P.; Rouzières, M.; Marvaud, V.; Stefańczyk, O.; Pinkowicz, D.; et al. Photoinduced Mo-CN bond breakage in octacyanomolybdate leading to spin triplet trapping. Angew. Chem. Int. Ed. 2020, 59, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Wyczesany, M.; Sieklucka, B. Lanthanide photoluminescence in heterometallic polycyanidometallates-based coordination networks. Molecules 2017, 22, 1902. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, H.; Ito, A.; Sakuda, E.; Kitamura, N.; Takayama, T.; Sekine, T.; Shinohara, A.; Yoshimura, T. Excited-state characteristics of tetracyanidonitridorhenium(V) and -technetium(V) complexes with N-heteroaromatic ligands. Inorg. Chem. 2013, 52, 6319–6327. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Takano, S.; Imoto, K.; Yoshikiyo, M.; Namai, A.; Tokoro, H. 90-degree optical switching of output second-harmonic light in chiral photomagnet. Nat. Photonics 2014, 8, 65–71. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Rams, M.; Mišek, M.; Kamenev, K.V.; Tomkowiak, H.; Katrusiak, A.; Sieklucka, B. Enforcing multifunctionality: A pressure-pressure induces spin-crossover photomagnet. J. Am. Chem. Soc. 2015, 137, 8795–8802. [Google Scholar] [CrossRef]
- Yersin, H.; Trümbach, D.; Strasser, J.; Patterson, H.H.; Assefa, Z. Tunable radiationless energy transfer in Eu[Au(CN)2]3·3H2O by high pressure. Inorg. Chem. 1998, 37, 3209–3216. [Google Scholar] [CrossRef]
- Mahfoud, T.; Molnár, G.; Bonhommeau, S.; Cobo, S.; Salmon, L.; Demont, P.; Tokoro, H.; Ohkoshi, S.; Boukheddaden, K.; Bousseksou, A. Electric-field-induced charge-transfer phase transition: A promising approach toward electrically switchable devices. J. Am. Chem. Soc. 2009, 131, 15049–15054. [Google Scholar] [CrossRef]
- Jankowski, R.; Reczyński, M.; Chorazy, S.; Zychowicz, M.; Arczyński, M.; Kozieł, M.; Ogorzały, K.; Makowski, W.; Pinkowicz, D.; Sieklucka, B. Guest-dependent pressure-induced spin crossover in FeII4[MIV(CN)8]2 (M=Mo,W) cluster-based material showing persistent solvent-driven structural transformations. Chem. Eur. J. 2020, 26, 11187–11198. [Google Scholar] [CrossRef]
- Milon, J.; Daniel, M.-C.; Kaiba, A.; Guionneau, P.; Brandès, S.; Sutter, J.-P. Nanoporous magnets of chiral and racemic [{Mn(HL)}2Mn{Mo(CN)7}2] with switchable ordering temperatures (TC = 85 K ⇿ 106 K) driven by H2O sorption (L = N,N-Dimethylalaninol). J. Am. Chem. Soc. 2007, 129, 13872–13878. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Southerland, H.I.; Avendaño, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clérac, R.; Nehrkom, J.; et al. Cyanide single-molecule magnets exhibiting solvent dependent reversible “on” and “off” exchange bias behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef] [PubMed]
- Magott, M.; Reczyński, M.; Gaweł, B.; Sieklucka, B.; Pinkowicz, D. A photomagnetic sponge: High-temperature light-induced ferrimagnet controlled by water sorption. J. Am. Chem. Soc. 2018, 140, 15876–15882. [Google Scholar] [CrossRef]
- Tokoro, H.; Ohkoshi, S.; Matsuda, T.; Hashimoto, K. A large thermal hysteresis loop produced by a charge-transfer phase transition in a rubidium manganese hexacyanoferrate. Inorg. Chem. 2004, 43, 5231–5236. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Liberka, M.; Zychowicz, M.; Chorazy, S. Diverse physical functionalities of rare-earth hexacyanidometallate frameworks and their molecular analogues. Inorg. Chem. Front. 2021, 8, 452–483. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Sieklucka, B.; Chorazy, S. Europium(III) photoluminescence governed by d8–d10 heterometallophilic interactions in trimetallic cyanido-bridged coordination frameworks. Inorg. Chem. 2020, 59, 1393–1404. [Google Scholar] [CrossRef]
