Bis(dicarbollide) Complexes of Transition Metals as a Platform for Molecular Switches. Study of Complexation of 8,8′-Bis(methylsulfanyl) Derivatives of Cobalt and Iron Bis(dicarbollides)
Abstract
:1. Introduction
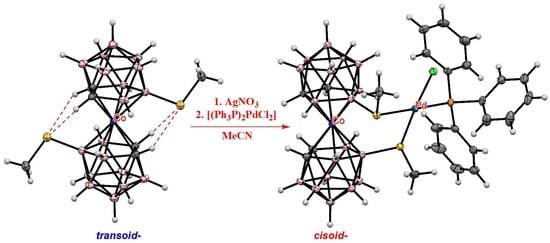
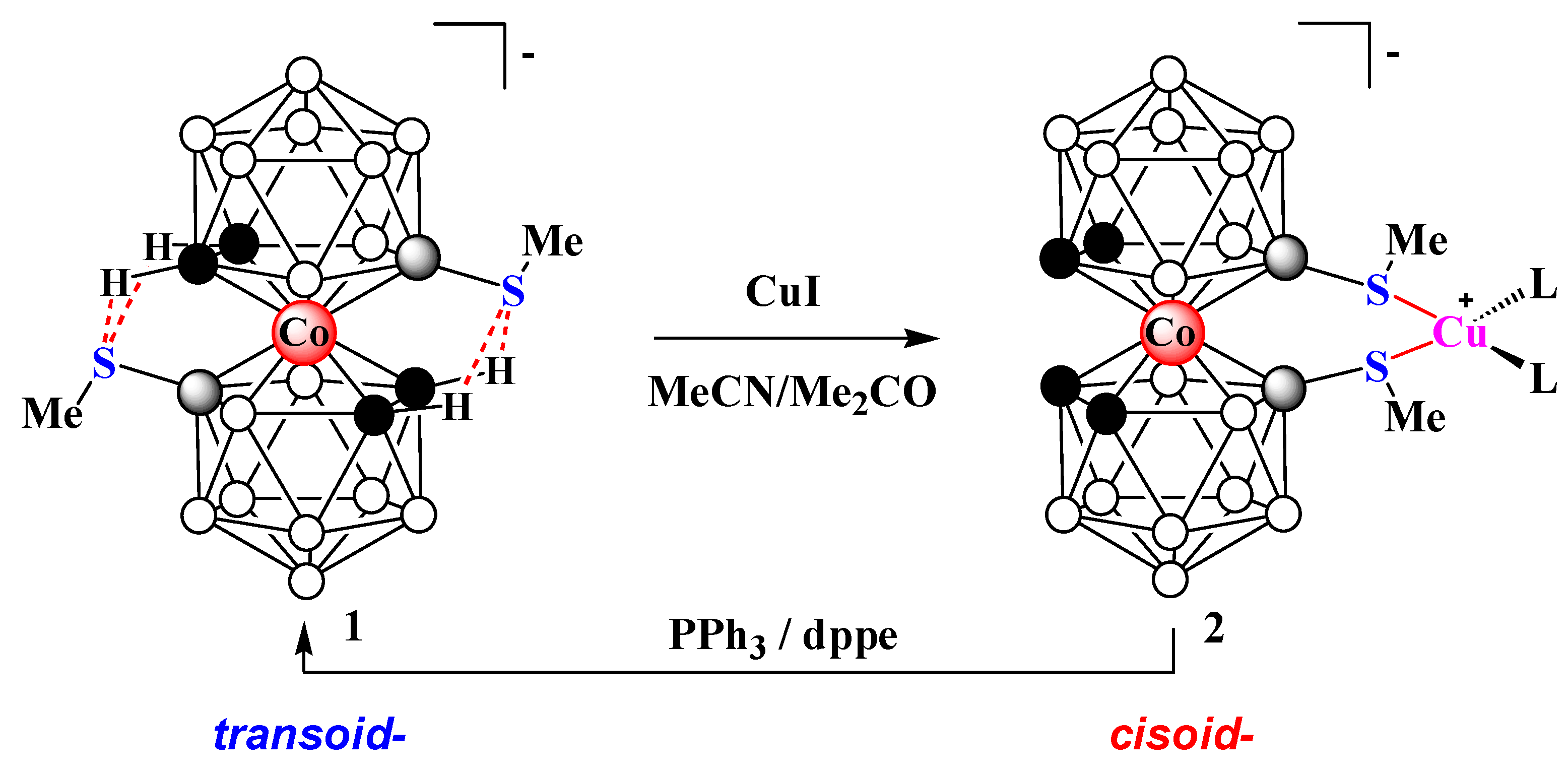
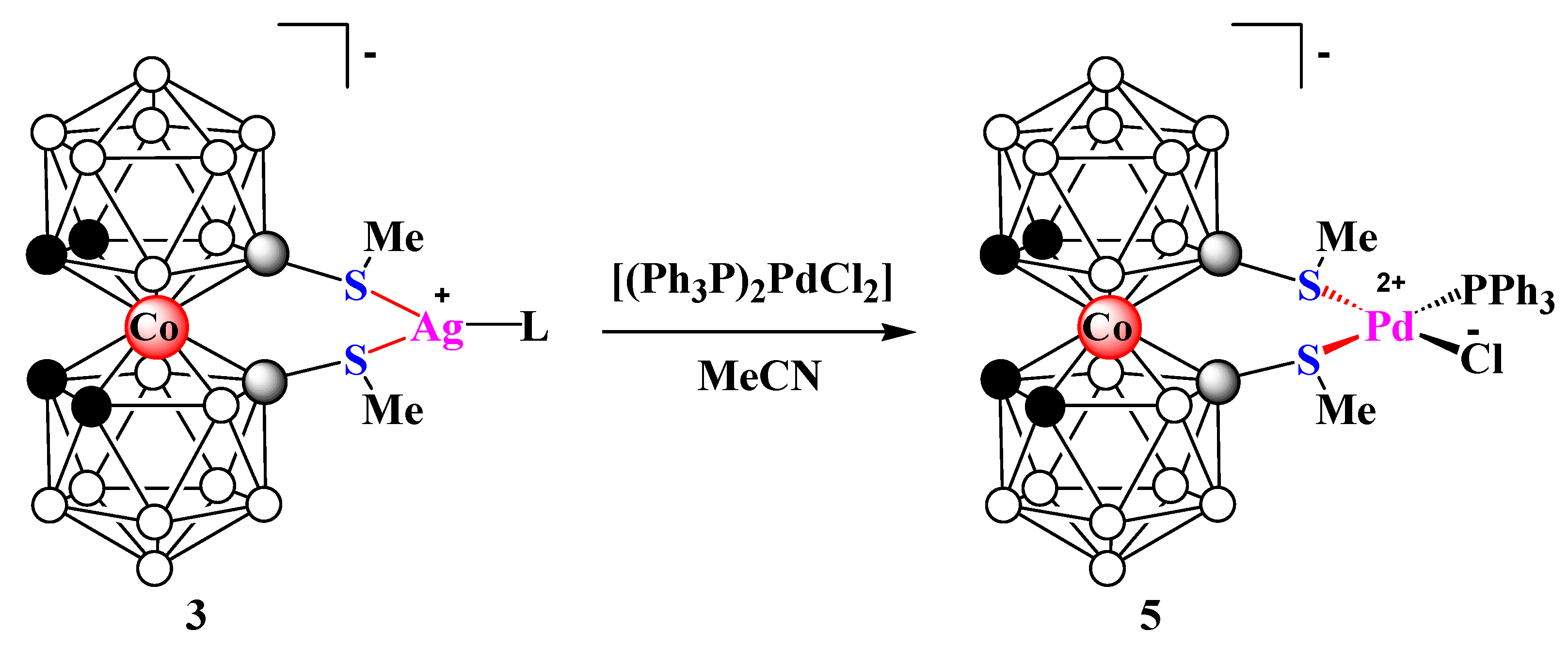
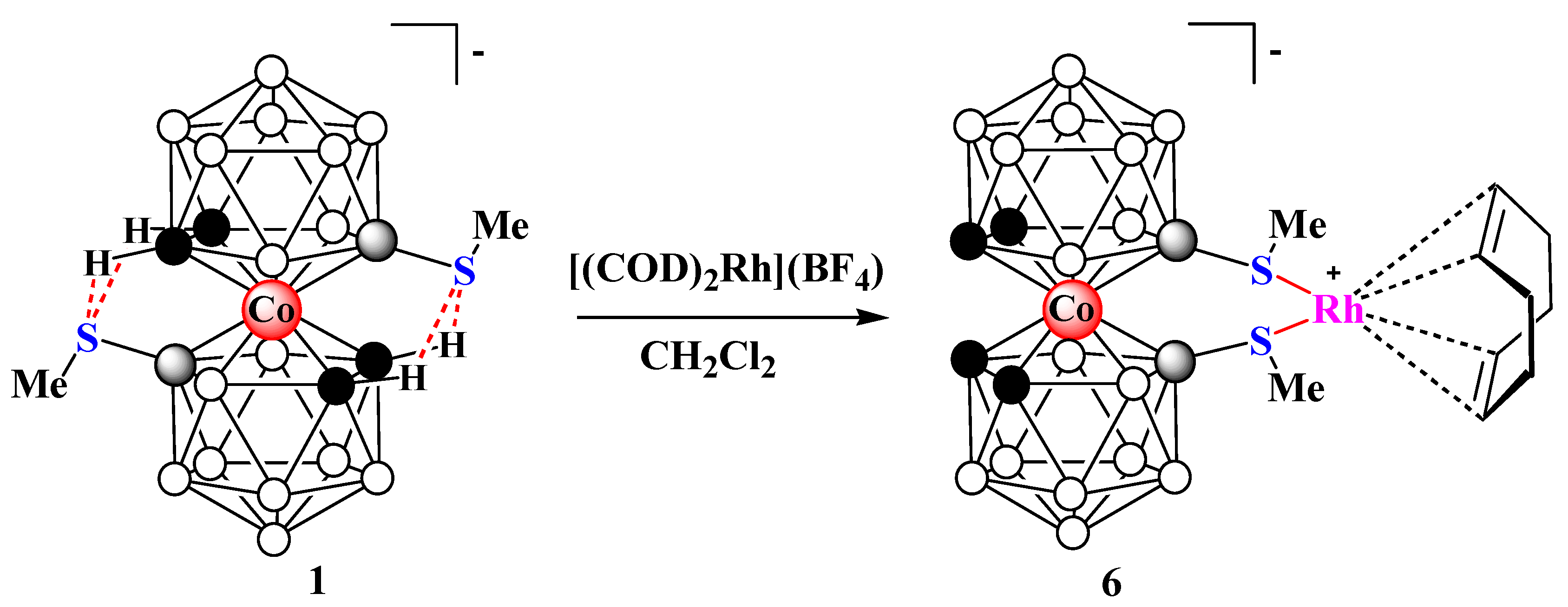
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Complex 2
3.3. Synthesis of Complex 3
3.4. Synthesis of Complex 4
3.5. Synthesis of Complex 5
3.6. Synthesis of Complex 6
3.7. Synthesis of Complex 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sauvage, J.-P. Molecular Machines and Motors (Structure and Bonding, Volume 99); Springer: Berlin, Germany, 2001; 302p, ISBN 978-354-041-382-0. [Google Scholar]
- Credi, A.; Silvi, S.; Venturi, M. Molecular Machines and Motors: Recent Advances and Perspectives (Topics in Current Chemistry, Volume 354); Springer: Heidelberg, Germany, 2014; 342p, ISBN 978-331-908-677-4. [Google Scholar]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial molecular machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Design of collective motions from synthetic molecular switches, rotors, and motors. Chem. Rev. 2020, 120, 310–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvage, J.-P. From chemical topology to molecular machines (Nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feringa, B.L. The art of building small: From molecular switches to motors (Nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddard, J.F. Mechanically interlocked molecules (MIMs)-molecular shuttles, switches, and machines (Nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–12125. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2006, 46, 72–191. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L.; Browne, W.R. Molecular Switches, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; 792p, ISBN 978-352-731-365-5. [Google Scholar]