- Jankowski, R.; Zakrzewski, J.J.; Surma, O.; Ohkoshi, S.; Chorazy, S.; Sieklucka, B. Near-infrared emissive Er(III) and Yb(III) molecular nanomagnets in metal-organic chains functionalized by octacyanidometallates(IV). Inorg. Chem. Front. 2019, 6, 2423–2434. [Google Scholar] [CrossRef]
- Davies, G.M.; Pope, S.J.A.; Adams, H.; Faulkner, S.; Ward, M.D. Structural and photophysical properties of coordination networks combining [Ru(bipy)(CN)4]2− anions and lanthanide(III) cations: Rates of photoinduced Ru-to-lanthanide energy transfer and sensitized near-infrared luminescence. Inorg. Chem. 2005, 44, 4656–4665. [Google Scholar] [CrossRef]
- Lazarides, T.; Davies, G.M.; Adams, H.; Sabatini, C.; Barigelletti, F.; Barbieri, A.; Pope, S.J.A.; Faulkner, S.; Ward, M.D. Ligand-field excited states of hexacyanochromate and hexacyanocobaltate as sensitizers for near-infrared luminescence from Nd(III) and Yb(III) in cyanide-bridged d-f assemblies. Photochem. Photobiol. Sci. 2007, 6, 1152–1157. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Larochelle, C.L.; Petterson, H.H. Tunable energy transfer from dicyanoaurate(I) and dicyanoargentate(I) donor ions to terbium(III) acceptor ions in pure crystals. Inorg. Chem. 2000, 39, 4527–4534. [Google Scholar] [CrossRef]
- Maynard, B.A.; Kalachnikova, K.; Whitehead, K.; Assefa, Z.; Sykora, R.E. Intramolecular energy transfer in a one-dimensional europium tetracyanoplatinate. Inorg. Chem. 2008, 47, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, J.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Dehydration-hydration switching of single-molecule magnet behavior and visible photoluminescence in a cyanido-bridged DyIIICoIII framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Proton conductive luminescent thermometer based on near-infrared emissive {YbCo2} molecular nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zakrzewski, J.J.; Zychowicz, M.; Vieru, V.; Chibotaru, L.F.; Nakabayashi, K.; Chorazy, S.; Ohkoshi, S. Holmium(III) molecular nanomagnets for optical thermometry exploring the luminescence re-absorption effect. Chem. Sci. 2021, 12, 730–741. [Google Scholar] [CrossRef]
- Errulat, D.; Marin, R.; Gálico, D.A.; Harriman, K.L.M.; Pialat, A.; Gabidullin, B.; Iikawa, F.; Couto, O.D.D., Jr.; Moilanen, J.O.; Hemmer, E.; et al. A luminescent thermometer exhibiting slow relaxation of the magnetization: Toward self-monitored building blocks for next-generation optomagnetic devices. ACS Cent. Sci. 2019, 5, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-X.; Zhang, W.-Y.; Wu, Z.-G.; Zheng, Y.-X.; Fu, D.-W. Enantiomorphic perovskite ferroelectrics with circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 4756–4761. [Google Scholar] [CrossRef]
- Kobayashi, A.; Imada, S.; Shigeta, Y.; Nagao, Y.; Yoshida, M.; Kato, M. Vapochromic luminescent proton conductors: Switchable vapochromism and proton conduction of luminescent Pt(II) complexes with proton-exchangeable sites. J. Mater. Chem. C 2019, 7, 14923–14931. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Reczyński, M.; Nakabayashi, K.; Ohkoshi, S.; Sieklucka, B. Humidity driven molecular switch based on photoluminescent DyIIICoIII single-molecule magnets. J. Mater. Chem. C 2019, 7, 4164–4172. [Google Scholar] [CrossRef]
- Petoud, S.; Cohen, S.M.; Bünzli, J.-C.G.; Raymond, K.N. Stable lanthanide luminescence agents highly emissive in aqueous solution: Multidentate 2-hydroxyisophthalamide complexes of Sm3+, Eu3+, Tb3+, Dy3+. J. Am. Chem. Soc. 2003, 125, 13324–13325. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.B.D.; Gonçalves, S.M.C.; Júnior, S.A.; Simas, A.M. A comprehensive strategy to boost the quantum yield of luminescence of europium complexes. Sci. Rep. 2013, 3, 2395. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Duan, C.-K.; Tanner, P.A. Luminescence and crystal field analysis of Eu3+ in Eu[Co(CN)6]·4H2O. J. Phys. Chem. Sol. 2007, 68, 1921–1925. [Google Scholar] [CrossRef]
- Kumar, K.; Chorazy, S.; Nakabayashi, K.; Sato, H.; Sieklucka, B.; Ohkoshi, S. TbCo and Tb0.5Dy0.5Co layered cyanido-bridged frameworks for construction of colorimetric and ratiometric luminescent thermometers. J. Mater. Chem. C 2018, 6, 8372–8384. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Optical properties of GdIII[MIII(CN)6] with M = Cr and Co. Phosphorescence form ligand-field states of [M(CN)6]3− under ambient conditions. Inorg. Chem. Commun. 2004, 7, 770–772. [Google Scholar] [CrossRef]
- Pandey, P.; Samanta, A.K.; Bandyopadhyay, B.; Chakraborty, T. Infrared spectroscopy of 2-pyrrolidinone and its hydrogen bonded dimers in a cold (8 K) inert gas matrix. Vib. Spectrosc. 2011, 50, 126–131. [Google Scholar] [CrossRef]
- Chorazy, S.; Rams, M.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S. White Light Emissive DyIII Single-Molecule Magnets Sensitized by Diamagnetic [CoIII(CN)6]3− Linkers. Chem. Eur. J. 2016, 22, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Wang, J.; Ohkoshi, S.; Sieklucka, B. Incorporation of hexacyanidoferrate(III) ion in photoluminescent trimetallic Eu(3-pyridone)[Co1−xFex(CN)6] chains exhibiting tunable visible light absorption and emission properties. CrystEngComm 2018, 20, 5695–5706. [Google Scholar] [CrossRef]



| Compound | 1 | 2 |
|---|---|---|
| formula | Eu1Co1C22H38N10O9 | Eu1Co1C22H36N10O8 |
| formula weight/g·mol−1 | 797.51 | 779.5 |
| T/K | 100(2) | |
| λ/Å | 0.71073 (Mo Kα) | |
| crystal system | triclinic | |
| space group | ||
| a/Å | 9.0135(5) | 9.032(1) |
| b/Å | 12.0910(7) | 12.089(2) |
| c/Å | 14.3010(8) | 14.023(2) |
| α/° | 90.199(2) | 95.815(4) |
| β/° | 92.429(2) | 90.186(4) |
| γ/° | 90.912(2) | 90.138(4) |
| V/Å3 | 1556.9 (2) | 1523.2(4) |
| Z | 2 | |
| ρcalc/g·cm−3 | 1.701 | 1.700 |
| μ/cm−1 | 2.592 | 2.645 |
| F(000) | 804 | 784 |
| crystal type | colorless plate | |
| crystal size/mm × mm × mm | 0.35 × 0.23 × 0.05 | |
| θ range/° | 2.725–25.026 | 2.345–25.027 |
| limiting indices | −10 < h < 10−14 < k < 14−15 < l < 17 | −10 < h < 10−14 < k < 14−16 < l < 16 |
| collected reflections | 15,970 | 16,135 |
| unique reflections | 5485 | 5354 |
| Rint | 0.0185 | 0.0346 |
| completeness/% | 99.7 | 99.5 |
| data/restraints/parameters | 5485/15/428 | 5354/13/411 |
| GOF on F2 | 1.097 | 1.153 |
| R1 [I ≥ 2σ(I)] | 0.0179 | 0.0319 |
| wR2 (all data) | 0.0444 | 0.0891 |
| largest diff. peak/hole/e∙Å−3 | 0.842/−0.408 | 1.896/−1.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, J.J.; Heczko, M.; Jankowski, R.; Chorazy, S. Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies. Molecules 2021, 26, 1102. https://doi.org/10.3390/molecules26041102
Zakrzewski JJ, Heczko M, Jankowski R, Chorazy S. Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies. Molecules. 2021; 26(4):1102. https://doi.org/10.3390/molecules26041102
Chicago/Turabian StyleZakrzewski, Jakub J., Michal Heczko, Robert Jankowski, and Szymon Chorazy. 2021. "Reversible Humidity-Driven Transformation of a Bimetallic {EuCo} Molecular Material: Structural, Sorption, and Photoluminescence Studies" Molecules 26, no. 4: 1102. https://doi.org/10.3390/molecules26041102