- Canary, J.W.; Mortezaei, S.; Liang, J. Transition metal-based chiroptical switches for nanoscale electronics and sensors. Coord. Chem. Rev. 2010, 254, 2249–2266. [Google Scholar] [CrossRef]
- Perera, U.G.E.; Ample, F.; Kersell, H.; Zhang, Y.; Vives, G.; Echeverria, J.; Grisolia, M.; Rapenne, G.; Joachim, C.; Hla, S.-W. Controlled clockwise and anticlockwise rotational switching of a molecular motor. Nat. Nanotech. 2013, 8, 46–51. [Google Scholar] [CrossRef]
- Bianchi, A.; Delgado-Pinar, E.; García-Espana, E.; Giorgi, C.; Pina, F. Highlights of metal ion-based photochemical switches. Coord. Chem. Rev. 2014, 260, 156–215. [Google Scholar] [CrossRef]
- Collins, B.S.L.; Kistemaker, J.C.M.; Otten, E.; Feringa, B.L. A chemically powered unidirectional rotary molecular motor based on a palladium redox cycle. Nat. Chem. 2016, 8, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Goswami, A.; Saha, S.; Biswas, P.K.; Schmittel, M. (Nano)mechanical motion triggered by metal coordination: From functional devices to networked multicomponent catalytic machinery. Chem. Rev. 2020, 120, 125–199. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, I.B. Ferrocene and transition metal bis(dicarbollides) as platform for design of rotatory molecular switches. Molecules 2017, 22, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czech. Chem. Commun. 1999, 64, 783–805. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of nickel and iron bis(dicarbollides). A review. J. Organomet. Chem. 2000, 614–615, 27–36. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849–850, 170–194. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 2017, 4444–4451. [Google Scholar] [CrossRef] [Green Version]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Laskova, J.N.; Godovikov, A.A.; Fabrizi de Biani, F.; Corsini, M.; Sivaev, I.B.; Bregadze, V.I. Synthesis and structure of bis(methylsulfanyl) derivatives of iron bis(dicarbollide). J. Organomet. Chem. 2018, 865, 239–246. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Suponitsky, K.Y.; Filippov, O.A.; Sivaev, I.B. Synthesis and structure of methylsulfanyl derivatives of nickel bis(dicarbollide). Molecules 2019, 24, 4449. [Google Scholar] [CrossRef] [Green Version]
- Timofeev, S.M.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of cobalt bis(8-methylthio-1,2- dicarbollide)-pentacarbonyltungsten complexes. Russ. Chem. Bull. 2018, 67, 570–572. [Google Scholar] [CrossRef]
- Long, N.J.; Martin, J.; Opromolla, G.; White, A.J.P.; Williams, D.J.; Zanello, P. Synthesis and characterisation of 1-(diphenylphosphino)-1′-(methylsulfanyl)ferrocene and a series of metal (CuI, AgI)–ferrocenylene complexes. J. Chem. Soc. Dalton Trans. 1999, 1981–1986. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, S.; Akabori, S.; Habata, Y. Copper(I), silver(I), mercury(II), and palladium(II) complexes of tetrathia[n]ferrocenophanes. Bull. Chem. Soc. Jpn. 1986, 59, 1515–1519. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Suzuki, K.; Akabori, S. The preparation and some properties of 1,4,7-trithia[7]- and 1,5,9-trithia[9]-ferrocenophanes and their copper(I), silver(I), mercury(II), and palladium(II) complexes. Bull. Chem. Soc. Jpn. 1986, 59, 3611–3615. [Google Scholar] [CrossRef]
- Sato, M.; Anano, H. The transition-metal complexes of the thiamacrocycle containing two ferrocene nuclei in the main chain. Synthesis, properties, and molecular structure of Ag(I), Cu(I), Pd(II), and Pt(II) complexes of 1,5,16,21-tetrathia[5.5]ferrocenophane. J. Organomet. Chem. 1998, 555, 167–175. [Google Scholar] [CrossRef]
- Balagurova, E.V.; Pisareva, I.V.; Godovikov, I.A.; Smol’yakov, A.F.; Dolgushin, F.M.; Chizhevsky, I.T. Formation of “three-bridge” cobalt-copper commo-cluster with the (B-H)3...Cu bond in the reaction of [Cs][commo-3,3′-Co(1,2-C2B9H11)2] with copper(I) and copper(II) compounds. Russ. Chem. Bull. 2013, 62, 548–553. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Isomerism in complexes with the decahydro-closo-decaborate anion. Polyhedron 2016, 105, 205–221. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Silver and copper complexes with closo-polyhedral borane, carborane and metallacarborane anions: Synthesis and X-ray structure. Crystals 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, V.V.; Malinina, E.A.; Churakov, A.V.; Polyakova, I.N.; Kuznetsov, N.T. Ligand metathesis in copper(I) complex [Cu2(CH3CN)4[B10H10]] to form [Cu2L4[B10H10]] (L = Ph3P, 5Nphen). Polyhedron 2019, 169, 144–150. [Google Scholar] [CrossRef]
- Tsang, C.-W.; Sun, J.; Xie, Z. Monomeric and polymeric structures of silver salts of cobalt(III) bis(dicarbollide) ions. J. Organomet. Chem. 2000, 613, 99–104. [Google Scholar] [CrossRef]
- Westcott, A.; Whitford, N.; Hardie, M.J. Coordination polymers with carborane anions: Silver dinitrile complexes. Inorg. Chem. 2004, 43, 3663–3672. [Google Scholar] [CrossRef]
- Cunha-Silva, L.; Hardie, M.J. Exploring Ag···H-B interactions in coordination polymers: Silver- alkanedinitrile networks with cobalt carbaborane anions. CrystEngComm 2012, 14, 3367–3372. [Google Scholar] [CrossRef]
- Kauffman, G.B. Il’ya Il’ich Chernyaev (1893–1966) and the trans effect. J. Chem. Educ. 1977, 54, 86–89. [Google Scholar] [CrossRef]
- Appleton, T.G.; Clark, H.C.; Manzer, L.E. The trans-influence: Its measurement and significance. Coord. Chem. Rev. 1973, 10, 335–422. [Google Scholar] [CrossRef]
- Itatani, H.; Bailar, J.C. Homogenous catalysis in the reactions of olefinic substances. V. Hydrogenation of soybean oil methyl ester with triphenylphosphine and triphenylarsine palladium catalysts. J. Am. Oil Chem. Soc. 1967, 44, 147–151. [Google Scholar] [CrossRef]
- Chelucci, G.; Marchetti, M.; Sechi, B. Enantioselective hydroformylation of vinyl-aromatics using a rhodium complex with the (1R,2R,4R)-2-(2-diphenylphosphinyl-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl pyridine. J. Mol. Cat. A 1997, 122, 111–114. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Burlington, NJ, USA, 2009. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]




Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. Bis(dicarbollide) Complexes of Transition Metals as a Platform for Molecular Switches. Study of Complexation of 8,8′-Bis(methylsulfanyl) Derivatives of Cobalt and Iron Bis(dicarbollides). Molecules 2020, 25, 5745. https://doi.org/10.3390/molecules25235745
Anufriev SA, Timofeev SV, Anisimov AA, Suponitsky KY, Sivaev IB. Bis(dicarbollide) Complexes of Transition Metals as a Platform for Molecular Switches. Study of Complexation of 8,8′-Bis(methylsulfanyl) Derivatives of Cobalt and Iron Bis(dicarbollides). Molecules. 2020; 25(23):5745. https://doi.org/10.3390/molecules25235745
Chicago/Turabian StyleAnufriev, Sergey A., Sergey V. Timofeev, Alexei A. Anisimov, Kyrill Yu. Suponitsky, and Igor B. Sivaev. 2020. "Bis(dicarbollide) Complexes of Transition Metals as a Platform for Molecular Switches. Study of Complexation of 8,8′-Bis(methylsulfanyl) Derivatives of Cobalt and Iron Bis(dicarbollides)" Molecules 25, no. 23: 5745. https://doi.org/10.3390/molecules25235745